|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Delaware
|
|
20-8099512
|
||
|
(State of incorporation)
|
|
(I.R.S. Employer Identification No.)
|
||
|
Securities registered pursuant to Section (12(b) of the Exchange Act:
|
||||
|
Title of Each Class
|
Trading Symbol(s)
|
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock, $0.0001 Par Value per share
|
SLS
|
|
The Nasdaq Capital Market
|
|
|
Securities registered pursuant to Section (12(g) of the Exchange Act: None
|
||||
|
Large accelerated filer
|
o
|
Accelerated filer
|
o
|
Non-accelerated filer
|
x
|
|||||
|
Smaller reporting company
|
x
|
Emerging growth company
|
o
|
|||||||
|
Page
|
|||
|
PART I
|
|||
|
Item 1
|
|||
|
Item 1A
|
|||
|
Item 1B
|
|||
|
Item 2
|
|||
|
Item 3
|
|||
|
Item 4
|
|||
|
PART II
|
|||
|
Item 5
|
|||
|
Item 6
|
|||
|
Item 7
|
|||
|
Item 7A
|
|||
|
Item 8
|
|||
|
Item 9
|
|||
|
Item 9A
|
|||
|
Item 9B
|
|||
|
PART III
|
|||
|
Item 10
|
|||
|
Item 11
|
|||
|
Item 12
|
|||
|
Item 13
|
|||
|
Item 14
|
|||
|
PART IV
|
|||
|
Item 15
|
|||
|
Item 16
|
|||


|
Key features of an Optimal Cancer
Active Immunizer Therapeutic
|
GPS Properties and Clinical Strategy
|
|
|
Selecting the right target antigen and
epitopes within that antigen
|
Four peptides and 25 epitopes selected optimally with the objective of ensuring:
- optimal MHC complex presentation;
- specificity across different HLA types;
- production of both CD4 and CD8 activated cells; and
- the ability to apply the heteroclitic principle, as described above, to overcome tolerance.
|
|
|
Optimal T-cell engagement leading to
cancer cell destruction
|
Immune response data from the final analysis of the Phase I clinical study of GPS in MM in 12 evaluable patients that were presented at the 44
th
Annual Meeting of the European Society for Blood and Marrow Transplantation, or EBMT, in 2018 (Dr. Kohne et al.) showed 75% frequency of either CD8+ or CD4+ responses to an all-pool mixture of WT1-derived antigens after completion of the 12 vaccinations per the study protocol. This evidence of multi-epitope, broad cross-reactivity along the full-length of the WT1 protein is suggestive of epitope spreading, as it emerged across epitopes against which the patients were not specifically immunized. These data corroborate the results of an earlier analysis in mid-2017 and strongly suggest stimulation of T cells towards intracellular antigen fragments from GPS-induced destruction of tumor cells, which effect is a hallmark of an effective vaccine, e.g., that it is targeting the right epitopes chosen by design.
|
|
|
Overcoming the barriers of an
adverse/immunosuppressive tumor
micro-environment, or TME
|
The GPS monotherapy clinical studies are in the setting of complete remission, or CRem, and minimal residual disease, or MRD, whereby no bulky or measurable tumor deposits exist. This is typically seen after successful frontline therapy in select cancer types for which such debulking standard therapies exist (
e.g.
, AML or MPM). In these settings, the tumor micro-environment, or TME, is substantially absent. We are also pursuing combination therapy with checkpoint inhibitors in tumor settings whereby measurable disease exists, as contemporaneous checkpoint inhibition would abrogate the immunosuppressive effects of the TME.
|
|
|
Overcoming or mitigating immune
tolerance
|
Heteroclitic peptides are those in which mutations have been deliberately introduced in the amino acid sequence. The use of heteroclitic peptide in an active immunizer, such as GPS, increases immunogenicity without changes in the antigenicity profile, as well as strengthens MHC binding of the peptide to produce cytotoxic CD8 cells that continue to recognize the corresponding native peptide sequence. This is believed to be a key factor differentiating GPS from essentially all previously developed peptide vaccines, and applies a highly innovative technology platform, peptide heteroclicity, in a clinical late-stage cancer immunotherapeutic candidate product.
|
|
|
Addressing the broadest possible
candidate patient population
|
GPS has activity across multiple HLA types that could allow treatment of a vast majority of global patient populations harboring WT1-positive malignancies.
|
|
|
•
|
heteroclitic peptides may offer increased immune response and less potential for tolerance;
|
|
•
|
multivalent oligopeptide mixture potentially drives differentiated immunotherapeutic efficacy, targeting 25 key epitopes of WT1;
|
|
•
|
potentially applicable to 20 or more cancer types worldwide and the vast majority of HLA types;
|
|
•
|
CRem or MRD status (after initial tumor debulking with preceding standard therapy) is the preferred setting for GPS monotherapy;
|
|
•
|
not directly competitive with current clinical standard of care therapies, but rather believed to complement them in the maintenance setting;
|
|
•
|
potential for combination approaches with other cancer immunotherapies, due to tolerable adverse event profile;
|
|
•
|
anticipated cost-effective manufacturing; allogeneic, “off-the-shelf,” vialed subcutaneously administered drug that is not patient-specific; and
|
|
•
|
positive Phase 2 clinical data on effectiveness (based on overall survival, or OS, in AML and progression-free survival, or PFS, in MM) with good tolerability and a favorable safety profile.
|
|
•
|
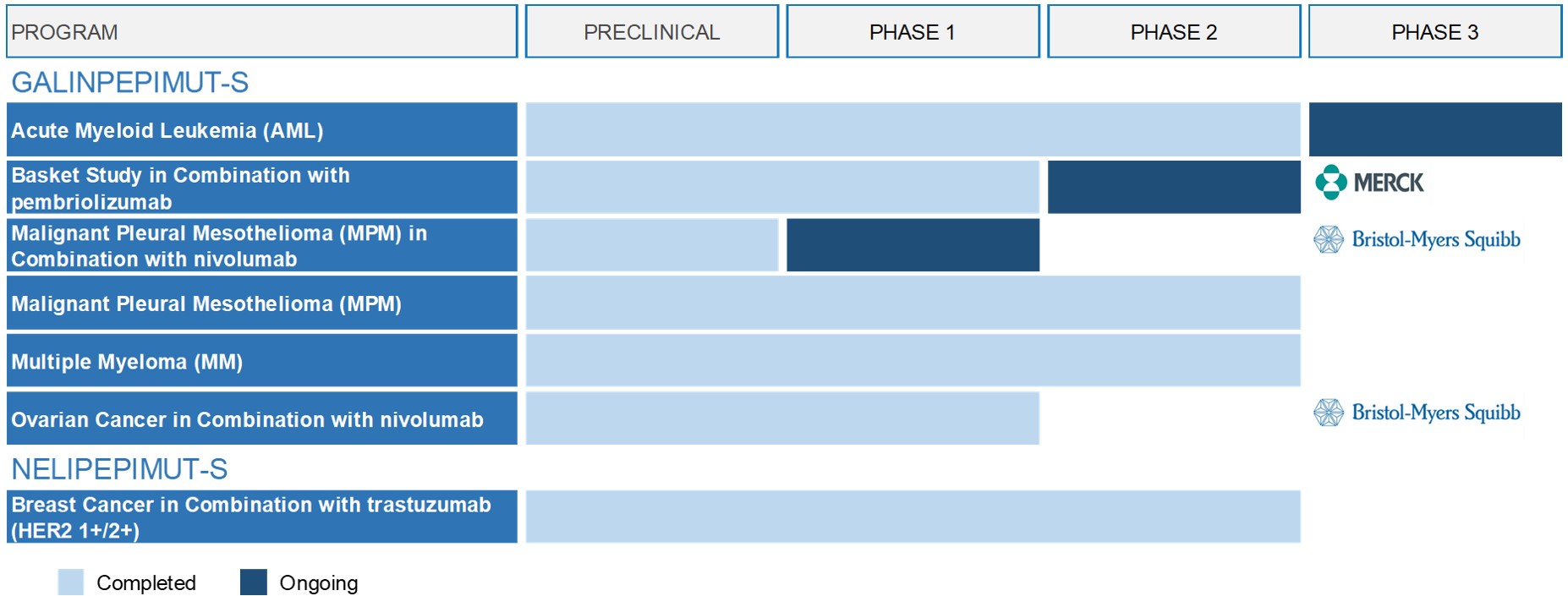
GPS monotherapy
: Phase 2 clinical trials of GPS as monotherapy for AML, MPM and MM have been completed. In January 2020, we commenced the Phase 3 clinical trial of GPS in AML, also known as the REGAL Study. The pivotal Phase 3 REGAL study is a 1:1 randomized, open-label study comparing GPS monotherapy in the maintenance setting to investigators’ choice of best available treatment in AML patients who have achieved hematologic complete remission, with or without thrombocytopenia (CRem2/CRem2p), after second-line antileukemic therapy and who are deemed ineligible for or unable to undergo allogeneic stem-cell transplantation. The study is expected to enroll approximately 116 patients across approximately 50 clinical sites in the United States and Europe. The primary endpoint is overall survival, or OS
,
from the time of study entry. The Phase 2 study in AML CRem2 patients, which is the same indication as the Phase 3 REGAL study, showed a median OS of 21.0 months, at a median follow-up of 30.8 months, in patients receiving GPS compared to 5.4 months in contemporaneously treated patients with best standard therapy. See data set forth in “- AML Clinical Data.”
|
|
•
|
GPS combination therapy
:
|
|
◦
|
Combination basket study:
In December 2018, we initiated a Phase 1/2 multi-arm ("basket" type) clinical trial of GPS in combination with the anti-PD-1 therapy Keytruda (pembrolizumab) in collaboration with a Merck & Co., Inc., Kenilworth, N.J., U.S. subsidiary (known as MSD outside the United States and Canada), or Merck. The purpose of the basket trial is to determine if the administration of GPS in combination with pembrolizumab has the potential to demonstrate clinical activity in the presence of macroscopic disease, where monotherapy with either agent would have a more limited effect.
