|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Delaware
|
|
20-8099512
|
|
(State of incorporation)
|
|
(I.R.S. Employer Identification No.)
|
|
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
||
|
Title of Each Class
|
|
Name of Exchange on Which Registered
|
|
Common Stock, $0.0001 Par Value per Share
|
|
The NASDAQ Capital Market
|
|
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
||
|
None
|
||
|
Large accelerated filer
|
¨
|
Accelerated filer
|
þ
|
|||
|
Non-accelerated filer
|
¨
|
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
¨
|
||
|
Part No.
|
Item No.
|
Description
|
Page No.
|
||
|
I
|
1
|
Business
|
|||
|
1A
|
Risk Factors
|
||||
|
1B
|
Unresolved Staff Comments
|
||||
|
2
|
Properties
|
||||
|
3
|
Legal Proceedings
|
||||
|
4
|
Mine Safety Disclosures
|
||||
|
II
|
5
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|||
|
6
|
Selected Financial Data
|
||||
|
7
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
|
||||
|
7A
|
Quantitative and Qualitative Disclosures About Market Risk
|
||||
|
II
|
8
|
Financial Statements and Supplementary Data
|
|||
|
9
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosures
|
||||
|
9A
|
Controls and Procedures
|
||||
|
9B
|
Other Information
|
||||
|
III
|
10
|
Directors, Executive Officers and Corporate Governance
|
|||
|
11
|
Executive Compensation
|
||||
|
12
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
||||
|
13
|
Certain Relationships and Related Transactions, and Director Independence
|
||||
|
14
|
Principal Accountant Fees and Services
|
||||
|
Index to Exhibits
|
|||||
|
EX-10.34
|
|||||
|
EX-10.35
|
|||||
|
EX-10.36
|
|||||
|
EX-10.37
|
|||||
|
EX-10.38
|
|||||
|
EX-10.39
|
|||||
|
EX-23.1
|
|||||
|
EX-31.1
|
|||||
|
EX-31.2
|
|||||
|
EX-32.1
|
|||||

|
•
|
Develop novel cancer immunotherapies to address unmet medical needs through the use of peptide-based vaccines targeting well-established tumor antigens. One of our key strategies is to target the adjuvant setting in patients with higher risk of recurrence, who had their primary treatment for cancer and have no evidence of disease, and are more likely to benefit from treatment via immunotherapy. Our immunotherapy programs are currently targeting two key areas: secondary prevention intended to significantly decrease the risk of disease recurrence in breast, gastric, and ovarian cancers; and primary prevention intended to cease or delay ductal carcinoma
in situ
(DCIS) from becoming invasive breast cancer.
|
|
•
|
Expand our development pipeline by enhancing the clinical and geographic footprint of our technologies. We intend to accomplish this through the initiation of new clinical trials and potentially through acquisition of additional oncology programs.
|
|
•
|
Leverage partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner.
|
|
•
|
Focus our resources on our valuable and expanding clinical development programs. On November 19, 2015 we sold our Abstral
®
(fentanyl) Sublingual Tablets product and related assets and on December 24, 2015 we sold Zuplenz (ondansetron) Oral Soluble Film product and related assets, and as of December 31, 2015, we ceased our commercial operations.
|

|
•
|
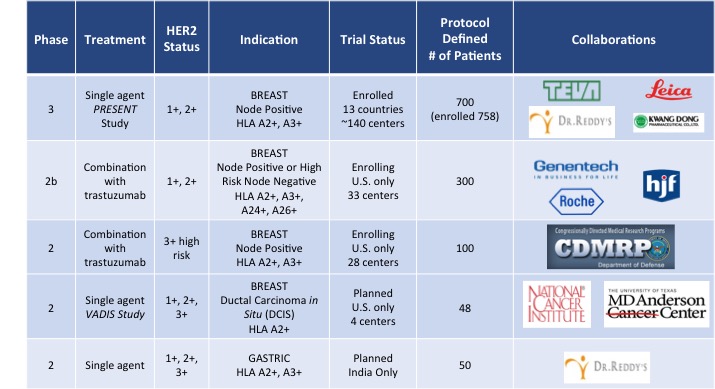
Phase 3 Ongoing: Our Phase 3 PRESENT (
P
revention of
R
ecurrence in
E
arly-
S
tage, Node-Positive Breast Cancer with Low to Intermediate HER2
E
xpression with
NeuVax T
reatment) study targeted enrollment of 700 HER2 1+/2+ patients who are HLA A2 or A3 positive under a Special Protocol Assessment (SPA) granted by the U.S. Food and Drug Administration (FDA). The multinational, multicenter, randomized, double-blinded PRESENT trial is ongoing in North America, Western and Eastern Europe, and Israel. The trial is fully enrolled with 758 patients.
|
|
•
|
Phase 2b Ongoing: A randomized, multicenter, investigator-sponsored, 300 patient Phase 2b clinical trial is enrolling HER2 1+/2+ node-positive and high-risk node-negative breast cancer patients who are HLA A2, A3, A24 and/or A26 positive to study NeuVax in combination with trastuzumab in the adjuvant setting. This investigator sponsored trial (IST) is co-funded by Genentech/Roche (providing both trastuzumab and monetary support) and Galena (providing NeuVax and monetary support).
|
|
•
|
Phase 2 Ongoing: An IST is also ongoing to study NeuVax in combination with trastuzumab. The study will enroll 100 node positive and negative HER2 IHC 3+ patients or HER2 gene-amplified breast cancer patients who are HLA A2 and/or HLA A3 positive and are determined to be at high-risk for recurrence. Partial funding for this trial comes from the Department of Defense (DoD) through the Congressionally Directed Medical Research Program via legislation known as the Defense Appropriations Act. The grant was awarded under a Breast Cancer Research Program with the Breakthrough Award given to the lead investigator for the trial.
|
|
•
|
Phase 2 Planned: A clinical trial, entitled, VADIS: Phase 2 trial of the Nelipepimut-S Peptide
VA
ccine in Women with
D
C
IS
of the Breast is planned to initiate in Q1 2016. The Phase 2 trial will be a single-blind, double arm, randomized, controlled trial in pre- or post-menopausal patients with DCIS who are HLA-A2 positive with HER2 expression of IHC 1+, 2+, or 3+. VADIS will be co-funded and run in collaboration with the National Cancer Institute (NCI).
|
|
•
|
Phase 2 Planned: A Phase 2 clinical trial in patients with gastric cancer is expected to initiate in 2016. The trial will be run in India by our partner, Dr. Reddy’s Laboratories, Ltd., as part of our NeuVax commercialization agreement in that region with Dr. Reddy’s.
|
|
Drug Candidate
|
Indication
|
Scope
|
Estimated
Exclusivity
Period
|
|
NeuVax™ (nelipepimut-S)
|
Breast cancer recurrence
|
Filed and pending or issued
worldwide
|
2028
|
|
NeuVax™ (nelipepimut-S)
|
Gastric
|
Filed and pending or issued
worldwide
|
2028
|
|
NeuVax™ (nelipepimut-S)
|
DCIS
|
Filed and pending or issued
worldwide
|
2028
|
|
NeuVax™ in combination with trastuzumab
|
Breast cancer
|
Filed and pending or issued
worldwide
|
2026
|
|
NeuVax™ in combination with other compounds
|
Breast cancer
|
Filed and pending or issued
worldwide
|
2037
|
|
GALE-301 & GALE-302
|
Breast, ovarian and endometrial cancer
|
Filed and pending or issued
worldwide
|
2035
|
|
GALE-401 (Anagrelide Controlled Release)
|
Platelet Lowering
|
Filed and pending or issued
worldwide
|
2029
|
|
•
|
difficulties or delays in enrolling patients in our Phase 1/2 clinical trials of GALE-301 and GALE-302 folate binding protein, or other clinical trials in conformity with required protocols or projected timeline or in our other NeuVax clinical trials;
|
|
•
|
conditions imposed on us by the FDA, including the possibility that the FDA would require an additional Phase 3 trial of NeuVax, or comparable foreign authorities regarding the scope or design of our clinical trials;
|
|
•
|
difficulties or delays in arranging for third parties to conduct clinical trials of our product candidates;
|
|
•
|
problems in engaging IRBs to oversee trials or problems in obtaining or maintaining IRB approval of studies;
|
|
•
|
third-party contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner;
|
|
•
|
our drug candidates having very different chemical and pharmacological properties in humans than in laboratory testing and interacting with human biological systems in unforeseen, ineffective or harmful ways, and the possibility that our previous Phase 2 trials will not be indicative of our drug candidates’ performance in larger patient populations;
|
|
•
|
the need to suspend or terminate our clinical trials if the participants are being exposed to unacceptable health risks;
|
|
•
|
insufficient or inadequate supply or quality of our drug candidates or other necessary materials necessary to conduct our clinical trials;
|
|
•
|
disruption at our foreign clinical trial sites resulting from local social or political unrest or other geopolitical factors;
|
|
•
|
effects of our drug candidates not being the desired effects or including undesirable side effects or the drug candidates having other unexpected characteristics;
|
|
•
|
negative or inconclusive results from our clinical trials or the clinical trials of others for drug candidates similar to our own or inability to generate statistically significant data confirming the efficacy of the product being tested;
|
|
•
|
adverse results obtained by other companies developing similar drugs;
|
|
•
|
modification of the drug during testing; and
|
|
•
|
reallocation of our financial and other resources to other clinical programs.