|
|
◦
|
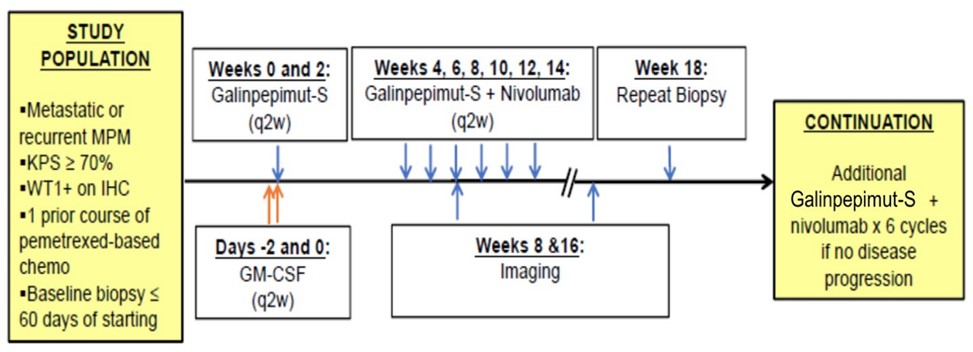
Mesothelioma
:
A Phase I open-label investigator-sponsored clinical trial of GPS, in combination with Bristol-Myers Squibb’s anti-PD-1 therapy, nivolumab, in patients with MPM who harbor relapsed or refractory disease after having received frontline standard of care multimodality therapy was commenced in February 2020 at MSK. The study drug is being provided by us and Bristol-Myers Squibb. The principal investigator for the study is Dr. Marjorie G. Zauderer, MD, Co-Director, Mesothelioma Program and Associate Attending Physician in the Thoracic Oncology Service, Department of Medicine at MSK.
|
|
•
|
AML presents a clinical setting in which CRem status (specifically CRem2) can be achieved with standard antileukemic therapy;
|
|
•
|
the high degree of unmet medical need in recurrent/relapsed AML and the absence of an effective maintenance therapy over the decades after salvage re-induction until and immediately after achievement of CRem2 status, especially considering that most patients in this clinical scenario are older than 60 years of age;
|
|
•
|
the almost universal expression of WT1 in leukemic blasts, which are AML’s replicating malignant cells, as well as leukemic stem cells, or LSCs, cells that are or become extremely resistant to standard chemotherapy or targeted agent approaches and which can be realistically eradicated only with immunotherapy methods (including allo-HSCT). LSCs have been shown to be susceptible to targeting by cytotoxic T cells (CD8 and CD4 cells) stimulated against leukemia-associated antigens and we predicted this would be the case for GPS;
|
|
•
|
the fact that WT1 has been associated with the actual development of leukemia;
|
|
•
|
the positive correlation between the level of expression of WT1 and the prognosis in AML;
|
|
•
|
the fact that the level of expression of WT1 can be followed over time in patients during and after therapy, including immunotherapy, as a method of monitoring for MRD;
|
|
•
|
early evidence from mouse models that vaccination with peptides against select WT1 antigenic epitopes leads to detection of immune response;
|
|
•
|
early evidence that human immunocytes sensitized ex-vivo to peptides contained in GPS were able to recognize naturally presented WT1 peptides on the surface of several leukemia cell lines;
|
|
•
|
early anecdotal (at the time) clinical data showing antileukemic activity of WT1 monovalent vaccines in the CRem1 maintenance setting in Japanese population (albeit restricted to HLA-A*2401 type), as well as a dendritic cell vaccine in the Netherlands (independent of HLA haplotype) in the same setting;
|
|
•
|
a predictive assumption of very low to negligible degree of clinical toxicity with a WT1-targeted immunotherapy such as GPS, due to the fact that WT1 in normal, non-cancerous, tissues is both expressed at extremely low levels and limited in number of organs and tissues, but also due to the fact that WT1 fragments, or peptide epitopes, in normal cells are presented to host APCs in a different manner than are WT1 fragments produced in cancer cells; of note, WT1 expression in normal tissues of adults is limited to the podocyte layer of the glomerulus (kidney), Sertoli cells (testis), granulosa cells (ovary), decidual cells (uterus), mesothelial cells (peritoneum, pleura), mammary duct and lobule (breast), and blood-forming (hematopoietic) progenitor cells (CD34+ cells in the bone marrow); and
|
|
•
|
the advent of modern immunotherapeutics in cancer and the promise of an innovative, off-the-shelf potentially effective, low adverse event burden immunotherapy to prevent or delay relapse in patients once they achieve CRem status in AML, a disease that has historically been associated with dearth of deep and sustained responses to checkpoint inhibitors.
|

|
•
|
ovarian cancer presents a clinical setting whereby MRD status can be achieved with standard upfront therapy both immediately after first line therapy, but also after effective debulking of the “first relapse.” The latter subgroup of patients (after successful second line treatment/first salvage, lacking demonstrable macroscopic residual disease) would be optimal candidates for GPS therapy, as no standard maintenance therapy exists for such patients and the subsequent relapse patterns and metrics are known and predictable;
|
|
•
|
the high levels of expression of WT1 in ovarian cancer cells. In fact, WT1 expression is so frequent that pathologists routinely use immunohistochemical stains for WT1 (with a standardized convention for describing expression and determining as “positive” or “negative”) to help distinguish epithelial ovarian cancers from other tumors;
|
|
•
|
preliminary evidence that WT1 expression may be linked to prognosis in ovarian cancer and that it may play an anti-apoptotic role in ovarian cancer cell lines;
|
|
•
|
the high degree of unmet medical need in ovarian cancer patients after first (or subsequent) successful “salvage” debulking therapy and the absence of effective therapies for such patients; and
|
|
•
|
a predictive assumption of very low to negligible degree of clinical toxicity with a WT1-targeted immunotherapy such as GPS due to the fact that WT1 in normal, non-cancerous tissues is both expressed at extremely low levels and limited in number of organs and tissues, but also due to the fact that WT1 fragments, or peptide epitopes, in normal cells are presented to host APCs in a different manner than are WT1 fragments produced in cancer cells.
|

|
•
|
a clinically meaningful difference in median DFS in favor of the active arm (NPS + trastuzumab), a primary endpoint of the study, with hazard ratios of 0.67 and 0.61 in the intent to treat, or ITT, and modified ITT, or mITT, populations (i.e., those who received at least one dose of vaccine or control) as well as a 34.9% and 39.5% reduction in relative risk of recurrence in the active versus control arms in the ITT and mITT populations, respectively;
|
|
•
|
a clinically meaningful and also statistically significant difference between the two arms in the cohort of patients (n= 98) with TNBC, with a hazard ratio of 0.26 and a p-value of 0.023 in favor of the NPS + trastuzumab combination with a 70.4% reduction in relative risk of recurrence in the active arm versus control;
|
|
•
|
a clinically meaningful and statistically significant difference between the two arms in favor of the combination in the cohort of patients not receiving hormonal therapy (n = 110), with a hazard ratio of 0.24 and a p-value of 0.009 with a 74.1% reduction in relative risk of recurrence in the active arm versus control;
|
|
•
|
no notable differences between treatment arms; and
|
|
•
|
that the addition of NPS to trastuzumab did not result in any additional cardiotoxicity compared to trastuzumab alone.
|
|
•
|
clinically and statistically significant efficacy in the TNBC cohort, with a p-value of 0.013 and a 75.2% reduction in risk of relapse or death;
|
|
•
|
clinically meaningful difference in median DFS in favor of the active arm (patients treated with NPS + trastuzumab) in the TNBC cohort at 24 months for patients of 92.6% compared to 70.2% for those treated with trastuzumab alone; and
|
|
•
|
in all HER2 low-expressing breast cancer patients, the DFS rate was also in favor of the NPS plus trastuzumab combination (89.8%) as compared to trastuzumab alone (83.8%).
|
|
•
|
the data analysis confirmed the therapeutic potential of NPS in patients with early-stage TNBC in the adjuvant setting across HLA types A-02, -03, -24 and -26, which cover approximately 80-85% of the North American/European populations and 86-90% of Asian/Pacific basin populations;
|
|
•
|
in the subgroup of TNBC patients with the HLA-A24+ allele type, which is highly prevalent in the Asian population, treated with the combination of NPS and trastuzumab (n=47), the p-value is 0.003 with a 90.6% relative reduction in risk of relapse or death at 24 months and a hazard ratio of 0.08 in favor of the active (combination) arm;
|
|
•
|
TNBC patients with the HLA-A24+ allele type had a significant improvement in DFS both by log-rank and landmark (24 month) analysis despite the lowest predicted binding potential between the E75 (NPS) antigen and this HLA-type; and
|
|
•
|
a clinically meaningful and statistically significant decrease in the number of clinically detectable relapses in the TNBC cohort with the combination of trastuzumab and NPS (7.5%) versus trastuzumab alone (27.3%) (p=0.004).