|
|
•
|
differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries;
|
|
•
|
unexpected changes in tariffs, trade barriers and regulatory requirements; economic weakness, including inflation, or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; foreign taxes, including withholding of payroll taxes;
|
|
•
|
foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations related to doing business or operating in another country;
|
|
•
|
workforce uncertainty in countries where labor unrest is more common than in the U.S.;
|
|
•
|
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
|
|
•
|
business interruptions resulting from geopolitical actions, including war and terrorism.
|
|
•
|
reports of the results of our clinical trials regarding the safety or efficacy of our product candidates and surrogate markers;
|
|
•
|
announcements of regulatory developments or technological innovations by us or our competitors;
|
|
•
|
announcements of business or strategic transactions;
|
|
•
|
announcements of legal or regulatory actions against us or any adverse outcome of any such actions;
|
|
•
|
changes in our relationship with our licensors, licensees and other strategic partners;
|
|
•
|
our quarterly operating results;
|
|
•
|
developments in patent or other technology ownership rights;
|
|
•
|
public concern regarding the safety of our product candidates;
|
|
•
|
additional funds may not be available on terms that are favorable to us and, in the case of equity financings, may result in dilution to our stockholders;
|
|
•
|
general changes in the economy, the financial markets or the pharmaceutical or biotechnology industries.
|
|
High
|
Low
|
||||||
|
2014
|
|||||||
|
First Quarter
|
$
|
7.77
|
|
$
|
2.15
|
|
|
|
Second Quarter
|
3.58
|
|
1.66
|
|
|||
|
Third Quarter
|
3.36
|
|
2.00
|
|
|||
|
Fourth Quarter
|
2.26
|
|
1.48
|
|
|||
|
2015
|
|||||||
|
First Quarter
|
$
|
2.12
|
|
$
|
1.33
|
|
|
|
Second Quarter
|
2.39
|
|
1.27
|
|
|||
|
Third Quarter
|
1.92
|
|
1.10
|
|
|||
|
Fourth Quarter
|
1.83
|
|
1.37
|
|
|||
|
(a)
|
(b)
|
Number of
Securities
Remaining
Available for
Issuance
Under Equity
Compensation
Plans
(Excluding
Securities
Reflected in
Column (a))
|
|||||||
|
Plan Category
|
Number of
Securities to be
Issued Upon
Exercise of
Outstanding
Options,
Warrants and
Rights
|
Weighted-
Average
Exercise Price
of Outstanding
Options,
Warrants and
Rights
|
|||||||
|
Equity compensation plans approved by our security holders:
|
|||||||||
|
Amended and Restated 2007 Incentive Plan
|
13,261,950
|
|
$
|
2.58
|
|
8,177,252
|
|
||
|
Equity compensation plans not approved by our security holders:
|
|||||||||
|
Employee Stock Purchase Plan
|
NA
|
|
NA
|
|
528,131
|
|
|||
|
Outstanding warrants
(1)
|
482,186
|
|
$
|
3.40
|
|
—
|
|
||
|
Total
|
13,744,136
|
|
$
|
2.61
|
|
8,705,383
|
|
||
|
(1)
|
The warrants shown were issued in discrete transactions from time to time as compensation for services rendered by consultants, advisers or other third parties, and do not include warrants sold in private placement or public offering transactions. The material terms of such warrants were determined based upon arm’s-length negotiations with the services providers. The warrant exercise prices approximated the market price of our common stock at or about the date of grant, and the warrant terms range from three to ten years from the grant date.
|

|
As of December 31,
|
|||||||||||||||||||||||
|
2010
|
2011
|
2012
|
2013
|
2014
|
2015
|
||||||||||||||||||
|
Galena Biopharma, Inc.
(1)
|
$
|
100.00
|
|
$
|
18.22
|
|
$
|
59.30
|
|
$
|
192.25
|
|
$
|
58.53
|
|
$
|
56.98
|
|
|||||
|
S&P 500
|
100.00
|
|
102.09
|
|
118.31
|
|
156.21
|
|
177.32
|
|
179.76
|
|
|||||||||||
|
NASDAQ Composite
|
100.00
|
|
99.23
|
|
116.80
|
|
163.38
|
|
187.42
|
|
200.70
|
|
|||||||||||
|
NASDAQ Biotechnology
|
100.00
|
|
112.06
|
|
148.73
|
|
146.78
|
|
331.57
|
|
370.60
|
|
|||||||||||
|
Years Ended December 31,
|
|||||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
2011
|
|||||||||||||||
|
Operating expenses:
|
|||||||||||||||||||
|
Research and development
(1)
|
$
|
23,611
|
|
$
|
27,674
|
|
$
|
20,424
|
|
$
|
14,614
|
|
$
|
3,851
|
|
||||
|
General, and administrative
(1)
|
10,609
|
|
16,226
|
|
8,065
|
|
6,585
|
|
8,635
|
|
|||||||||
|
Non-operating income (loss)
(1)
|
(4,371
|
)
|
15,616
|
|
(41,786
|
)
|
(13,178
|
)
|
9,079
|
|
|||||||||
|
Loss from continuing operations
(1)
|
(38,956
|
)
|
(28,284
|
)
|
(71,327
|
)
|
(33,325
|
)
|
(3,407
|
)
|
|||||||||
|
Loss from continuing operations per share, basic and diluted
(1)
|
$
|
(0.25
|
)
|
$
|
(0.24
|
)
|
$
|
(0.79
|
)
|
$
|
(0.53
|
)
|
$
|
(0.09
|
)
|
||||
|
As of December 31,
|
|||||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
2011
|
|||||||||||||||
|
Total assets
(1)
|
$
|
82,144
|
|
$
|
80,488
|
|
$
|
87,976
|
|
$
|
54,986
|
|
$
|
30,968
|
|
||||
|
Total debt
(1)
|
4,739
|
|
8,402
|
|
9,892
|
|
—
|
|
—
|
|
|||||||||
|
Other long-term obligations
(1)
|
11,560
|
|
11,704
|
|
11,874
|
|
11,311
|
|
9,654
|
|
|||||||||
|
Total stockholders' equity
(1)
|
13,513
|
|
37,059
|
|
5,886
|
|
27,756
|
|
10,112
|
|
|||||||||
|
•
|
Develop novel cancer immunotherapies to address unmet medical needs through the use of peptide-based vaccines targeting well-established tumor antigens. One of our key strategies is to target the adjuvant setting in patients with higher risk of recurrence, who had their primary treatment for cancer and have no evidence of disease, and are more likely to benefit from treatment via immunotherapy. Our immunotherapy programs are currently targeting two key areas: secondary prevention intended to significantly decrease the risk of disease recurrence in breast, gastric, and ovarian cancers; and primary prevention intended to cease or delay ductal carcinoma
in situ
(DCIS) from becoming invasive breast cancer.
|
|
•
|
Expand our development pipeline by enhancing the clinical and geographic footprint of our technologies. We intend to accomplish this through the initiation of new clinical trials and potentially through acquisition of additional oncology programs.
|
|
•
|
Leverage partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner.
|
|
•
|
Focus our resources on our valuable and expanding clinical development programs. On November 19, 2015 we sold our Abstral
®
(fentanyl) Sublingual Tablets product and related assets and on December 24, 2015 we sold Zuplenz (ondansetron) Oral Soluble Film product and related assets, and as of December 31, 2015, we ceased our commercial operations.
|
|
2015
|
2014
|
|||||
|
Risk free interest rate
|
1.67
|
%
|
2.01
|
%
|
||
|
Volatility
|
73.97
|
%
|
79.37
|
%
|
||
|
Expected lives (years)
|
6.16
|
|
6.16
|
|
||
|
Expected dividend yield
|
0.00
|
%
|
0.00
|
%
|
||
|
2015
|
2014
|
|||
|
Risk free interest rate
|
1.41%
|
NA
|
||
|
Volatility
|
73.41%
|
NA
|
||
|
Expected lives (years)
|
5
|
NA
|
||
|
Expected dividend yield
|
0.00%
|
NA
|
||
|
•
|
significant changes in the manner of its use of acquired assets or the strategy for its overall business;
|
|
•
|
significant negative industry or economic trends;
|
|
•
|
significant decline in stock price for a sustained period; and
|
|
•
|
significant decline in market capitalization relative to net book value.
|
|
Year Ended December 31,
|
Year Ended December 31,
|
||||||||||||||||||||
|
2015
|
2014
|
% Change
|
2014
|
2013
|
% Change
|
||||||||||||||||
|
Research and development expense
|
$
|
23,611
|
|
$
|
27,674
|
|
(15
|
)%
|
$
|
27,674
|
|
$
|
20,424
|
|
35
|
%
|
|||||
|
Year Ended December 31,
|
||||||||||
|
2015
|
2014
|
% Change
|
||||||||
|
General and administrative expense
|
$
|
10,609
|
|
$
|
16,226
|
|
(35
|
)%
|
||
|
Year Ended December 31,
|
||||||||||
|
2014
|
2013
|
% Change
|
||||||||
|
General and administrative expense
|
$
|
16,226
|
|
$
|
8,065
|
|
101
|
%
|
||
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
$ Change
|
||||||||||
|
Non-operating income (expense):
|
||||||||||||
|
Litigation settlement
|
$
|
(5,282
|
)
|
$
|
—
|
|
$
|
(5,282
|
)
|
|||
|
Change in fair value of warrants potentially settleable in cash
|
1,162
|
|
16,556
|
|
(15,394
|
)
|
||||||
|
Interest income (expense), net
|
(760
|
)
|
(1,110
|
)
|
350
|
|
||||||
|
Other income (expense)
|
509
|
|
170
|
|
339
|
|
||||||
|
Total non-operating income (expense), net
|
$
|
(4,371
|
)
|
$
|
15,616
|
|
$
|
(19,987
|
)
|
|||
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
$ Change
|
||||||||||
|
Non-operating income (expense):
|
||||||||||||
|
Change in fair value of warrants potentially settleable in cash
|
$
|
16,556
|
|
$
|
(44,001
|
)
|
$
|
60,557
|
|
|||
|
Interest income (expense), net
|
(1,110
|
)
|
(807
|
)
|
(303
|
)
|
||||||
|
Other income (expense)
|
170
|
|
3,022
|
|
(2,852
|
)
|
||||||
|
Total non-operating income (expense), net
|
$
|
15,616
|
|
$
|
(41,786
|
)
|
$
|
57,402
|
|
|||
|
2015
|
2014
|
2013
|
|||||||||
|
Net revenue
|
$
|
9,734
|
|
$
|
9,319
|
|
$
|
2,487
|
|
||
|
Cost of revenue
|
(1,780
|
)
|
(1,403
|
)
|
(520
|
)
|
|||||
|
Amortization of certain acquired intangible assets
|
(921
|
)
|
(440
|
)
|
(131
|
)
|
|||||
|
Research and development
|
(355
|
)
|
(680
|
)
|
(651
|
)
|
|||||
|
Selling, general, and administrative
|
(17,655
|
)
|
(15,118
|
)
|
(6,536
|
)
|
|||||
|
Impairment charge from classification as held for sale
|
(8,071
|
)
|
—
|
|
—
|
|
|||||
|
Loss on sale of commercial business assets
|
(4,549
|
)
|
—
|
|
—
|
|
|||||
|
Severance and exit costs
|
(1,349
|
)
|
—
|
|
—
|
|
|||||
|
Loss from discontinued operations
|
$
|
(24,946
|
)
|
$
|
(8,322
|
)
|
$
|
(5,351
|
)
|
||
|
For the Year Ended December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Cash flows from continuing operations:
|
||||||||
|
Cash flows used in continuing operating activities
|
$
|
(38,802
|
)
|
$
|
(37,037
|
)
|
||
|
Cash flows provided by (used in) continuing investing activities
|
(354
|
)
|
(2,472
|
)
|
||||
|
Cash flows provided by continuing financing activities
|
43,845
|
|
24,260
|
|
||||
|
Total cash flows provided by (used in) continuing operations
|
4,689
|
|
(15,249
|
)
|
||||
|
Cash flows from discontinued operations:
|
||||||||
|
Cash flows used in discontinued operating activities
|
(9,358
|
)
|
(5,832
|
)
|
||||
|
Cash flows provided by (used in) discontinued investing activities
|
10,749
|
|
—
|
|
(3,056
|
)
|
||
|
Total cash flows provided by (used in) discontinued operations
|
1,391
|
|
(8,888
|
)
|
||||
|
Total cash flows:
|
||||||||
|
Cash flows used in operating activities
|
(48,160
|
)
|
(42,869
|
)
|
||||
|
Cash flows provided by (used in) investing activities
|
10,395
|
|
(5,528
|
)
|
||||
|
Cash flows provided by financing activities
|
43,845
|
|
24,260
|
|
||||
|
Total increase (decrease) in cash and cash equivalents
|
$
|
6,080
|
|
$
|
(24,137
|
)
|
||
|
Payment Due by Period
|
||||||||||||||||
|
Less than 1 Year
|
1 to 3 Years
|
3 to 5 Years
|
Total
|
|||||||||||||
|
Long-term debt
(1)
|
$
|
4,739
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4,739
|
|
||||
|
Cancelable license agreements
(2)
|
350
|
|
700
|
|
7,165
|
|
8,215
|
|
||||||||
|
Non-cancelable employment agreements
(2)
|
850
|
|
—
|
|
—
|
|
850
|
|
||||||||
|
Non-cancelable operating leases
(2)
|
316
|
|
639
|
|
487
|
|
1,442
|
|
||||||||
|
Total
|
$
|
6,255
|
|
$
|
1,339
|
|
$
|
7,652
|
|
$
|
15,246
|
|
||||
|
Page No.
|
|
|
December 31, 2015
|
December 31, 2014
|
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
29,730
|
|
$
|
23,650
|
|
|
|
Restricted cash
|
401
|
|
200
|
|
|||
|
Litigation settlement insurance recovery
|
21,700
|
|
—
|
|
|||
|
Prepaid expenses and other current assets
|
1,398
|
|
1,237
|
|
|||
|
Current assets of discontinued operations
|
392
|
|
27,013
|
|
|||
|
Total current assets
|
53,621
|
|
52,100
|
|
|||
|
Equipment and furnishings, net
|
335
|
|
285
|
|
|||
|
GALE-401 rights
|
9,255
|
|
9,255
|
|
|||
|
In-process research and development
|
12,864
|
|
12,864
|
|
|||
|
Goodwill
|
5,898
|
|
5,897
|
|
|||
|
Deposits and other assets
|
171
|
|
87
|
|
|||
|
Total assets
|
$
|
82,144
|
|
$
|
80,488
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
1,597
|
|
$
|
1,886
|
|
|
|
Accrued expenses and other current liabilities
|
5,292
|
|
8,885
|
|
|||
|
Litigation settlement payable
|
25,000
|
|
—
|
|
|||
|
Fair value of warrants potentially settleable in cash
|
14,518
|
|
5,383
|
|
|||
|
Current portion of long-term debt
|
4,739
|
|
3,910
|
|
|||
|
Current liabilities of discontinued operations
|
5,925
|
|
7,169
|
|
|||
|
Total current liabilities
|
57,071
|
|
27,233
|
|
|||
|
Deferred tax liability
|
5,418
|
|
5,053
|
|
|||
|
Contingent purchase price consideration
|
6,142
|
|
6,651
|
|
|||
|
Long-term debt, net of current portion
|
—
|
|
4,492
|
|
|||
|
Total liabilities
|
68,631
|
|
43,429
|
|
|||
|
Commitments and contingencies
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value; 275,000,000 shares authorized, 162,581,753 shares issued and 161,906,753 shares outstanding at December 31, 2015; 200,000,000 shares authorized, 130,146,341 shares issued and 129,471,341 shares outstanding at December 31, 2014
|
15
|
|
12
|
|
|||
|
Additional paid-in capital
|
296,730
|
|
256,377
|
|
|||
|
Accumulated deficit
|
(279,383
|
)
|
(215,481
|
)
|
|||
|
Less treasury shares at cost, 675,000 shares
|
(3,849
|
)
|
(3,849
|
)
|
|||
|
Total stockholders’ equity
|
13,513
|
|
37,059
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
82,144
|
|
$
|
80,488
|
|
|
|
For the Year Ended December 31,
|
|||||||||||
|
2015
|
2014
|
2013
|
|||||||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
$
|
23,611
|
|
$
|
27,674
|
|
$
|
20,424
|
|
||
|
General and administrative
|
10,609
|
|
16,226
|
|
8,065
|
|
|||||
|
Total operating expenses
|
34,220
|
|
43,900
|
|
28,489
|
|
|||||
|
Operating loss
|
(34,220
|
)
|
(43,900
|
)
|
(28,489
|
)
|
|||||
|
Non-operating income (expense):
|
|||||||||||
|
Litigation settlement
|
(5,282
|
)
|
—
|
|
—
|
|
|||||
|
Gain (loss) on warrant derivative liability
|
1,162
|
|
16,556
|
|
(44,001
|
)
|
|||||
|
Interest expense, net
|
(760
|
)
|
(1,110
|
)
|
(807
|
)
|
|||||
|
Other income
|
509
|
|
170
|
|
3,022
|
|
|||||
|
Total non-operating income (expense), net
|
(4,371
|
)
|
15,616
|
|
(41,786
|
)
|
|||||
|
Loss from continuing operations before income taxes
|
(38,591
|
)
|
(28,284
|
)
|
(70,275
|
)
|
|||||
|
Income tax expense
|
365
|
|
—
|
|
1,052
|
|
|||||
|
Loss from continuing operations
|
(38,956
|
)
|
(28,284
|
)
|
(71,327
|
)
|
|||||
|
Loss from discontinued operations
|
(24,946
|
)
|
(8,322
|
)
|
(5,351
|
)
|
|||||
|
Net loss
|
$
|
(63,902
|
)
|
$
|
(36,606
|
)
|
$
|
(76,678
|
)
|
||
|
Net loss per common share:
|
|||||||||||
|
Basic and diluted per share, continuing operations