|
|
Product Candidate
|
Product Candidate Component
|
Jurisdiction
|
Indication
|
Claims
|
Scope
|
Latest Estimated Patent Exclusivity Period
|
|
GPS
|
Peptide WT1-A1
|
United States
|
Any
|
Composition of Matter
|
1 issued
|
03/22/2026*
|
|
GPS
|
Peptide WT1-A1
|
Australia, Switzerland, Germany, Spain, France, Great Britain, Italy
|
Any
|
Composition of Matter
|
8 issued
|
11/30/2024
|
|
GPS
|
Peptide WT1-A1
|
Canada
|
Any
|
Composition of Matter and Method of Use
|
1 issued
|
11/30/2024
|
|
GPS
|
Peptides WT1-427 long and WT1-331 long
|
United States
|
Any
|
Composition of Matter
|
1 issued
|
10/26/2031*
|
|
GPS
|
Peptides WT1-427 long and WT1-331 long
|
United States
|
WT1-expressing cancer
|
Method of Use
|
1 issued
|
10/17/2026
|
|
GPS
|
Peptides WT1-427 long and WT1-331 long
|
United States
|
Any
|
Composition of Matter and Method of Use
|
1 issued
|
10/17/2026
|
|
GPS
|
Peptides WT1-427 long and WT1-331 long
|
United States
|
Composition of Matter and Method of Use
|
1 pending
|
10/17/2026**
|
|
|
GPS
|
Peptide WT1-427 long
|
Australia, Switzerland, Germany, Spain, France, Great Britain, Ireland, Italy
|
Any
|
Composition of Matter and Method of Use
|
9 issued
|
10/17/2026
|
|
GPS
|
Peptide WT1-331 long
|
Switzerland, Germany, Spain, France, Great Britain, Ireland, Italy
|
Any
|
Composition of Matter and Method of Use
|
8 issued
|
10/17/2026
|
|
GPS
|
Peptide WT1-427 long
|
Canada
|
Any
|
Composition of Matter and Method of Use
|
1 issued
|
10/17/2026
|
|
GPS
|
Peptides WT1-427 long and WT1-331 long
|
Canada
|
Any
|
Composition of Matter and Method of Use
|
1 pending
|
10/17/2026**
|
|
GPS
|
Non-product peptide
|
United States
|
Any
|
Composition of Matter
|
1 issued
|
12/21/2026
|
|
GPS
|
Peptide WT1-122A1 long
|
United States
|
Any
|
Composition of Matter
|
1 issued
|
02/20/2033*
|
|
GPS
|
Peptide WT1-122A1 long
|
United States
|
Any
|
Composition of Matter and Method of Use
|
1 pending
|
04/10/2027**
|
|
GPS
|
Peptide WT1-122A1 long
|
Austria, Belgium, Switzerland, Germany, Spain, Finland, France, Great Britain, Greece, Ireland, Italy, Netherlands, Poland, Romania, Turkey
|
Any
|
Composition of Matter and Method of Use
|
15 issued
|
4/10/2027
|
|
GPS
|
Peptide WT1-122A1 long
|
Europe, Canada, Hong Kong
|
Any
|
Composition of Matter and Method of Use
|
3 pending
|
4/10/2027
|
|
GPS
|
Product peptides plus checkpoint inhibitors
|
United States, Australia, Canada, China, Europe, Hong Kong, Japan, South Korea
|
Any
|
Composition of Matter and Method of Use
|
8 pending
|
11/18/2036**
|
|
GPS
|
Product peptides plus other WT1 peptides
|
United States
|
Any
|
Composition of Matter and Method of Use
|
1 pending
|
2040***
|
|
Not applicable
|
Non-product peptide
|
United States
|
Any
|
Composition of Matter and Method of Use
|
1 issued, 1 pending
|
1/15/2034
|
|
Not applicable
|
Non-product peptide
|
Australia, China, Macau, Japan
|
Any
|
Composition of Matter and Method of Use
|
4 issued
|
1/15/2034
|
|
Not applicable
|
Non-product peptide
|
Australia, Canada, China, Europe, Japan
|
Composition of Matter and Method of Use
|
6 pending
|
1/15/2034**
|
|
|
NeuVax™
|
United States, Australia, Canada, China, Europe, Hong Kong, Japan, Korea
|
Recurrence of cancers expressing low to intermediate levels of HER2/neu
|
Methods of Use
|
6 pending and 12 issued
|
2028
|
|
|
and Mexico
|
||||||
|
NeuVax™ in combination with trastuzumab
|
United States and Australia
|
HER2/neu expressing cancer
|
Methods of Use
|
2 issued
|
2026
|
|
|
NeuVax™ in combination with trastuzumab
|
United States and PCT
|
Triple-negative breast cancer
|
Methods of Use
|
2 pending
|
2039
|
|
|
GALE-301
|
United States, Europe, and Hong Kong
|
Cancers expressing low levels of FBP (IHC 0 or 1+)
|
Dosage Regimen
|
3 pending
|
2037
|
|
|
GALE-301 & GALE-302 Combination
|
United States, Canada, Europe, Hong Kong, and Japan
|
Cancers expressing Folate Binding Protein (FBP)
|
Compositions & Methods of Use
|
1 pending and 9 issued
|
2022
|
|
|
GALE-301 & GALE-302 Combination
|
United States
|
Cancers expressing Folate Binding Protein (FBP)
|
Combination Dosage Regimen
|
1 issued and 1 pending
|
2036
|
|
|
*
|
Includes patent term adjustment
|
|
**
|
Projected expiration date of pending application, if granted
|
|
***
|
Projected expiration date of non-provisional application to be filed from provisional application
|
|
•
|
completion of extensive preclinical laboratory tests and animal studies performed in accordance with the FDA’s current Good Laboratory Practices, or GLP, regulations or other applicable regulations;
|
|
•
|
submission to the FDA of an IND application, which must become effective before clinical trials may begin and must be updated annually or when significant changes are made;
|
|
•
|
approval by an independent Institutional Review Board, or IRB, or ethics committee at each clinical site before the trial is begun;
|
|
•
|
performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, and other clinical-trial related regulations to establish the safety, purity and potency of the investigational biologic product candidate for its proposed indication;
|
|
•
|
preparation of and submission to the FDA of a BLA, after completion of all pivotal clinical trials;
|
|
•
|
satisfactory completion of an FDA Advisory Committee review, if applicable;
|
|
•
|
a determination by the FDA within 60 days of its receipt of a BLA to file the application for review;
|
|
•
|
satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with current Good Manufacturing Practices, or cGMP, and to assure that the facilities, methods and controls are adequate to preserve the biological product’s continued safety, purity and potency;
|
|
•
|
potential audit of selected clinical trial sites to assess compliance with current GCP and the integrity of the clinical data submitted in support of the BLA; and
|
|
•
|
FDA review and approval of the BLA to permit commercial marketing of the product for particular indications for use in the United States.
|
|
•
|
Phase 1
-The investigational product is initially introduced into healthy human subjects or patients with the target disease or condition. These studies are designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness.
|
|
•
|
Phase 2
-The investigational product is administered to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
|
|
•
|
Phase 3
-The investigational product is administered to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval and labeling. These trials may include comparisons with placebo and/or other comparator treatments. The duration of treatment is often extended to mimic the actual use of a product during marketing.
|
|
•
|
Phase 4
-In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved to gain additional information and experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow up. These so-called Phase 4 studies may be made a condition to approval of the BLA.
|
|
•
|
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
|
|
•
|
fines, warning letters or other enforcement-related letters or clinical holds on post-approval clinical trials;
|
|
•
|
refusal of the FDA to approve pending BLAs or supplements to approved BLAs, or suspension or revocation of product approvals;
|
|
•
|
product seizure or detention, or refusal to permit the import or export of products;
|
|
•
|
injunctions or the imposition of civil or criminal penalties; and
|
|
•
|
consent decrees, corporate integrity agreements, debarment, or exclusion from federal health care programs; or mandated modification of promotional materials and labeling and the issuance of corrective information.
|
|
•
|
the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior;
|
|
•
|
the applicant consents to a second orphan medicinal product application; or
|
|
•
|
the applicant cannot supply enough orphan medicinal product.
|
|
•
|
conduct additional clinical trials of our lead product, GPS, including the Phase 3 clinical trial evaluating GPS for AML and the Phase 1/2 basket study in combination with pembrolizumab;
|
|
•
|
hire additional clinical, manufacturing, quality control, quality assurance and scientific personnel;
|
|
•
|
seek marketing approval for any product candidates that successfully complete clinical trials;
|
|
•
|
develop our outsourced manufacturing and commercial activities and establish sales, marketing and distribution capabilities, if we receive, or expect to receive, marketing approval for any product candidates;
|
|
•
|
in-license or acquire the rights to, and pursue development of, other products, product candidates or technologies;
|
|
•
|
maintain, expand and protect our intellectual property portfolio; and
|
|
•
|
add operational, financial and management information systems and personnel.
|
|
•
|
the scope, progress, results and costs of our ongoing and planned development programs for our product candidates, as well as any additional clinical trials we undertake to obtain data sufficient to seek marketing approval for our product candidates in any indication;
|
|
•
|
the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates if our clinical trials are successful;
|
|
•
|
the cost of commercialization activities for our product candidates, if any of these product candidates are approved for sale, including marketing, sales and distribution costs;
|
|
•
|
the cost of manufacturing our product candidates for clinical trials in preparation for regulatory approval, including the cost and timing of process development, manufacturing scale-up and validation activities;
|
|
•
|
our ability to establish and maintain strategic licensing or other arrangements and the financial terms of such agreements;
|
|
•
|
the costs to in-license future product candidates or technologies;
|
|
•
|
the costs involved in preparing, filing, prosecuting, maintaining, expanding, defending and enforcing patent claims, including litigation costs and the outcome of such litigation;
|
|
•
|
the costs in defending and resolving future derivative and securities class action litigation;
|
|
•
|
our operating expenses; and
|
|
•
|
the emergence of competing technologies or other adverse market developments.
|
|
•
|
successfully complete development activities, including the necessary clinical trials;
|
|
•
|
complete and submit either BLAs or NDAs to the FDA and obtain U.S. regulatory approval for indications for which there is a commercial market;
|
|
•
|
complete and submit applications to foreign regulatory authorities in Europe, Asia and other jurisdictions;
|
|
•
|
obtain regulatory approval in territories with viable market sizes;
|
|
•
|
obtain coverage and adequate reimbursement from third parties, including government and private payors;
|
|
•
|
set commercially viable prices for our products, if any;
|
|
•
|
establish and maintain supply and manufacturing relationships with reliable third parties and/or build our own manufacturing facility and ensure adequate, legally globally compliant manufacturing of bulk drug substances and drug products to maintain that supply;
|
|
•
|
develop distribution processes for our product candidates;
|
|
•
|
develop commercial quantities of our product candidates, once approved, at acceptable cost levels; obtain additional funding, if required to develop and commercialize our product candidates;
|
|
•
|
develop a commercial organization capable of sales, marketing and distribution for any products we intend to sell ourselves, in the markets in which we choose to commercialize on our own;
|
|
•
|
achieve market acceptance of our products;
|
|
•
|
attract, hire and retain qualified personnel; and
|
|
•
|
protect our rights in our intellectual property portfolio.
|
|
•
|
designing, conducting and successfully completing preclinical development activities, including preclinical efficacy and IND-enabling studies, for our product candidates or product candidates we are interested in in-licensing or acquiring, including product candidates;
|
|
•
|
designing, conducting and completing clinical trials for our product candidates with positive results;
|
|
•
|
receipt of regulatory approvals from applicable authorities;
|
|
•
|
obtaining and maintaining patent and trade secret protection and regulatory exclusivity for our product candidates;
|
|
•
|
making arrangements with third-party manufacturers, receiving regulatory approval of our manufacturing processes and our third-party manufacturers’ facilities from applicable regulatory authorities and ensuring adequate supply of drug product;
|
|
•
|
manufacturing our product candidates at an acceptable cost;
|
|
•
|
effectively launching commercial sales of our product candidates, if approved, whether alone or in collaboration with others;
|
|
•
|
achieving acceptance of our product candidates, if approved, by patients, the medical community and third-party payors;
|
|
•
|
effectively competing with other therapies;
|
|
•
|
if our products candidates are approved, obtaining and maintaining coverage and adequate reimbursement by third-party payors, including government payors, for our product candidates;
|
|
•
|
complying with all applicable regulatory requirements, including FDA current Good Clinical Practices, or GCP, current Good Manufacturing Practices, or cGMP, and standards, rules and regulations governing promotional and other marketing activities;
|
|
•
|
maintaining a continued acceptable safety profile of the products during development and following approval; and
|
|
•
|
maintaining and growing an organization of scientists and business people who can develop and commercialize our products and technology.
|
|
•
|
obtaining regulatory approval from the FDA and other regulatory authorities, which have very limited experience with the development and commercialization of WT1 cancer immunotherapies;
|
|
•
|
obtaining the components required for the administration of GPS (i.e., GPS, granulocyte macrophage-colony stimulating factor, or GM-CSF, and Montanide) from three separate sources, the subsequent separate storage requirements for each of these components and the delivery of these components to the administration location;
|
|
•
|
utilizing GPS in combination with other therapies, which may increase the risk of adverse side effects;
|
|
•
|
sourcing clinical and, if approved, commercial supplies for the materials used to manufacture and process GPS;
|
|
•
|
developing a manufacturing process used in connection with GPS that will yield a satisfactory product that is safe, effective, scalable and profitable;
|
|
•
|
establishing sales and marketing capabilities after obtaining any regulatory approval to gain market acceptance; and
|
|
•
|
obtaining coverage and adequate reimbursement from third-party payors and government authorities.
|
|
•
|
disruptions caused by the global coronavirus pandemic;
|
|
•
|
eligibility criteria of our ongoing and planned clinical trials with specific characteristics appropriate for inclusion in our clinical trials;
|
|
•
|
design of the clinical trial;
|
|
•
|
size and nature of the patient population;
|
|
•
|
patients’ perceptions as to risks and benefits of the product candidate under study and the participation in a clinical trial generally in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating;
|
|
•
|
the availability and efficacy of competing therapies and clinical trials;
|
|
•
|
pendency of other trials underway in the same patient population;
|
|
•
|
willingness of physicians to participate in our planned clinical trials;
|
|
•
|
severity of the disease under investigation;
|
|
•
|
proximity of patients to clinical sites;
|
|
•
|
patients who do not complete the trials for personal reasons; and
|
|
•
|
issues with contract research organizations, or CROs, and/or with other vendors that handle our clinical trials.
|
|
•
|
delay or failure in reaching agreement with the FDA or a comparable foreign regulatory authority on a clinical trial design that we are able to execute;
|
|
•
|
delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a trial;
|
|
•
|
delay or failure in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
•
|
delay or failure in obtaining IRB approval or the approval of other reviewing entities, including comparable foreign regulatory authorities, to conduct a clinical trial at each site;
|
|
•
|
withdrawal of clinical trial sites from our clinical trials or the ineligibility of a site to participate in our clinical trials;
|
|
•
|
delay or failure in recruiting and enrolling suitable subjects to participate in a trial;
|
|
•
|
delay or failure in subjects completing a trial or returning for post-treatment follow-up;
|
|
•
|
clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial;
|
|
•
|
inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs, including some that may be for the same indication;
|
|
•
|
failure of our third-party clinical trial managers, CROs, clinical trial sites, contracted laboratories or other third-party vendors to satisfy their contractual duties, meet expected deadlines or return trustworthy data;
|
|
•
|
delay or failure in adding new trial sites;
|
|
•
|
interim results or data that are ambiguous or negative or are inconsistent with earlier results or data;
|
|
•
|
alteration of trial design necessitated by re-evaluation of design assumptions based upon observed data;
|
|
•
|
feedback from the FDA, the IRB, DSMB or a comparable foreign regulatory authority, or results from earlier stage or concurrent preclinical studies and clinical trials, that might require modification to the protocol for a trial;
|
|
•
|
a decision by the FDA, the IRB, a comparable foreign regulatory authority, or us, or a recommendation by a DSMB or comparable foreign regulatory authority, to suspend or terminate clinical trials at any time for safety issues or for any other reason;
|
|
•
|
unacceptable risk-benefit profile, unforeseen safety issues or adverse side effects;
|
|
•
|
failure to demonstrate a benefit from using a product candidate;
|
|
•
|
difficulties in manufacturing or obtaining from third parties sufficient quantities of a product candidate to start or to use in clinical trials;
|
|
•
|
lack of adequate funding to continue a trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional studies or increased expenses associated with the services of our CROs and other third parties; or
|
|
•
|
changes in governmental regulations or administrative actions or lack of adequate funding to continue a clinical trial.
|
|
•
|
differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries; more stringent privacy requirements for data to be supplied to our operations in the United States,
e.g.
, General Data Protection Regulation in the European Union;
|
|
•
|
unexpected changes in tariffs, trade barriers and regulatory requirements; economic weakness, including inflation, or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; foreign taxes, including withholding of payroll taxes;
|
|
•
|
differing payor reimbursement regimes, governmental payors or patient self-pay systems and price controls;
|
|
•
|
foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country;
|
|
•
|
workforce uncertainty in countries where labor unrest is more common than in the United States;
|
|
•
|
uncertainties related to the withdrawal of the United Kingdom from the European Union (known as "Brexit") and its financial, trade, regulatory and legal implications, which could lead to legal uncertainty and potentially divergent national laws and regulations as the United Kingdom determines which EU laws to replace or replicate, and which may further create global economic uncertainty, which could materially adversely affect our business, business opportunities, results of operations, financial condition, and cash flows;
|
|
•
|
the recent outbreak of an infection caused by COVID-19, a novel coronavirus strain commonly referred to as coronavirus, which has resulted in global travel restrictions and as of March 2020, has spread to over 50 countries, including the United States and the Lombardy region of Italy, which is where our APIs are manufactured for our GPS study;
|
|
•
|
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad, including those that may result from the recent coronavirus outbreak; and
|
|
•
|
business interruptions resulting from geopolitical actions, including war and terrorism.
|
|
•
|
we may be forced to suspend marketing of such product;
|
|
•
|
regulatory authorities may withdraw their approvals of such product;
|
|
•
|
regulatory authorities may require additional warnings on the label that could diminish the usage or otherwise limit the commercial success of such products;
|
|
•
|
we may be required to conduct post-marketing studies;
|
|
•
|
we may be required to change the way the product is administered;
|
|
•
|
we could be sued and held liable for harm caused to subjects or patients; and
|
|
•
|
our reputation may suffer.
|
|
•
|
disagreement with the regulatory authorities regarding the scope, design or implementation of our clinical trials;
|
|
•
|
failure to demonstrate that a product candidate is safe and effective for our proposed indication;
|
|
•
|
failure of clinical trials to meet the level of statistical significance required for approval;
|
|
•
|
failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks;
|
|
•
|
disagreement with our interpretation of data from preclinical studies or clinical trials;
|
|
•
|
the insufficiency of data collected from clinical trials of our product candidates to support the submission and filing of a BLA, NDA or other submission or to obtain regulatory approval;
|
|
•
|
the insufficiency of a single Phase 3 clinical trial of GPS in AML for regulatory approval in that indication;
|
|
•
|
failure to obtain approval of our manufacturing processes or facilities of third-party manufacturers with whom we contract for clinical and commercial supplies or our own manufacturing facility; or
|
|
•
|
changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval.
|
|
•
|
issue warning letters or untitled letters;
|
|
•
|
mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners;
|
|
•
|
require us to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance;
|
|
•
|
seek an injunction or impose civil or criminal penalties or monetary fines;
|
|
•
|
suspend or withdraw regulatory approval;
|
|
•
|
suspend any ongoing clinical trials;
|
|
•
|
refuse to approve pending applications or supplements to applications filed by us;
|
|
•
|
suspend or impose restrictions on operations, including costly new manufacturing requirements; or
|
|
•
|
seize or detain products, refuse to permit the import or export of products, or require us to initiate a product recall.
|
|
•
|
issue warning letters;
|
|
•
|
impose civil or criminal penalties;
|
|
•
|
suspend or withdraw regulatory approval;
|
|
•
|
suspend any of our ongoing clinical trials;
|
|
•
|
refuse to approve pending applications or supplements to approved applications submitted by us;
|
|
•
|
impose restrictions on our operations, including closing our contract manufacturers' facilities; or
|
|
•
|
require a product recall.