|
$
|
(0.25
|
)
|
$
|
(0.24
|
)
|
$
|
(0.79
|
)
|
||
|
Basic and diluted loss per share, discontinued operations
|
$
|
(0.16
|
)
|
$
|
(0.07
|
)
|
$
|
(0.06
|
)
|
||
|
Basic and diluted net loss per share
|
$
|
(0.41
|
)
|
$
|
(0.31
|
)
|
$
|
(0.85
|
)
|
||
|
Weighted-average common shares outstanding: basic and diluted
|
155,264,729
|
|
119,388,366
|
|
90,181,501
|
|
|||||
|
Comprehensive loss
|
|||||||||||
|
Net loss
|
$
|
(63,902
|
)
|
$
|
(36,606
|
)
|
$
|
(76,678
|
)
|
||
|
Reclassification of unrealized gain upon sale of marketable securities
|
—
|
|
—
|
|
(2,678
|
)
|
|||||
|
Tax effect of reclassification of unrealized gain upon sale of marketable securities
|
—
|
|
—
|
|
1,052
|
|
|||||
|
Total comprehensive loss
|
$
|
(63,902
|
)
|
$
|
(36,606
|
)
|
$
|
(78,304
|
)
|
||
|
Common Stock
|
Additional Paid-In Capital
|
Accumulated Other Comprehensive Income (Loss)
|
Accumulated Deficit
|
Treasury Stock
|
Total
|
|||||||||||||||||||||
|
Shares Issued
|
Amount
|
|||||||||||||||||||||||||
|
Balance at December 31, 2012
|
83,595,837
|
|
$
|
8
|
|
$
|
132,168
|
|
$
|
1,626
|
|
$
|
(102,197
|
)
|
$
|
(3,849
|
)
|
$
|
27,756
|
|
||||||
|
Issuance of common stock
|
20,125,000
|
|
2
|
|
37,537
|
|
—
|
|
—
|
|
—
|
|
37,539
|
|
||||||||||||
|
Common stock warrants issued in connection with September 2013 common stock offering
|
—
|
|
—
|
|
(8,238
|
)
|
—
|
|
—
|
|
—
|
|
(8,238
|
)
|
||||||||||||
|
Issuance of common stock upon exercise of warrants
|
5,320,669
|
|
—
|
|
22,064
|
|
—
|
|
—
|
|
—
|
|
22,064
|
|
||||||||||||
|
Issuance of common stock in settlement of contingent purchase price consideration
|
492,988
|
|
—
|
|
1,247
|
|
—
|
|
—
|
|
—
|
|
1,247
|
|
||||||||||||
|
Issuance of common stock warrants with long-term debt financing
|
—
|
|
—
|
|
351
|
|
—
|
|
—
|
|
—
|
|
351
|
|
||||||||||||
|
Issuance of common stock in exchange for services
|
99,998
|
|
—
|
|
211
|
|
—
|
|
—
|
|
—
|
|
211
|
|
||||||||||||
|
Issuance of common stock in connection with employee stock purchase plan
|
52,532
|
|
—
|
|
163
|
|
—
|
|
—
|
|
—
|
|
163
|
|
||||||||||||
|
Stock based compensation for directors and employees
|
—
|
|
—
|
|
1,886
|
|
—
|
|
—
|
|
—
|
|
1,886
|
|
||||||||||||
|
Stock based compensation for services
|
—
|
|
—
|
|
644
|
|
—
|
|
—
|
|
—
|
|
644
|
|
||||||||||||
|
Reclassification of unrealized gain upon the sale of marketable securities, net of tax of $1,052
|
—
|
|
—
|
|
—
|
|
(1,626
|
)
|
—
|
|
—
|
|
(1,626
|
)
|
||||||||||||
|
Exercise of stock options
|
413,677
|
|
—
|
|
567
|
|
—
|
|
—
|
|
—
|
|
567
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(76,678
|
)
|
—
|
|
(76,678
|
)
|
||||||||||||
|
Balance at December 31, 2013
|
110,100,701
|
|
$
|
10
|
|
$
|
188,600
|
|
$
|
—
|
|
$
|
(178,875
|
)
|
$
|
(3,849
|
)
|
$
|
5,886
|
|
||||||
|
Issuance of common stock
|
6,633,008
|
|
1
|
|
10,704
|
|
—
|
|
—
|
|
—
|
|
10,705
|
|
||||||||||||
|
Issuance of common stock under milestone achievement
|
4,381,215
|
|
—
|
|
9,340
|
|
—
|
|
—
|
|
—
|
|
9,340
|
|
||||||||||||
|
Issuance of common stock upon exercise of warrants
|
5,467.027
|
|
1
|
|
37,741
|
|
—
|
|
—
|
|
—
|
|
37,742
|
|
||||||||||||
|
Issuance of common stock in connection with employee stock purchase plan
|
114,630
|
|
—
|
|
263
|
|
—
|
|
—
|
|
—
|
|
263
|
|
||||||||||||
|
Stock based compensation for directors and employees
|
—
|
|
—
|
|
5,253
|
|
—
|
|
—
|
|
—
|
|
5,253
|
|
||||||||||||
|
Stock based compensation for services
|
—
|
|
—
|
|
134
|
|
—
|
|
—
|
|
—
|
|
134
|
|
||||||||||||
|
Exercise of stock options
|
3,449,760
|
|
—
|
|
4,342
|
|
—
|
|
—
|
|
—
|
|
4,342
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(36,606
|
)
|
—
|
|
(36,606
|
)
|
||||||||||||
|
Balance at December 31, 2014
|
130,146,341
|
|
$
|
12
|
|
$
|
256,377
|
|
$
|
—
|
|
$
|
(215,481
|
)
|
$
|
(3,849
|
)
|
$
|
37,059
|
|
||||||
|
Issuance of common stock
|
32,158,685
|
|
3
|
|
47,413
|
|
—
|
|
—
|
|
—
|
|
47,416
|
|
||||||||||||
|
Common stock warrants issued in connection with March 2015 common stock offering
|
—
|
|
—
|
|
(10,296
|
)
|
—
|
|
—
|
|
—
|
|
(10,296
|
)
|
||||||||||||
|
Issuance of common stock in connection with employee stock purchase plan
|
231,312
|
|
—
|
|
309
|
|
—
|
|
—
|
|
—
|
|
309
|
|
||||||||||||
|
Stock based compensation for directors and employees
|
—
|
|
—
|
|
2,896
|
|
—
|
|
—
|
|
—
|
|
2,896
|
|
||||||||||||
|
Exercise of stock options
|
45,415
|
|
—
|
|
31
|
|
—
|
|
—
|
|
—
|
|
31
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(63,902
|
)
|
—
|
|
(63,902
|
)
|
||||||||||||
|
Balance at December 31, 2015
|
162,581,753
|
|
$
|
15
|
|
$
|
296,730
|
|
$
|
—
|
|
$
|
(279,383
|
)
|
$
|
(3,849
|
)
|
$
|
13,513
|
|
||||||
|
For the Year Ended December 31,
|
|||||||||||||
|
2015
|
2014
|
2013
|
|||||||||||
|
Cash flows from operating activities:
|
|||||||||||||
|
Cash flows from continuing operating activities:
|
|||||||||||||
|
Net loss from continuing operations
|
$
|
(38,956
|
)
|
$
|
(28,284
|
)
|
$
|
(71,327
|
)
|
||||
|
Adjustment to reconcile net loss to net cash used in operating activities:
|
|||||||||||||
|
Depreciation and amortization expense
|
355
|
|
362
|
|
286
|
|
|||||||
|
Gain on sale of marketable securities
|
—
|
|
—
|
|
(3,911
|
)
|
|||||||
|
Deferred taxes
|
365
|
|
—
|
|
1,052
|
|
|||||||
|
Non-cash stock-based compensation
|
1,931
|
|
4,666
|
|
2,307
|
|
|||||||
|
Litigation settlement payable in common stock
|
1,000
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Fair value of common stock issued in exchange for services
|
—
|
|
—
|
|
211
|
|
|||||||
|
Change in fair value of common stock warrants
|
(1,161
|
)
|
(16,556
|
)
|
44,001
|
|
|||||||
|
Change in fair value of contingent consideration
|
(509
|
)
|
(170
|
)
|
926
|
|
|||||||
|
Changes in operating assets and liabilities:
|
|||||||||||||
|
Prepaid expenses and other assets
|
(245
|
)
|
(1,078
|
)
|
437
|
|
|||||||
|
Litigation settlement insurance recovery
|
(21,700
|
)
|
—
|
|
—
|
|
|||||||
|
Litigation settlement payable
|
24,000
|
|
—
|
|
—
|
|
|||||||
|
Accounts payable
|
(289
|
)
|
(21
|
)
|
(69
|
)
|
|||||||
|
Accrued expenses and other current liabilities
|
(3,593
|
)
|
4,044
|
|
2,811
|
|
|||||||
|
Net cash used in continuing operating activities
|
(38,802
|
)
|
(37,037
|
)
|
(23,276
|
)
|
|||||||
|
Cash flows from discontinued operating activities:
|
|||||||||||||
|
Net loss from discontinued operations
|
(24,946
|
)
|
(8,322
|
)
|
(5,351
|
)
|
|||||||
|
Loss on sale of commercial assets
|
4,549
|
|
—
|
|
—
|
|
|||||||
|
Impairment charge from classification of assets held for sale
|
8,071
|
|
—
|
|
—
|
|
|||||||
|
Changes in operating assets and liabilities attributable to discontinued operations
|
2,968
|
|
2,490
|
|
(302
|
)
|
|||||||
|
Net cash used in discontinued operating activities
|
(9,358
|
)
|
(5,832
|
)
|
(5,653
|
)
|
|||||||
|
Net cash used in operating activities
|
(48,160
|
)
|
(42,869
|
)
|
(28,929
|
)
|
|||||||
|
Cash flows from investing activities:
|
|||||||||||||
|
Change in restricted cash
|
(201
|
)
|
—
|
|
(99
|
)
|
|||||||
|
Cash paid for acquisition of GALE-401
|
—
|
|
(2,415
|
)
|
—
|
|
|||||||
|
Purchase of short-term investments
|
—
|
|
—
|
|
3,911
|
|
|||||||
|
Cash paid for purchase of equipment and furnishings
|
(153
|
)
|
(57
|
)
|
(320
|
)
|
|||||||
|
Net cash provided by (used in) continuing investing activities
|
(354
|
)
|
(2,472
|
)
|
3,492
|
|
|||||||
|
Net proceeds received from sale of commercial assets
|
11,283
|
|
—
|
|
—
|
|
|||||||
|
Cash paid for commercial assets
|
(534
|
)
|
(3,056
|
)
|
(15,532
|
)
|
|||||||
|
Net cash provided by (used in) discontinued investing activities
|
10,749
|
|
—
|
|
(3,056
|
)
|
—
|
|
(15,532
|
)
|
|||
|
Net cash provided by (used in) investing activities
|
10,395
|
|
(5,528
|
)
|
(12,040
|
)
|
|||||||
|
Cash flows from financing activities:
|
|||||||||||||
|
Net proceeds from issuance of common stock
|
47,416
|
|
10,704
|
|
37,539
|
|
|||||||
|
Net proceeds from exercise of stock options
|
31
|
|
4,342
|
|
567
|
|
|||||||
|
Proceeds from exercise of warrants
|
—
|
|
10,717
|
|
7,815
|
|
|||||||
|
Proceeds from common stock issued in connection with ESPP
|
309
|
|
263
|
|
163
|
|
|||||||
|
Net proceeds from issuance of long-term debt
|
—
|
|
—
|
|
9,865
|
|
|||||||
|
Principal payments on long-term debt
|
(3,911
|
)
|
(1,766
|
)
|
—
|
|
|||||||
|
Net cash provided by financing activities
|
43,845
|
|
24,260
|
|
55,949
|
|
|||||||
|
Net increase (decrease) in cash and cash equivalents
|
6,080
|
|
(24,137
|
)
|
14,980
|
|
|||||||
|
Cash and cash equivalents at the beginning of period
|
23,650
|
|
47,787
|
|
32,807
|
|
|||||||
|
Cash and cash equivalents at end of period
|
$
|
29,730
|
|
$
|
23,650
|
|
$
|
47,787
|
|
||||
|
For the Year Ended December 31,
|
|||||||||||
|
2015
|
2014
|
2013
|
|||||||||
|
Supplemental disclosure of cash flow information:
|
|||||||||||
|
Cash received during the periods for interest
|
$
|
18
|
|
$
|
15
|
|
$
|
19
|
|
||
|
Cash paid during the periods for interest
|
$
|
541
|
|
$
|
800
|
|
$
|
547
|
|
||
|
Supplemental disclosure of non-cash investing and financing activities:
|
|||||||||||
|
Fair value of warrants issued in connection with common stock recorded as cost of equity
|
$
|
10,296
|
|
$
|
—
|
|
$
|
8,238
|
|
||
|
Reclassification of warrant liabilities upon exercise
|
$
|
—
|
|
$
|
27,026
|
|
$
|
14,249
|
|
||
|
Common stock issued in settlement of contingent purchase price consideration
|
$
|
—
|
|
$
|
—
|
|
$
|
1,247
|
|
||
|
Change in fair value of marketable securities before settlement
|
$
|
—
|
|
$
|
—
|
|
$
|
(2,678
|
)
|
||
|
Issuance of common stock in settlement of GALE-401 milestone
|
$
|
—
|
|
$
|
6,840
|
|
$
|
—
|
|
||
|
Fair value of shares issued to acquire Zuplenz rights
|
$
|
—
|
|
$
|
2,500
|
|
$
|
—
|
|
||
|
Future obligations for Zuplenz rights included in accrued expenses
|
$
|
—
|
|
$
|
2,716
|
|
$
|
—
|
|
||
|
•
|
Develop novel cancer immunotherapies to address unmet medical needs through the use of peptide-based vaccines targeting well-established tumor antigens. One of our key strategies is to target the adjuvant setting in patients with higher risk of recurrence, who had their primary treatment for cancer and have no evidence of disease, and are more likely to benefit from treatment via immunotherapy. Our immunotherapy programs are currently targeting two key areas: secondary prevention intended to significantly decrease the risk of disease recurrence in breast, gastric, and ovarian cancers; and primary prevention intended to cease or delay ductal carcinoma
in situ
(DCIS) from becoming invasive breast cancer.
|
|
•
|
Expand our development pipeline by enhancing the clinical and geographic footprint of our technologies. We intend to accomplish this through the initiation of new clinical trials and potentially through acquisition of additional oncology programs.
|
|
•
|
Leverage partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner.
|
|
•
|
Focus our resources on our valuable and expanding clinical development programs. On November 19, 2015 we sold our Abstral
®
(fentanyl) Sublingual Tablets product and related assets and on December 24, 2015 we sold Zuplenz (ondansetron) Oral Soluble Film product and related assets, and as of December 31, 2015, we ceased our commercial operations.
|
|
December 31, 2015
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
29,171
|
|
$
|
29,171
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total assets measured and recorded at fair value
|
$
|
29,171
|
|
$
|
29,171
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Warrants potentially settleable in cash
|
$
|
14,518
|
|
$
|
—
|
|
$
|
14,518
|
|
$
|
—
|
|
|||
|
Contingent purchase price consideration
|
6,142
|
|
—
|
|
—
|
|
6,142
|
|
|||||||
|
Total liabilities measured and recorded at fair value
|
$
|
20,660
|
|
$
|
—
|
|
$
|
14,518
|
|
$
|
6,142
|
|
|||
|
December 31, 2014
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
19,477
|
|
$
|
19,477
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total assets measured and recorded at fair value
|
$
|
19,477
|
|
$
|
19,477
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Warrants potentially settleable in cash
|
$
|
5,383
|
|
$
|
—
|
|
$
|
5,383
|
|
$
|
—
|
|
|||
|
Contingent purchase price consideration
|
6,651
|
|
—
|
|
—
|
|
6,651
|
|
|||||||
|
Total liabilities measured and recorded at fair value
|
$
|
12,034
|
|
$
|
—
|
|
$
|
5,383
|
|
$
|
6,651
|
|
|||
|
|
Fair Value
Measurements
Using Significant
Unobservable
Inputs
(Level 3)
|
||
|
Balance, January 1, 2014
|
$
|
6,821
|
|
|
Change in the estimated fair value of the contingent purchase price consideration
|
(170
|
)
|
|
|
Balance, December 31, 2014
|
6,651
|
|
|
|
Change in the estimated fair value of the contingent purchase price consideration
|
(509
|
)
|
|
|
Balance at December 31, 2015
|
$
|
6,142
|
|
|
December 31,
|
|||||||
|
2015
|
2014
|
||||||
|
Clinical development expense
|
$
|
3,294
|
|
$
|
6,967
|
|
|
|
Compensation and related benefits
|
1,535
|
|
1,040
|
|
|||
|
Professional fees
|
435
|
|
821
|
|
|||
|
Interest expense
|
28
|
|
57
|
|
|||
|
Accrued expenses and other current liabilities
|
$
|
5,292
|
|
$
|
8,885
|
|
|
|
2016 Principal Payments
|
$
|
4,254
|
|
|
|
Add - 5.5% cash final payment (less Unamortized debt issuance costs)
|
485
|
|
||
|
Carrying value of long-term debt
|
$
|
4,739
|
|
|
|
Amount
|
|||
|
Class action settlement
|
$
|
20,000
|
|
|
Derivative settlement
|
5,000
|
|
|
|
Total settlement payable
|
25,000
|
|
|
|
Payable by the insurance carriers
|
21,700
|
|
|
|
Payable by the company in cash
|
2,300
|
|
|
|
Payable by the company in common stock
|
1,000
|
|
|
|
Total settlement payable
|
$
|
25,000
|
|
|
Operating
Leases
(1)
|
Non-Cancelable
Employment
Agreements
(2)
|
Subtotal
|
Cancelable
License
Agreements
(3)
|
Total
|
|||||||||||||||
|
2016
|
$
|
316
|
|
$
|
850
|
|
$
|
1,166
|
|
$
|
350
|
|
$
|
1,516
|
|
||||
|
2017
|
323
|
|
—
|
|
323
|
|
350
|
|
673
|
|
|||||||||
|
2018
|
316
|
|
—
|
|
316
|
|
350
|
|
666
|
|
|||||||||
|
2019
|
251
|
|
—
|
|
251
|
|
4,200
|
|
4,451
|
|
|||||||||
|
2020 and thereafter
|
236
|
|
—
|
|
236
|
|
2,965
|
|
3,201
|
|
|||||||||
|
Total
|
$
|
1,442
|
|
$
|
850
|
|
$
|
2,292
|
|
$
|
8,215
|
|
$
|
10,507
|
|
||||
|
(1)
|
Operating leases are primarily facility and equipment related obligations with third party vendors. Operating lease expenses during the years ended December 31, 2015, 2014, and 2013 were approximately
$116,000
,
$72,000
and
$77,000
, respectively.
|
|
(2)
|
Employment agreement obligations include management contracts, as well as scientific advisory board member compensation agreements. Certain agreements, which have been revised from time to time, provide for minimum salary levels, adjusted annually at the discretion of the Compensation Committee, as well as for minimum bonuses that are payable.