|
|
•
|
product loss due to contamination, equipment failure or improper installation or operation of equipment, or vendor or operator error;
|
|
•
|
product loss or manufacturing failure due to failure of temperature controls in production, storage or transit;
|
|
•
|
reduced production yields, product defects, and other supply disruptions due to deviations, even minor, from normal manufacturing and distribution processes;
|
|
•
|
unexpected product defects;
|
|
•
|
microbial, viral, or other contaminations in our product candidates or in the manufacturing facilities in which our product candidates are made, which may result in the closure of such manufacturing facilities for an extended period of time to allow for the investigation and remediation of the contamination;
|
|
•
|
adverse impact on the active ingredient of GPS as a result of potential contamination from the presence of heavy metals which can lead to higher than acceptable rates of impurities resulting in the active ingredient being unacceptable for use; and
|
|
•
|
adverse impact on the manufacturing of GPS as a result of potential contamination from excess water and oxygen which can lead to higher than acceptable levels of impurities resulting in the drug product being unacceptable for use.
|
|
•
|
the efficacy and safety of such product candidates as demonstrated in clinical trials;
|
|
•
|
the clinical indications and patient populations for which the product candidate is approved;
|
|
•
|
acceptance by physicians, major cancer treatment centers and patients of the drug as a safe and effective treatment;
|
|
•
|
the adoption of novel immunotherapies by physicians, hospitals and third-party payors;
|
|
•
|
the potential and perceived advantages of product candidates over alternative treatments;
|
|
•
|
the safety of product candidates seen in a broader patient group, including our use outside the approved indications;
|
|
•
|
any restrictions on use together with other medications;
|
|
•
|
the prevalence and severity of any side effects;
|
|
•
|
product labeling or product insert requirements of the FDA or other regulatory authorities;
|
|
•
|
the timing of market introduction of our products as well as competitive products;
|
|
•
|
the development of manufacturing and distribution processes for commercial scale manufacturing for our novel WT1 peptide cancer immunotherapy product candidate;
|
|
•
|
the cost of treatment in relation to alternative treatments;
|
|
•
|
the availability of coverage and adequate reimbursement from third-party payors and government authorities;
|
|
•
|
relative convenience and ease of administration; and
|
|
•
|
the effectiveness of our sales and marketing efforts and those of our collaborators.
|
|
•
|
the demand for our product candidates, if we obtain regulatory approval;
|
|
•
|
our ability to receive or set a price that we believe is fair for our products;
|
|
•
|
our ability to generate revenue and achieve or maintain profitability;
|
|
•
|
our ability to enjoy or maintain market exclusivity;
|
|
•
|
the level of taxes that we are required to pay; and
|
|
•
|
the availability of capital.
|
|
•
|
the federal healthcare Anti-Kickback Statute which prohibits, among other things, individuals and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare or Medicaid;
|
|
•
|
federal civil and criminal false claims laws, including the federal False Claims Act that can be enforced through civil whistleblower or qui tam actions, and civil monetary penalty laws, prohibit individuals or entities from knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment or approval that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
•
|
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for
|
|
•
|
the federal physician sunshine requirements under the ACA which requires certain manufacturers of drugs, devices, biologics and medical supplies, with certain exceptions, to report annually to HHS information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations;
|
|
•
|
analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; some state laws which require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or pricing information; and certain state and local laws which require the registration of pharmaceutical sales representatives; and
|
|
•
|
state and foreign laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
|
|
•
|
decreased demand for any product candidates or products that we may develop;
|
|
•
|
termination of clinical trial sites or entire clinical trial programs;
|
|
•
|
injury to our reputation and significant negative media attention;
|
|
•
|
withdrawal of clinical trial participants;
|
|
•
|
significant costs to defend the related litigation;
|
|
•
|
substantial monetary awards to trial subjects or patients;
|
|
•
|
loss of revenue;
|
|
•
|
diversion of management and scientific resources from our business operations; and
|
|
•
|
the inability to commercialize any products that we may develop.
|
|
•
|
managing our clinical trials effectively;
|
|
•
|
identifying, recruiting, maintaining, motivating and integrating additional employees;
|
|
•
|
managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties;
|
|
•
|
improving our managerial, development, operational, information technology, human resources and finance systems; and
|
|
•
|
expanding our facilities.
|
|
•
|
reports of the results of our clinical trials regarding the safety or efficacy of our product candidates and surrogate markers;
|
|
•
|
announcements of regulatory developments or technological innovations by us or our competitors;
|
|
•
|
announcements of business or strategic transactions or our success in finalizing such a transaction;
|
|
•
|
announcements of legal or regulatory actions against us or any adverse outcome of any such actions;
|
|
•
|
changes in our relationships with our licensors, licensees and other strategic partners;
|
|
•
|
low volume in the number of shares of our common stock traded on Nasdaq;
|
|
•
|
our quarterly operating results;
|
|
•
|
announcements of dilutive financing;
|
|
•
|
announcements of additional potential reverse stock split;
|
|
•
|
developments in patent or other technology ownership rights;
|
|
•
|
additional funds may not be available on terms that are favorable to us and, in the case of equity financings, may result in dilution to our stockholders;
|
|
•
|
government regulation of drug pricing; and
|
|
•
|
general changes in the economy, the financial markets or the pharmaceutical or biotechnology industries.
|
|
•
|
divide our Board of Directors into three classes, with members of each class to be elected for staggered three-year terms;
|
|
•
|
limit the right of securityholders to remove directors;
|
|
•
|
prohibit stockholders from acting by written consent;
|
|
•
|
regulate how stockholders may present proposals or nominate directors for election at annual meetings of stockholders; and
|
|
•
|
authorize our Board to issue preferred stock in one or more series, without stockholder approval.
|
|
Plan Category
|
Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights
|
Weighted Average Exercise Price of Outstanding Options, Warrants and Rights
|
Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans (Excluding Securities Reflected in Previous Columns)
|
|||||||
|
Equity compensation plans approved by security holders
|
||||||||||
|
2017 Equity Incentive Plan
|
21,520
|
|
$
|
112.81
|
|
—
|
|
|||
|
2019 Equity Incentive Plan
|
—
|
|
N/A
|
|
202,684
|
|
||||
|
Employee Stock Purchase Plan
|
—
|
|
N/A
|
|
5,302
|
|
||||
|
Equity compensation plans not approved by security holders
|
||||||||||
|
None
|
—
|
|
—
|
|
—
|
|
||||
|
Total
|
21,520
|
|
$
|
112.81
|
|
207,986
|
|
|||
|
•
|
complete our Phase 3 clinical trial and our Phase 1/2 basket study;
|
|
•
|
continue the research, development and scale-up of manufacturing capabilities to optimize products and dose forms for which we may obtain regulatory approval;
|
|
•
|
maintain, expand and protect our global intellectual property portfolio;
|
|
•
|
hire additional clinical, manufacturing, and scientific personnel; and
|
|
•
|
add, acquire of develop operational, financial and management information systems and personnel, including personnel to support our drug development and potential future commercialization efforts.
|
|
•
|
expenses incurred under agreements with CROs, as well as investigative sites and consultants that conduct our preclinical studies and clinical trials;
|
|
•
|
manufacturing expenses;
|
|
•
|
outsourced professional scientific development services;
|
|
•
|
employee-related expenses, which include salaries, benefits and stock-based compensation;
|
|
•
|
payments made under our license agreements, under which we acquired certain intellectual property;
|
|
•
|
expenses relating to certain regulatory activities, including filing fees paid to regulatory agencies;
|
|
•
|
laboratory materials and supplies used to support our research activities; and
|
|
•
|
allocated expenses, utilities and other facility-related costs.
|
|
•
|
the number of clinical sites included in the trials;
|
|
•
|
the length of time required to enroll suitable patients;
|
|
•
|
the number of patients that ultimately participate in the trials;
|
|
•
|
the number of doses patients receive;
|
|
•
|
the duration of patient follow-up;
|
|
•
|
the results of clinical trials;
|
|
•
|
the expenses associated with manufacturing;
|
|
•
|
the receipt of marketing approvals; and
|
|
•
|
the commercialization of current and future product candidates.
|
|
(dollars in thousands)
|
Year ended December 31,
|
||||||||||
|
2019
|
2018
|
Change
|
|||||||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
$
|
7,285
|
|
$
|
8,767
|
|
$
|
(1,482
|
)
|
||
|
General and administrative
|
9,923
|
|
12,772
|
|
(2,849
|
)
|
|||||
|
In-process research and development impairment charge
|
2,833
|
|
9,550
|
|
(6,717
|
)
|
|||||
|
Total operating expenses and operating loss
|
(20,041
|
)
|
(31,089
|
)
|
(11,048
|
)
|
|||||
|
Non-operating income
|
668
|
|
2,041
|
|
(1,373
|
)
|
|||||
|
Loss before income taxes
|
(19,373
|
)
|
(29,048
|
)
|
(9,675
|
)
|
|||||
|
Income tax expense (benefit)
|
(81
|
)
|
(1,378
|
)
|
1,297
|
|
|||||
|
Net loss
|
$
|
(19,292
|
)
|
$
|
(27,670
|
)
|
$
|
(8,378
|
)
|
||
|
Years Ended December 31,
|
|||||||||||
|
2019
|
2018
|
Change
|
|||||||||
|
Change in fair value of warrant liability
|
$
|
1,136
|
|
$
|
5,300
|
|
$
|
(4,164
|
)
|
||
|
Change in fair value of the contingent consideration
|
(586
|
)
|
(3,032
|
)
|
2,446
|
|
|||||
|
Loss on settlement of liability-classified warrants
|
—
|
|
(727
|
)
|
727
|
|
|||||
|
Gain on extinguishment of debt
|
—
|
|
766
|
|
(766
|
)
|
|||||
|
Interest income (expense), net
|
118
|
|
(266
|
)
|
384
|
|
|||||
|
Total non-operating income (expense), net
|
$
|
668
|
|
$
|
2,041
|
|
$
|
(1,373
|
)
|
||
|
For the December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Net cash (used in) provided by:
|
|||||||
|
Operating activities
|
(17,643
|
)
|
(30,422
|
)
|
|||
|
Financing activities
|
19,569
|
|
23,123
|
|
|||
|
Net increase (decrease) in cash, cash equivalents, restricted cash, and restricted cash equivalents
|
$
|
1,926
|
|
$
|
(7,299
|
)
|
|
|
Payments due by period
|
|||||||||||||||||||
|
Total
|
Less than 1 year
|
1-3 years
|
3-5 years
|
More than 5 years
|
|||||||||||||||
|
Contractual obligations
|
|||||||||||||||||||
|
Operating lease
(1)
|
$
|
224
|
|
$
|
224
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
•
|
estimates of revenue and operating profits related to products or product candidates;
|
|
•
|
the probability of success for unapproved product candidates considering their stages of development;
|
|
•
|
the time and resources needed to complete the development and approval of product candidates;
|
|
•
|
the life of the potential commercialized products and associated risks, including the inherent difficulties and uncertainties in developing a product candidate such as obtaining FDA and other regulatory approvals; and
|
|
•
|
risks related to the viability of and potential alternative treatments in any future target markets.
|
|
•
|
Vendors in connection with preclinical development activities;
|
|
•
|
the production of preclinical and clinical trial materials; and
|
|
•
|
CROs in connection with clinical trials; and investigative sites in connection with clinical trials.
|
|
2018
|
|||
|
Risk free interest rate
|
2.76
|
%
|
|
|
Volatility
|
83.14
|
%
|
|
|
Expected lives (years)
|
5.5
|
|
|
|
Expected dividend yield
|
—
|
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Cash and cash equivalents
|
$
|
7,277
|
|
$
|
5,337
|
|
|
|
Restricted cash and cash equivalents
|
100
|
|
114
|
|
|||
|
Total cash, cash equivalents, restricted cash, and restricted cash equivalents
|
$
|
7,377
|
|
$
|
5,451
|
|
|
|
Page
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
7,277
|
|
$
|
5,337
|
|
|
|
Restricted cash and cash equivalents
|
100
|
|
114
|
|
|||
|
Stock subscription receivable
|
308
|
|
—
|
|
|||
|
Prepaid expenses and other current assets
|
557
|
|
387
|
|
|||
|
Total current assets
|
8,242
|
|
5,838
|
|
|||
|
Operating lease right-of-use asset
|
217
|
|
—
|
|
|||
|
In-process research and development
|
5,700
|
|
8,500
|
|
|||
|
Goodwill
|
1,914
|
|
1,914
|
|
|||
|
Deposits and other assets
|
536
|
|
663
|
|
|||
|
Total assets
|
$
|
16,609
|
|
$
|
16,915
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
3,902
|
|
$
|
3,755
|
|
|
|
Accrued expenses and other current liabilities
|
1,171
|
|
2,219
|
|
|||
|
Operating lease liability
|
217
|
|
—
|
|
|||
|
Total current liabilities
|
5,290
|
|
5,974
|
|
|||
|
Deferred tax liability
|
262
|
|
357
|
|
|||
|
Warrant liability
|
52
|
|
1,013
|
|
|||
|
Contingent consideration
|
4,912
|
|
4,326
|
|
|||
|
Total liabilities
|
10,516
|
|
11,670
|
|
|||
|
Commitments and contingencies (Note 9)
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.0001 par value; 5,000,000 shares authorized; Series A convertible preferred stock, 17,500 shares designated; no shares issued and outstanding at December 31, 2019 and December 31, 2018
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value; 350,000,000 shares authorized, 5,080,100 shares issued and outstanding at December 31, 2019; 440,529 shares issued and outstanding at December 31, 2018
|
5
|
|
1
|
|
|||
|
Additional paid-in capital
|
107,235
|
|
87,099
|
|
|||
|
Accumulated deficit
|
(101,147
|
)
|
(81,855
|
)
|
|||
|
Total stockholders’ equity
|
6,093
|
|
5,245
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
16,609
|
|
$
|
16,915
|
|
|
|
Year Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Operating expenses:
|
|||||||
|
Research and development
|
$
|
7,285
|
|
$
|
8,767
|
|
|
|
General and administrative
|
9,923
|
|
12,772
|
|
|||
|
In-process research and development impairment charge
|
2,833
|
|
9,550
|
|
|||
|
Total operating expenses and operating loss
|
(20,041
|
)
|
(31,089
|
)
|
|||
|
Non-operating income (expense):
|
|||||||
|
Change in fair value of warrant liability
|
1,136
|
|
5,300
|
|
|||
|
Change in fair value of contingent consideration
|
(586
|
)
|
(3,032
|
)
|
|||
|
Loss on settlement of liability-classified warrants
|
—
|
|
(727
|
)
|
|||
|
Gain on extinguishment of debt
|
—
|
|
766
|
|
|||
|
Interest income (expense), net
|
118
|
|
(266
|
)
|
|||
|
Total non-operating income, net
|
668
|
|
2,041
|
|
|||
|
Loss before income taxes
|
(19,373
|
)
|
(29,048
|
)
|
|||
|
Income tax benefit
|
(81
|
)
|
(1,378
|
)
|
|||
|
Net loss
|
(19,292
|
)
|
(27,670
|
)
|
|||
|
Deemed dividend arising from warrant modifications
|
(8,416
|
)
|
—
|
|
|||
|
Deemed dividend arising from beneficial conversion feature of convertible preferred stock
|
—
|
|
(4,436
|
)
|
|||
|
Deemed dividend arising from the issuance of common stock to Series A convertible preferred stockholders under most favored nation provision
|
—
|
|
(8,654
|
)
|
|||
|
Impact of anti-dilution protection on liability-classified warrants
|
(243
|
)
|
(491
|
)
|
|||
|
Net loss attributable to common stockholders
|
$
|
(27,951
|
)
|
$
|
(41,251
|
)
|
|
|
Per share information:
|
|||||||
|
Net loss per common share attributable to common stockholders, basic and diluted
|
$
|
(10.92
|
)
|
$
|
(157.72
|
)
|
|
|
Weighted-average common shares outstanding, basic and diluted
|
2,558,755
|
|
261,542
|
|
|||
|
Preferred Stock
|
Common Stock
|
Additional Paid-In Capital
|
Accumulated Deficit
|
Total Stockholders' Equity
|
||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||
|
Balance at January 1, 2018
|
—
|
|
$
|
—
|
|
115,337
|
|
$
|
1
|
|
$
|
56,254
|
|
$
|
(54,185
|
)
|
$
|
2,070
|
|
|||||||
|
Issuance of common stock and common stock warrants, net of issuance costs
|
—
|
|
—
|
|
136,900
|
|
—
|
|
21,564
|
|
—
|
|
21,564
|
|
||||||||||||
|
Issuance of common stock upon exercise of pre-funded warrants
|
—
|
|
—
|
|
81,000
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||
|
Issuance of Series A convertible preferred stock, net of offering costs
|
10,700
|
|
—
|
|
—
|
|
—
|
|
9,647
|
|
—
|
|
9,647
|
|
||||||||||||
|
Fair value of liability-classified warrants issued in connection with Series A convertible preferred stock offering
|
—
|
|
—
|
|
—
|
|
—
|
|
(5,039
|
)
|
—
|
|
(5,039
|
)
|
||||||||||||
|
Beneficial conversion feature arising from Series A convertible preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,436
|
)
|
—
|
|
(4,436
|
)
|
||||||||||||
|
Deemed dividend arising from beneficial conversion feature of Series A convertible preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
4,436
|
|
—
|
|
4,436
|
|
||||||||||||
|
Conversion of Series A convertible preferred stock
|
(2,898
|
)
|
—
|
|
9,993
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||
|
Issuance of common stock to Series A convertible preferred stockholders under most favored nation provision
|
(7,802
|
)
|
—
|
|
74,963
|
|
—
|
|
(8,654
|
)
|
—
|
|
(8,654
|
)
|
||||||||||||
|
Deemed dividend arising from the issuance of common stock to Series A convertible preferred stockholders under most favored nation provision
|
—
|
|
—
|
|
—
|
|
—
|
|
8,654
|
|
—
|
|
8,654
|
|
||||||||||||
|
Impact of anti-dilution protection on liability-classified warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
(491
|
)
|
—
|
|
(491
|
)
|
||||||||||||
|
Convertible preferred stock dividends
|
—
|
|
—
|
|
—
|
|
—
|
|
(487
|
)
|
—
|
|
(487
|
)
|
||||||||||||
|
Issuance of common stock as repayment of principal and interest on long-term debt
|
—
|
|
—
|
|
14,305
|
|
—
|
|
2,896
|
|
—
|
|
2,896
|
|
||||||||||||
|
Issuance of common stock in connection with litigation settlements
|
—
|
|
—
|
|
4,573
|
|
—
|
|
1,250
|
|
—
|
|
1,250
|
|
||||||||||||
|
Issuance of common stock upon conversion of promissory notes
|
—
|
|
—
|
|
2,372
|
|
—
|
|
825
|
|
—
|
|
825
|
|
||||||||||||
|
Issuance of common stock in connection with warrant exchange agreements
|
—
|
|
—
|
|
1,086
|
|
—
|
|
285
|
|
—
|
|
285
|
|
||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
395
|
|
—
|
|
395
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(27,670
|
)
|
(27,670
|
)
|
||||||||||||
|
Balance at December 31, 2018
|
—
|
|
—
|
|
440,529
|
|
1
|
|
87,099
|
|
(81,855
|
)
|
5,245
|
|
||||||||||||
|
Issuance of common stock and common stock warrants, net of issuance costs
|
—
|
|
—
|
|
1,051,441
|
|
1
|
|
16,143
|
|
—
|
|
16,144
|
|
||||||||||||
|
Issuance of common stock for exercise of warrants, net of offering costs
|
—
|
|
—
|
|
2,102,744
|
|
2
|
|
3,656
|
|
—
|
|
3,658
|
|