|
|
(3)
|
License agreements generally relate to the company’s obligations with The Board of Regents, University of Texas and Henry M. Jackson Foundation for our oncology therapies. The company continually assesses the progress of its licensed technology and the progress of its research and development efforts as it relates to its licensed technology and may terminate with notice to the licensor at any time. In the event these licenses are terminated, no amounts will be due.
|
|
March
2015 Warrants |
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
Other Warrant Issuances
|
Consultant
and Oxford Warrants
|
Total
|
||||||||||||||
|
Outstanding, January 1, 2014
|
—
|
|
6,442
|
|
4,917
|
|
1,158
|
|
1,444
|
|
889
|
|
14,850
|
|
||||||
|
Granted
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
300
|
|
300
|
|
||||||
|
Exercised
|
—
|
|
(2,469
|
)
|
(1,886
|
)
|
(543
|
)
|
(327
|
)
|
(469
|
)
|
(5,694
|
)
|
||||||
|
Expired
|
—
|
|
—
|
|
—
|
|
—
|
|
(916
|
)
|
—
|
|
(916
|
)
|
||||||
|
Outstanding, December 31, 2014
|
—
|
|
3,973
|
|
3,031
|
|
615
|
|
201
|
|
720
|
|
8,540
|
|
||||||
|
Granted
|
14,006
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
14,006
|
|
||||||
|
Expired
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(238
|
)
|
(238
|
)
|
||||||
|
Outstanding, December 31, 2015
|
14,006
|
|
3,973
|
|
3,031
|
|
615
|
|
201
|
|
482
|
|
22,308
|
|
||||||
|
Expiration
|
March 2020
|
September 2018
|
December 2017
|
April 2017
|
Varies 2014-2016
|
Varies 2014-2020
|
||||||||||||||
|
|
As of December 31, 2015
|
||||||||||||||||||||||
|
|
March
2015
Warrants
|
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
March
2011
Warrants
|
March
2010
Warrants
|
|||||||||||||||||
|
Strike price
|
$
|
2.08
|
|
$
|
2.50
|
|
$
|
1.83
|
|
$
|
0.65
|
|
$
|
0.65
|
|
$
|
2.02
|
|
|||||
|
Expected term (years)
|
4.22
|
|
2.72
|
|
1.98
|
|
1.31
|
|
0.18
|
|
0.24
|
|
|||||||||||
|
Volatility %
|
75.85
|
%
|
74.70
|
%
|
76.37
|
%
|
65.60
|
%
|
47.98
|
%
|
71.41
|
%
|
|||||||||||
|
Risk-free rate %
|
1.58
|
%
|
1.24
|
%
|
1.05
|
%
|
0.77
|
%
|
—
|
%
|
—
|
%
|
|||||||||||
|
|
As of December 31, 2014
|
||||||||||||||||||
|
|
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
March
2011
Warrants
|
March
2010
Warrants
|
||||||||||||||
|
Strike price
|
$
|
2.50
|
|
$
|
1.90
|
|
$
|
0.65
|
|
$
|
0.65
|
|
$
|
2.15
|
|
||||
|
Expected term (years)
|
3.72
|
|
2.98
|
|
2.31
|
|
1.18
|
|
1.24
|
|
|||||||||
|
Volatility %
|
75.60
|
%
|
76.85
|
%
|
78.24
|
%
|
77.38
|
%
|
77.12
|
%
|
|||||||||
|
Risk-free rate %
|
1.30
|
%
|
1.09
|
%
|
0.80
|
%
|
0.32
|
%
|
0.35
|
%
|
|||||||||
|
March
2015 Warrants |
September
2013
Warrants
|
December
2012
Warrants
|
April 2011
Warrants
|
Other Warrant Issuances
|
Total
|
||||||||||||||||||
|
Warrant liability, January 1, 2014
|
$
|
—
|
|
$
|
22,950
|
|
$
|
18,060
|
|
$
|
5,069
|
|
$
|
2,886
|
|
$
|
48,965
|
|
|||||
|
Fair value of warrants granted
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Fair value of warrants exercised
|
—
|
|
(12,713
|
)
|
(10,086
|
)
|
(2,906
|
)
|
(1,321
|
)
|
(27,026
|
)
|
|||||||||||
|
Change in fair value of warrants
|
—
|
|
(7,677
|
)
|
(5,947
|
)
|
(1,538
|
)
|
(1,394
|
)
|
(16,556
|
)
|
|||||||||||
|
Warrant liability, December 31, 2014
|
—
|
|
2,560
|
|
2,027
|
|
625
|
|
171
|
|
5,383
|
|
|||||||||||
|
Fair value of warrants granted
|
10,296
|
|
—
|
|
—
|
|
—
|
|
—
|
|
10,296
|
|
|||||||||||
|
Fair value of warrants exercised
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Change in fair value of warrants
|
41
|
|
(627
|
)
|
(462
|
)
|
(88
|
)
|
(25
|
)
|
(1,161
|
)
|
|||||||||||
|
Warrant liability, December 31, 2015
|
$
|
10,337
|
|
$
|
1,933
|
|
$
|
1,565
|
|
$
|
537
|
|
$
|
146
|
|
$
|
14,518
|
|
|||||
|
2015
|
2014
|
2013
|
|||||||||
|
Research and development
|
$
|
350
|
|
$
|
484
|
|
$
|
754
|
|
||
|
General and administrative
|
1,591
|
|
4,903
|
|
2,150
|
|
|||||
|
Total stock-based compensation
|
$
|
1,941
|
|
$
|
5,387
|
|
$
|
2,904
|
|
||
|
2015
|
2014
|
2013
|
||||||
|
Risk free interest rate
|
1.67
|
%
|
2.01
|
%
|
1.57
|
%
|
||
|
Volatility
|
73.97
|
%
|
79.37
|
%
|
77.98
|
%
|
||
|
Expected lives (years)
|
6.16
|
|
6.16
|
|
6.25
|
|
||
|
Expected dividend yield
|
0.00
|
%
|
0.00
|
%
|
0.00
|
%
|
||
|
Total
Number of
Shares
(In Thousands)
|
Weighted
Average
Exercise
Price
|
|||||
|
Outstanding at December 31, 2014
|
8,590
|
|
$
|
3.25
|
|
|
|
Granted
|
6,743
|
|
1.63
|
|
||
|
Exercised
|
(39
|
)
|
0.79
|
|
||
|
Cancelled
|
(2,032
|
)
|
2.31
|
|
||
|
Outstanding at December 31, 2015
|
13,262
|
|
$
|
2.58
|
|
|
|
Options exercisable at December 31, 2015
|
7,192
|
|
$
|
3.22
|
|
|
|
Year Ended December 31,
|
||||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Realized gain on sale of marketable securities
|
$
|
—
|
|
$
|
—
|
|
$
|
3,911
|
|
|||
|
Change in fair value of the contingent purchase price liability
|
509
|
|
170
|
|
(926
|
)
|
||||||
|
Miscellaneous other income
|
—
|
|
—
|
|
37
|
|
||||||
|
Total other income
|
$
|
509
|
|
$
|
170
|
|
$
|
3,022
|
|
|||
|
|
December 31,
|
||||
|
|
2015
|
2014
|
|||
|
Warrants to purchase common stock
|
22,308
|
|
8,540
|
|
|
|
Options to purchase common stock
|
13,262
|
|
8,590
|
|
|
|
Total
|
35,570
|
|
17,130
|
|
|
|
|
As of December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Current
|
||||||||||||
|
Federal
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
State
|
—
|
|
—
|
|
—
|
|
||||||
|
Total current
|
—
|
|
—
|
|
—
|
|
||||||
|
Deferred expense (benefit)
|
||||||||||||
|
Federal
|
332
|
|
—
|
|
894
|
|
||||||
|
State
|
33
|
|
—
|
|
158
|
|
||||||
|
Total deferred
|
365
|
|
—
|
|
1,052
|
|
||||||
|
Total income tax expense (benefit)
|
$
|
365
|
|
$
|
—
|
|
$
|
1,052
|
|
|||
|
|
As of December 31,
|
|||||||
|
|
2015
|
2014
|
||||||
|
Net operating loss carryforwards
|
$
|
75,221
|
|
$
|
53,950
|
|
||
|
Tax credit carryforwards
|
3,866
|
|
3,590
|
|
||||
|
Stock based compensation
|
5,050
|
|
4,676
|
|
||||
|
Other
|
1,430
|
|
190
|
|
||||
|
Licensing deduction deferral
|
9,910
|
|
8,919
|
|
||||
|
Gross deferred tax assets
|
95,477
|
|
71,325
|
|
||||
|
Valuation allowance
|
(95,477
|
)
|
(71,325
|
)
|
||||
|
Net deferred tax asset
|
$
|
—
|
|
$
|
—
|
|
||
|
|
As of December 31,
|
|||||||
|
|
2015
|
2014
|
||||||
|
In-process research and development not subject to future amortization for tax purposes
|
$
|
5,418
|
|
$
|
5,053
|
|
||
|
Gross deferred tax liability
|
$
|
5,418
|
|
$
|
5,053
|
|
||
|
|
As of December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Expected federal income tax benefit
|
$
|
(21,603
|
)
|
$
|
(12,447
|
)
|
$
|
(25,713
|
)
|
|||
|
State income taxes after credits
|
(2,375
|
)
|
(1,283
|
)
|
(3,676
|
)
|
||||||
|
Unrealized gain on marketable securities
|
—
|
|
—
|
|
1,052
|
|
||||||
|
Changes in warrant value
|
(456
|
)
|
(6,503
|
)
|
17,283
|
|
||||||
|
Stock compensation
|
508
|
|
3,996
|
|
813
|
|
||||||
|
Effect of change in valuation allowance
|
24,029
|
|
17,275
|
|
11,408
|
|
||||||
|
Income tax credits
|
(276
|
)
|
(42
|
)
|
(240
|
)
|
||||||
|
Other
|
538
|
|
(996
|
)
|
125
|
|
||||||
|
$
|
365
|
|
$
|
—
|
|
$
|
1,052
|
|
||||
|
Sale of Abstral and related assets on November 19, 2015
|
Sale of Zuplenz and related assets on December 24, 2015
|
||||||
|
Net proceeds from sales
|
|||||||
|
Total consideration
|
$
|
8,348
|
|
$
|
3,750
|
|
|
|
Less selling costs*
|
(815
|
)
|
(1,050
|
)
|
|||
|
Proceeds from sale, net of selling costs
|
$
|
7,533
|
|
$
|
2,700
|
|
|
|
2015
|
2014
|
||||||
|
Carrying amounts of assets included as part of discontinued operations:
|
|||||||
|
Accounts receivable
|
$
|
392
|
|
$
|
1,535
|
|
|
|
Inventories
|
—
|
|
655
|
|
|||
|
Prepaid expenses and other current assets
|
—
|
|
1,747
|
|
|||
|
Equipment and furnishings, net
|
—
|
|
270
|
|
|||
|
Abstral rights, net
|
—
|
|
14,533
|
|
|||
|
Zuplenz rights
|
—
|
|
8,101
|
|
|||
|
Goodwill
|
—
|
|
172
|
|
|||
|