||||||||||||
|
Issuance of common stock upon exercise of pre-funded warrants
|
—
|
|
—
|
|
1,485,156
|
|
1
|
|
7
|
|
—
|
|
8
|
|
||||||||||||
|
Impact of anti-diultion protection on liability-classified warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
(243
|
)
|
—
|
|
(243
|
)
|
||||||||||||
|
Issuance of common stock upon vesting of restricted stock units
|
—
|
|
—
|
|
230
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
573
|
|
—
|
|
573
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(19,292
|
)
|
(19,292
|
)
|
||||||||||||
|
Balance at December 31, 2019
|
—
|
|
$
|
—
|
|
5,080,100
|
|
$
|
5
|
|
$
|
107,235
|
|
$
|
(101,147
|
)
|
$
|
6,093
|
|
|||||||
|
Year ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Cash flows from operating activities:
|
|||||||
|
Net loss
|
$
|
(19,292
|
)
|
$
|
(27,670
|
)
|
|
|
Adjustment to reconcile net loss to net cash used in operating activities:
|
|||||||
|
Gain on extinguishment of debt
|
—
|
|
(766
|
)
|
|||
|
Non-cash In-process research and development impairment charge
|
2,833
|
|
9,100
|
|
|||
|
Non-cash interest expense
|
—
|
|
134
|
|
|||
|
Deferred income taxes
|
(95
|
)
|
(1,316
|
)
|
|||
|
Non-cash stock-based compensation
|
573
|
|
395
|
|
|||
|
Fair value of common stock issued in connection with litigation settlements
|
—
|
|
1,250
|
|
|||
|
Change in fair value of common stock warrants
|
(1,136
|
)
|
(5,302
|
)
|
|||
|
Change in fair value of contingent consideration
|
586
|
|
3,032
|
|
|||
|
Loss on settlement of liability-classified warrants
|
—
|
|
727
|
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Prepaid expenses and other assets
|
(76
|
)
|
212
|
|
|||
|
Accounts payable
|
12
|
|
(7,936
|
)
|
|||
|
Accrued expenses and other current liabilities
|
(1,048
|
)
|
(2,282
|
)
|
|||
|
Net cash used in operating activities
|
(17,643
|
)
|
(30,422
|
)
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Proceeds from issuance of Series A convertible preferred stock and common stock warrants, net of issuance costs
|
—
|
|
9,647
|
|
|||
|
Proceeds from issuance of common stock, net of issuance costs
|
15,971
|
|
21,564
|
|
|||
|
Proceeds from exercise of warrants
|
3,598
|
|
—
|
|
|||
|
Dividends paid
|
—
|
|
(487
|
)
|
|||
|
Principal payments on long-term debt
|
—
|
|
(7,601
|
)
|
|||
|
Net cash provided by financing activities
|
19,569
|
|
23,123
|
|
|||
|
Net increase (decrease) in cash, cash equivalents, restricted cash, and restricted cash equivalents
|
1,926
|
|
(7,299
|
)
|
|||
|
Cash, cash equivalents, restricted cash, and restricted cash equivalents at the beginning of year
|
5,451
|
|
12,750
|
|
|||
|
Cash, cash equivalents, restricted cash, and restricted cash equivalents at the end of year
|
$
|
7,377
|
|
$
|
5,451
|
|
|
|
Supplemental disclosure of cash flow information:
|
|||||||
|
Cash received during the year for interest
|
$
|
118
|
|
$
|
186
|
|
|
|
Cash paid during the year for interest
|
$
|
—
|
|
$
|
321
|
|
|
|
Supplemental disclosure of non-cash investing and financing activities:
|
|||||||
|
Fair value of liability-classified warrants issued in connection with Series A convertible preferred stock recorded as issuance cost
|
$
|
—
|
|
$
|
5,350
|
|
|
|
Deemed dividend arising from beneficial conversion feature of Series A convertible preferred stock
|
$
|
—
|
|
$
|
4,436
|
|
|
|
Repayment of interest and principal on long-term debt through issuance of common stock
|
$
|
—
|
|
$
|
3,721
|
|
|
|
Stock subscription receivable
|
$
|
308
|
|
$
|
—
|
|
|
|
Reclassification of warrant liabilities upon exchange for shares of common stock
|
$
|
68
|
|
$
|
285
|
|
|
|
Impact of anti-dilution protection on liability-classified warrants
|
$
|
243
|
|
$
|
491
|
|
|
|
Debt issued in connection with warrant exchange agreements
|
$
|
—
|
|
$
|
966
|
|
|
|
Deferred offering costs included in accounts payable and accrued expenses
|
$
|
135
|
|
$
|
—
|
|
|
|
Right-of-use assets recorded upon adoption of ASC 842
|
$
|
549
|
|
$
|
—
|
|
|
|
Year Ended December 31,
|
|||||
|
2019
|
2018
|
||||
|
Common stock warrants
|
302
|
|
356
|
|
|
|
Stock options
|
22
|
|
8
|
|
|
|
324
|
|
364
|
|
||
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Cash and cash equivalents
|
$
|
7,277
|
|
$
|
5,337
|
|
|
|
Restricted cash and cash equivalents
|
100
|
|
114
|
|
|||
|
Total cash, cash equivalents, restricted cash, and restricted cash equivalents
|
$
|
7,377
|
|
$
|
5,451
|
|
|
|
Year Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
NPS
|
$
|
5,700
|
|
$
|
5,700
|
|
|
|
GALE-301 and GALE-302
|
—
|
|
2,800
|
|
|||
|
$
|
5,700
|
|
$
|
8,500
|
|
||
|
Description
|
December 31, 2019
|
Quoted Prices In
Active Markets
(Level 1)
|
Significant Other
Observable
Inputs (Level 2)
|
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
7,027
|
|
$
|
7,027
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total assets measured and recorded at fair value
|
$
|
7,027
|
|
$
|
7,027
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Warrants potentially settleable in cash
|
$
|
52
|
|
$
|
—
|
|
$
|
—
|
|
$
|
52
|
|
|||
|
Contingent consideration
|
4,912
|
|
—
|
|
—
|
|
4,912
|
|
|||||||
|
Total liabilities measured and recorded at fair value
|
$
|
4,964
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4,964
|
|
|||
|
Description
|
December 31, 2018
|
Quoted Prices In
Active Markets
(Level 1)
|
Significant Other
Observable
Inputs (Level 2)
|
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
5,195
|
|
$
|
5,195
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total assets measured and recorded at fair value
|
$
|
5,195
|
|
$
|
5,195
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Warrants potentially settleable in cash
|
$
|
1,013
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,013
|
|
|||
|
Contingent consideration
|
4,326
|
|
—
|
|
—
|
|
4,326
|
|
|||||||
|
Total liabilities measured and recorded at fair value
|
$
|
5,339
|
|
$
|
—
|
|
$
|
—
|
|
$
|
5,339
|
|
|||
|
|
Fair Value
Measurements
Using Significant
Unobservable
Inputs
(Level 3)
|
||
|
Contingent consideration, January 1, 2018
|
$
|
1,294
|
|
|
Change in the estimated fair value of the contingent consideration
|
3,032
|
|
|
|
Contingent consideration, December 31, 2018
|
$
|
4,326
|
|
|
Change in the estimated fair value of the contingent consideration
|
586
|
|
|
|
Contingent consideration, December 31, 2019
|
$
|
4,912
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Clinical development
|
$
|
224
|
|
$
|
48
|
|
|
|
Insurance
|
200
|
|
154
|
|
|||
|
Professional fees
|
49
|
|
56
|
|
|||
|
Other
|
84
|
|
129
|
|
|||
|
Prepaid expenses and other current assets
|
$
|
557
|
|
$
|
387
|
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Compensation and related benefits
|
$
|
606
|
|
$
|
675
|
|
|
|
Clinical trial costs
|
371
|
|
858
|
|
|||
|
Professional fees
|
194
|
|
540
|
|
|||
|
Rebates and returns of former commercial products
|
—
|
|
138
|
|
|||
|
Other
|
—
|
|
8
|
|
|||
|
Accrued expenses and other current liabilities
|
$
|
1,171
|
|
$
|
2,219
|
|
|
|
Total minimum lease payments, all due in 2020
|
$
|
224
|
|
|
|
Less: imputed interest
|
(7
|
)
|
||
|
Operating lease liability
|
$
|
217
|
|
|
|
|
December 31, 2019
|
|
|
Warrants outstanding
|
302
|
|
|
Stock options outstanding
|
22
|
|
|
Options reserved for future issuance under the Company’s 2019 Equity Incentive Plan
|
203
|
|
|