Total current assets of discontinued operations
|
$
|
392
|
|
$
|
27,013
|
|
|
|
Carrying amounts of liabilities included as part of discontinued operations:
|
|||||||
|
Accounts payable
|
$
|
1,491
|
|
$
|
385
|
|
|
|
Accrued expenses and other current liabilities
|
4,434
|
|
6,784
|
|
|||
|
Total current liabilities of discontinued operations
|
$
|
5,925
|
|
$
|
7,169
|
|
|
|
2015
|
2014
|
2013
|
|||||||||
|
Net revenue
|
$
|
9,734
|
|
$
|
9,319
|
|
$
|
2,487
|
|
||
|
Cost of revenue
|
(1,780
|
)
|
(1,403
|
)
|
(520
|
)
|
|||||
|
Amortization of certain acquired intangible assets
|
(921
|
)
|
(440
|
)
|
(131
|
)
|
|||||
|
Research and development
|
(355
|
)
|
(680
|
)
|
(651
|
)
|
|||||
|
Selling, general, and administrative
|
(17,655
|
)
|
(15,118
|
)
|
(6,536
|
)
|
|||||
|
Impairment charge form classification as held for sale
|
(8,071
|
)
|
—
|
|
—
|
|
|||||
|
Loss on sale of commercial business assets
|
(4,549
|
)
|
—
|
|
—
|
|
|||||
|
Severance and exit costs
|
(1,349
|
)
|
—
|
|
—
|
|
|||||
|
Loss from discontinued operations
|
$
|
(24,946
|
)
|
$
|
(8,322
|
)
|
$
|
(5,351
|
)
|
||
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
|||||||||||||
|
2015
|
||||||||||||||||
|
Net revenue
|
$
|
2,750
|
|
$
|
3,382
|
|
$
|
2,166
|
|
$
|
1,436
|
|
||||
|
Gross profit on net revenue
(1)
|
$
|
2,357
|
|
$
|
2,914
|
|
$
|
1,454
|
|
$
|
1,229
|
|
||||
|
Net loss
|
$
|
(10,537
|
)
|
$
|
(15,660
|
)
|
$
|
(18,026
|
)
|
$
|
(19,678
|
)
|
||||
|
Net loss per share
|
$
|
(0.08
|
)
|
$
|
(0.10
|
)
|
$
|
(0.11
|
)
|
$
|
(0.12
|
)
|
||||
|
2014
|
||||||||||||||||
|
Net revenue
|
$
|
2,173
|
|
$
|
2,331
|
|
$
|
1,620
|
|
$
|
3,195
|
|
||||
|
Gross profit on net revenue
(1)
|
$
|
1,751
|
|
$
|
1,886
|
|
$
|
1,303
|
|
$
|
2,536
|
|
||||
|
Net loss
|
$
|
(2,536
|
)
|
$
|
(19,941
|
)
|
$
|
(6,173
|
)
|
$
|
(7,506
|
)
|
||||
|
Net loss per share
|
$
|
(0.02
|
)
|
$
|
(0.17
|
)
|
$
|
(0.05
|
)
|
$
|
(0.06
|
)
|
||||
|
GALENA BIOPHARMA, INC.
|
|||
|
By:
|
/s/ Mark W. Schwartz
|
||
|
Mark W. Schwartz, Ph.D.
|
|||
|
President and Chief Executive Officer
|
|||
|
Date: March 10, 2016
|
|||
|
By:
|
/s/ John T. Burns
|
||
|
John T. Burns
|
|||
|
Controller and Principal Accounting Officer
|
|||
|
Date: March 10, 2016
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Mark W. Schwartz
|
President, Chief Executive Officer and Director (Principal Executive Officer)
|
March 10, 2016
|
||
|
Mark W. Schwartz, Ph. D.
|
||||
|
/s/ John T. Burns.
|
Controller
(Principal Accounting Officer)
|
March 10, 2016
|
||
|
John T. Burns
|
||||
|
/s/ Sanford J. Hillsberg
|
Director, Chairman of the Board
|
March 10, 2016
|
||
|
Sanford J. Hillsberg
|
||||
|
/s/ William L. Ashton
|
Director
|
March 10, 2016
|
||
|
William L. Ashton
|
||||
|
/s/ Richard Chin
|
Director
|
March 10, 2016
|
||
|
Richard Chin, M.D.
|
||||
|
/s/ Irving M. Einhorn
|
Director
|
March 10, 2016
|
||
|
Irving M. Einhorn
|
||||
|
/s/ Stephen S. Galliker
|
Director
|
March 10, 2016
|
||
|
Stephen S. Galliker
|
||||
|
/s/ Steven A. Kriegsman
|
Director
|
March 10, 2016
|
||
|
Steven A. Kriegsman
|
||||
|
/s/ Rudolph Nisi
|
Director
|
March 10, 2016
|
||
|
Rudolph Nisi, M.D.
|
||||
|
Exhibit
Number
|
Description
|
||
|
1.1
|
At Market Issuance Sales Agreement dated May 24, 2013 between Galena Biopharma, Inc. and Maxim Group LLC.(25)
|
||
|
1.2
|
At Market Issuance Sales agreements dated May 24, 2013 between Galena Biopharma, Inc. and MLV & Co. LLC.(25)
|
||
|
2.1
|
Unit Purchase Agreement, dated as of January 12, 2014, between Galena Biopharma, Inc. and Mills Pharmaceuticals, LLC.+(19)
|
||
|
3.1
|
Amended and Restated Certificate of Incorporation of Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), as amended as of June 28, 2013.(2)
|
||
|
3.2
|
Certificate of Ownership and Merger.(11)
|
||
|
3.3
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation of Galena Biopharma, Inc.(26)
|
||
|
3.4
|
Amended and Restated By-Laws of Galena Biopharma, Inc., as amended as of August 6, 2013.(2)
|
||
|
4.1
|
Form of Warrant Agreement by and Galena Biopharma, Inc., Computershare Inc. and Computershare Trust Company, N.A.(1)
|
||
|
4.2
|
Form of Common Stock Purchase Warrant issued in March 2010.(14)
|
||
|
4.3
|
Form of Five-Year Common Stock Purchase Warrant issued in March 2011.(15)
|
||
|
4.4
|
Form of Common Stock Purchase Warrant issued in April 2011.(16)
|
||
|
4.5
|
Warrant No. 2012-1 in favor of Legend Securities, Inc. issued in February 2012.(4)
|
||
|
4.6
|
Form of December 2012 Warrant.(17)
|
||
|
4.7
|
Form of warrants granted on May 8, 2013 under the Loan and Security Agreement set forth as Exhibit 10.21.(18)
|
||
|
4.8
|
Warrant Agreement, dated as of March 18, 2015, by and among Galena Biopharma, Inc., Computershare, Inc. and Computershare Trust Company, N.A.(26)
|
||
|
4.9
|
Warrant Agreement, dated as of January 12, 2016, by and among Galena Biopharma, Inc., Computershare Inc. and Computershare Trust Company, N.A.**
|
||
|
10.1
|
Contingent Value Rights Agreement among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Computershare Trust Company, N.A., Computershare Inc., and Robert E Kennedy, dated April 13, 2011.(3)
|
||
|
10.2
|
First Amendment to Contingent Value Rights Agreement among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Computershare Trust Company, N.A., Computershare Inc., and Robert E Kennedy, dated February 15, 2012.(4)
|
||
|
10.3
|
Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(8)
|
||
|
10.4
|
Amendment to Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(9)
|
||
|
10.5
|
Patent and Technology License Agreement, dated September 11, 2006, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).+(5))
|
||
|
10.6
|
Amendment No. 1 to Patent and Technology License Agreement, dated December 21, 2007, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).(5)
|
||
|
10.7
|
Amendment No. 2 to Patent and Technology License Agreement, dated September 3, 2008, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).(5)
|
||
|
10.8
|
Amendment No. 3 to Patent and Technology License Agreement, dated July 8, 2009, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).(5)
|
||
|
10.9
|
Amendment No. 4 to Patent and Technology License Agreement, dated February 11, 2010, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).+(5)
|
||
|
10.10
|
Amendment No. 5 to Patent and Technology License Agreement, dated January 10, 2011, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.).+(5)
|
||
|
10.11
|
Scientific Advisory Agreement between Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) and George E. Peoples, Ph.D., dated May 1, 2011.(6)
|
||
|
10.12
|
Exclusive License Agreement, dated as of July 11, 2011, by and among The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) and its wholly-owned subsidiary, Apthera, Inc.+(5)
|
||
|
10.13
|
Agreement and Plan of Merger by and among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Diamondback Acquisition Corp., Apthera, Inc. and Robert E. Kennedy, in his capacity as the Stockholder Representative, dated March 31, 2011.(7)
|
||
|
10.14
|
Exclusive License Agreement, dated effective as of September 16, 2011, by and among The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), The Board of Regents of the University of Texas System and The University of Texas M.D. Anderson Cancer Center.+(10)
|
||
|
10.15
|
Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Employee Stock Purchase Plan.*(12)
|
||
|
10.16
|
License Agreement, effective as of April 30, 2009, between Kwangdong Pharmaceutical Co., Ltd. and Apthera, Inc.+(4)
|
||
|
10.17
|
Amendment No. 1 to License Agreement, dated as of January 13, 2012, by and among Apthera, Inc., Kwangdong Pharmaceutical Co., Ltd., and Galena Biopharma, Inc.(4)
|
||
|
10.18
|
Amendment to Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(22)
|
||
|
10.19
|
Amendment to Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan.*(23)
|
||
|
10.20
|
License and Supply Agreement, effective December 3, 2012, between Galena Biopharma, Inc. and ABIC Marketing Limited, a subsidiary of Teva Pharmaceuticals.+(6)
|
||
|
10.21
|
Loan and Security Agreement dated May 8, 2013 among Galena Biopharma, Inc., Apthera, Inc., Oxford Finance LLC and the Lenders listed on Schedule 1.1 thereto.(18)
|
||
|
10.22
|
License and Development Agreement, dated January 13, 2014, between Galena Biopharma, Inc. and Dr. Reddy’s Laboratories, Ltd.+(19)
|
||
|
10.23
|
Exclusive License Agreement, dated as of December 20, 2013, between Mills Pharmaceuticals, LLC and BioVascular, Inc.+(19)
|
||
|
10.24
|
License and Supply Agreement dated as of July 17, 2014 between Galena Biopharma, Inc. and MonoSol Rx, LLC.+(21)
|
||
|
10.25
|
Employment Agreement, dated September 16, 2014, between Galena Biopharma, Inc. and Mark W. Schwartz, Ph.D.*(20)
|
||
|
10.26
|
Purchase Agreement, dated as of November 18, 2014, by and between Galena Biopharma, Inc. and Lincoln Park Capital Fund, LLC.(24)
|
||
|
10.27
|
Form of Incentive Stock Option under the Galena Biopharma, Inc., Amended and Restated 2007 Incentive Plan.*(26)
|
||
|
10.28
|
Form of Nonstatutory Stock Option under the Galena Biopharma, Inc., Amended and Restated 2007 Incentive Plan.*(26)
|
||
|
10.29
|
Amendment to Galena Biopharma, Inc. Amended and Restated 2007 Incentive Plan.*(27)
|
||
|
10.30
|
Separation and Consulting Agreement, dated as of June 24, 2015, by and between Galena Biopharma, Inc. and Margaret Kivinski, and General Release, dated as of June 24, 2015, by Margaret Kivinski.*(26)
|
||
|
10.31
|
Employment Offer Letter effective June 25, 2015, between Galena Biopharma, Inc. and Thomas J. Knapp.*(26)
|
||
|
10.32
|
Settlement and License Agreement dated October 23, 2015 between Galena Biopharma, Inc., Actavis Laboratories FL, Inc., and Orexo AB.(28)+
|
||
|
10.33
|
Employment Agreement, dated as of October 30, 2015, between Galena Biopharma, Inc. and Bijan Nejadnik, M.D.* **
|
||
|
10.34
|
Asset Purchase Agreement, dated November 19, 2015, between Galena Biopharma, Inc. and Sentynl Therapeutics Inc.**
|
||
|
10.35
|
Amendment, dated as of December 16, 2015, to License and Supply Agreement dated as of July 17, 2014 between Galena Biopharma, Inc. and MonoSol Rx, LLC.**
|
||
|
10.36
|
Asset Purchase Agreement, dated December 17, 2015, between Galena Biopharma, Inc. and Midatech Pharma PLC.**
|
||
|
10.37
|
Separation Agreement and Releases, dated December 31, 2015, between Galena Biopharma, Inc. and Ryan Dunlap.** *
|
||
|
10.38
|
Amendment, dated December 31, 2015, to Employment Offer Letter effective June 25, 2015, between Galena Biopharma, Inc. and Thomas J. Knapp.* **
|
||
|
10.39
|
Form of Undertaking re Advancement of Expenses between Galena Biopharma, Inc. and certain of its Existing or Former Directors and Executive officers.* **
|
||
|
14.1
|
Code of Ethics and Conduct.(13)
|
||
|
21.1
|
Subsidiaries of the Registrant.(19)
|
||
|
23.1
|
Consent of Moss Adams LLP, Independent Registered Public Accounting Firm.**
|
||
|
31.1
|
Sarbanes-Oxley Act Section 302 Certification of Mark W. Schwartz, Ph.D.**
|
||
|
31.2
|
Sarbanes-Oxley Act Section 302 Certification of John T. Burns.**
|
||
|
32.1
|
Sarbanes-Oxley Act Section 906 Certification of Mark W. Schwartz, Ph.D., and John T. Burns.**
|
||
|
101.INS
|
XBRL Instance Document.
|
||
|
101.SCH
|
XBRL Taxonomy Extension Schema.
|
||
|
101.CAL
|
XBRL Taxonomy Extension Calculation.
|
||
|
101.DEF
|
XBRL Taxonomy Extension Definition.
|
||
|
101.LAB
|
XBRL Taxonomy Extension Label.
|
||
|
101.PRE
|
XBRL Taxonomy Extension Presentation.
|
||
|
101.PRE
|
XBRL Taxonomy Extension Presentation.
|
||
|
(1)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 13, 2013 (File No. 001-33958) and incorporated herein by reference.
|
|
(2)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on August 9, 2013 (File No. 001-33958) and incorporated herein by reference.
|
|
(3)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on April 14, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(4)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on March 28, 2012 (File No. 001-33958) and incorporated by reference herein.
|
|
(5)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on August 15, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(6)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on March 12, 2013 (File No. 001-33958) and incorporated by reference herein.
|
|
(7)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on April 5, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(8)
|
Previously filed as Annex A to the Company’s Proxy Statement on Schedule 14A filed on April 23, 2010 (File No. 001-33958) and incorporated by reference herein.
|
|
(9)
|
Previously filed as Annex A to the Company’s Proxy Statement on Schedule 14A filed on May 31, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(10)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 21, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(11)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 26, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(12)
|
Previously filed as Annex B to the Company's Proxy Statement on Schedule 14A, filed on April 23, 2010 (File No. 001-33958) and incorporated by reference herein.
|
|
(13)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on April 15, 2008 (File No. 001-33958) incorporated by reference herein.
|
|
(14)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on March 23, 2010 (File No. 001-33958) and incorporated by reference herein.
|
|
(15)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on March 1, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(16)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on April 15, 2011 (File No. 001-33958) and incorporated by reference herein.
|
|
(17)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on December 19, 2012 (File No. 001-33958) and incorporated by reference herein.
|
|
(18)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on May 9, 2013 (File No. 001-33958) and incorporated by reference herein.
|
|
(19)
|
Previously filed as an Exhibit to the Company’s Form 10-K filed on March 17, 2014 (File No. 001-33958) incorporated by reference herein.
|
|
(20)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on September 18, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(21)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on August 11, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(22)
|
Previously filed as Annex A to the Company’s Proxy Statement on Schedule 14A filed on April 30, 2012 (File No. 001-33958) and incorporated by reference herein.
|
|
(23)
|
Previously filed as Annex B to the Company’s Proxy Statement on Schedule 14A filed on April 29, 2013 (File No. 001-33958) and incorporated by reference herein.
|
|
(24)
|
Previously filed as an Exhibit to the Company’s Form 8-K filed on November 20, 2014 (File No. 001-33958) and incorporated by reference herein.
|
|
(25)
|
Previously filed as an Exhibit to the Company’s Registration Statement on Form S-3 filed on May 24, 2013 (File No. 333-188849) and incorporated by reference herein.
|
|
(26)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on August 6, 2015 (File No. 001-33958) and incorporated by reference herein.
|
|
(27)
|
Previously filed as Annex B to the Company’s Proxy Statement on Schedule 14A filed on April 30, 2015 and incorporated by reference herein by reference.
|
|
(28)
|
Previously filed as an Exhibit to the Company’s Form 10-Q filed on November 9, 2015 (File No. 001-33958) and incorporated by reference herein.
|
|
*
|
Indicates a management contract or compensatory plan or arrangement.
|
|
**
|
Filed herewith.
|
|
+
|
This exhibit was filed separately with the Commission pursuant to an application for confidential treatment. The confidential portions of the exhibit have been omitted and have been marked by an asterisk.
|