Shares reserved for future issuance under the Employee Stock Purchase Plan
|
5
|
|
|
Total shares of common stock reserved for future issuance
|
532
|
|
|
Warrant Issuance
|
Outstanding, December 31, 2018
|
Granted
|
Exercised
|
Canceled/Expired
|
Outstanding, December 31, 2019
|
Expiration
|
||||||||||
|
June 2019 Offering
|
—
|
|
2,000
|
|
(1,998
|
)
|
—
|
|
2
|
|
June 2024
|
|||||
|
Pre-funded June 2019 Offering
|
—
|
|
1,473
|
|
(1,473
|
)
|
—
|
|
—
|
|
June 2024
|
|||||
|
March 2019 Exercise Agreement
|
—
|
|
63
|
|
—
|
|
—
|
|
63
|
|
March 2024
|
|||||
|
July 2018 Offering
|
305
|
|
—
|
|
(97
|
)
|
—
|
|
208
|
|
July 2025
|
|||||
|
Pre-funded July 2018 Offering
|
13
|
|
—
|
|
(13
|
)
|
—
|
|
—
|
|
July 2023
|
|||||
|
Series A Convertible Preferred
|
28
|
|
—
|
|
(9
|
)
|
—
|
|
19
|
|
September 2023
|
|||||
|
2017 Equilibria
|
6
|
|
—
|
|
—
|
|
—
|
|
6
|
|
December 2022
|
|||||
|
Galena February 2017
|
1
|
|
—
|
|
—
|
|
—
|
|
1
|
|
February 2022
|
|||||
|
Galena Other
|
3
|
|
—
|
|
—
|
|
—
|
|
3
|
|
January 2022
|
|||||
|
356
|
|
3,536
|
|
(3,590
|
)
|
—
|
|
302
|
|
|||||||
|
Warrant Issuance
|
Outstanding, January 1, 2018
|
Granted
|
Exercised
|
Canceled/Expired
|
Outstanding, December 31, 2018
|
Expiration
|
||||||||||
|
July 2018 Offering
|
—
|
|
305
|
|
—
|
|
—
|
|
305
|
|
July 2025
|
|||||
|
Pre-funded July 2018 Offering
|
—
|
|
94
|
|
(81
|
)
|
—
|
|
13
|
|
July 2023
|
|||||
|
Series A Convertible Preferred
|
—
|
|
28
|
|
—
|
|
—
|
|
28
|
|
September 2023
|
|||||
|
2017 Equilibria
|
6
|
|
—
|
|
—
|
|
—
|
|
6
|
|
December 2022
|
|||||
|
Galena February 2017
|
12
|
|
—
|
|
—
|
|
(11
|
)
|
1
|
|
February 2022
|
|||||
|
Galena Other
|
3
|
|
—
|
|
—
|
|
—
|
|
3
|
|
January 2022
|
|||||
|
21
|
|
427
|
|
(81
|
)
|
(11
|
)
|
356
|
|
|||||||
|
As of December 31, 2019
|
||||||||||||||
|
Warrant Issuance
|
Outstanding (in thousands)
|
Strike price (per share)
|
Expected term (years)
|
Volatility %
|
Risk-free rate %
|
|||||||||
|
Series A Convertible Preferred
|
19
|
|
$
|
7.50
|
|
3.75
|
112.84
|
%
|
1.64
|
%
|
||||
|
Galena February 2017
|
1
|
|
$
|
1,650.00
|
|
2.12
|
114.91
|
%
|
1.64
|
%
|
||||
|
Galena Other
|
3
|
|
$
|
41,494.00
|
|
1.43
|
114.91
|
%
|
1.64
|
%
|
||||
|
As of December 31, 2018
|
||||||||||||||
|
Warrant Issuance
|
Outstanding (in thousands)
|
Strike price (per share)
|
Expected term (years)
|
Volatility %
|
Risk-free rate %
|
|||||||||
|
Series A Convertible Preferred
|
28
|
|
$
|
105.00
|
|
4.76
|
88.80
|
%
|
2.50
|
%
|
||||
|
Galena February 2017
|
1
|
|
$
|
1,650.00
|
|
3.12
|
94.12
|
%
|
2.46
|
%
|
||||
|
Galena Other
|
3
|
|
$
|
41,494.00
|
|
2.43
|
94.73
|
%
|
2.46
|
%
|
||||
|
Warrant Issuance
|
Warrant liability, December 31, 2018
|
Fair value of warrants granted
|
Fair value of warrants exercised
|
Adjustment to exercise price of warrants
|
Change in fair value of warrants
|
Warrant liability, December 31, 2019
|
|||||||||||||||||
|
Series A Convertible Preferred
|
$
|
1,010
|
|
$
|
—
|
|
$
|
(68
|
)
|
$
|
243
|
|
$
|
(1,133
|
)
|
$
|
52
|
|
|||||
|
Galena February 2017
|
3
|
|
—
|
|
—
|
|
—
|
|
(3
|
)
|
—
|
|
|||||||||||
|
$
|
1,013
|
|
$
|
—
|
|
$
|
(68
|
)
|
$
|
243
|
|
$
|
(1,136
|
)
|
$
|
52
|
|
||||||
|
Years Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Research and development
|
$
|
—
|
|
$
|
77
|
|
|
|
General and administrative
|
573
|
|
318
|
|
|||
|
Total stock-based compensation
|
$
|
573
|
|
$
|
395
|
|
|
|
Years Ended December 31,
|
|||||
|
2019
|
2018
|
||||
|
Risk free interest rate
|
2.49
|
%
|
2.73
|
%
|
|
|
Volatility
|
96.57
|
%
|
80.83
|
%
|
|
|
Expected lives (years)
|
6.15
|
|
6.20
|
|
|
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
|
|
Total
Number of
Shares
|
Weighted
Average
Exercise
Price
|
Weighted Average Remaining Contractual Term (in years)
|
Aggregate
Intrinsic
Value
(In Thousands)
|
|||||||||
|
Outstanding at January 1, 2018
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Granted
|
9,650
|
|
261.96
|
|
||||||||
|
Canceled
|
(2,000
|
)
|
264.77
|
|
||||||||
|
Outstanding at December 31, 2018
|
7,650
|
|
$
|
261.09
|
|
9.22
|
$
|
—
|
|
|||
|
Granted
|
18,800
|
|
69.00
|
|
||||||||
|
Canceled
|
(4,930
|
)
|
175.86
|
|
||||||||
|
Outstanding at December 31, 2019
|
21,520
|
|
$
|
112.81
|
|
8.98
|
—
|
|
||||
|
Vested and exercisable at December 31, 2019
|
1,906
|
|
$
|
261.03
|
|
8.26
|
$
|
—
|
|
|||
|
As of December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
U.S.
|
$
|
(8,477
|
)
|
$
|
(17,179
|
)
|
|
|
Non - U.S.
|
(10,895
|
)
|
(11,869
|
)
|
|||
|
$
|
(19,372
|
)
|
$
|
(29,048
|
)
|
||
|
As of December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Current
|
|||||||
|
Federal
|
$
|
—
|
|
$
|
(106
|
)
|
|
|
State
|
14
|
|
45
|
|
|||
|
Total current
|
14
|
|
(61
|
)
|
|||
|
Deferred expense
|
|||||||
|
Federal
|
(117
|
)
|
(747
|
)
|
|||
|
State
|
22
|
|
(570
|
)
|
|||
|
Total deferred
|
(95
|
)
|
(1,317
|
)
|
|||
|
Total income tax expense (benefit)
|
$
|
(81
|
)
|
$
|
(1,378
|
)
|
|
|
As of December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Net operating loss carryforwards
|
$
|
6,720
|
|
$
|
4,464
|
|
|
|
Tax credit carryforwards
|
—
|
|
134
|
|
|||
|
Stock-based compensation
|
2,059
|
|
2,223
|
|
|||
|
Licensing deduction deferral
|
5,139
|
|
5,961
|
|
|||
|
Lease liability
|
47
|
|
—
|
|
|||
|
Other
|
27
|
|
56
|
|
|||
|
Gross deferred tax assets
|
13,992
|
|
12,838
|
|
|||
|
Valuation allowance
|
(12,987
|
)
|
(11,410
|
)
|
|||
|
Net deferred tax asset
|
$
|
1,005
|
|
$
|
1,428
|
|
|
|
As of December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
In-process research and development not subject to future amortization for tax purposes
|
$
|
1,220
|
|
$
|
1,785
|
|
|
|
Right of use asset
|
$
|
47
|
|
$
|
—
|
|
|
|
Gross deferred tax liability
|
$
|
1,267
|
|
$
|
1,785
|
|
|
|
As of December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Net deferred tax asset
|
$
|
1,005
|
|
$
|
1,428
|
|
|
|
Gross deferred tax liability
|
1,267
|
|
1,785
|
|
|||
|
Net deferred tax liability
|
$
|
262
|
|
$
|
357
|
|
|
|
As of December 31,
|
|||||
|
2019
|
2018
|
||||
|
U.S. federal statutory income tax rate
|
(21.0
|
)%
|
(21.0
|
)%
|
|
|
State and local taxes, net of federal benefit
|
(1.1
|
)%
|
(1.0
|
)%
|
|
|
Foreign rate differential
|
11.8
|
%
|
8.6
|
%
|
|
|
Permanent differences
|
1.1
|
%
|
1.8
|
%
|
|
|
Tax rate change and true-up
|
—
|
%
|
(13.9
|
)%
|
|
|
Fair value change warrants
|
(6.5
|
)%
|
0.5
|
%
|
|
|
Contingent consideration
|
4.5
|
%
|
(3.8
|
)%
|
|
|
Other
|
0.8
|
%
|
0.1
|
%
|
|
|
Valuation allowance
|
9.9
|
%
|
23.5
|
%
|
|
|
Tax credits
|
—
|
%
|
0.5
|
%
|
|
|
Effective income tax rate
|
(0.5
|
)%
|
(4.7
|
)%
|
|
|
As of December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Beginning of the Year - unrecognized tax benefits
|
$
|
55
|
|
$
|
72
|
|
|
|
Increase/(Decrease) - prior year tax positions
|
(55
|
)
|
(17
|
)
|
|||
|
End of the Year - unrecognized tax benefits
|
$
|
—
|
|
$
|
55
|
|
|
|
Exhibit
Number
|
Description
|
Form
|
Exhibit
|
Filing Date
|
|
2.1^
|
8-K
|
2.1
|
August 8, 2017
|
|
|
3.1
|
10-K
|
3.1
|
April 13, 2018
|
|
|
3.2
|
8-K
|
3.1
|
March 12, 2018
|
|
|
3.3
|
8-K
|
3.1
|
November 6, 2019
|
|
|
3.4
|
8-K
|
3.3
|
January 5, 2018
|
|
|
4.1*
|
10-K
|
4.1
|
April 13, 2018
|
|
|
4.2
|
8-K
|
10.1
|
April 14, 2011
|
|
|
4.3
|
10-K
|
10.2
|
March 28, 2012
|
|
|
4.4
|
10-Q
|
10.3
|
May 10, 2016
|
|
|
4.5
|
10-Q
|
4.2
|
May 10, 2016
|
|
|
4.6
|
10-Q
|
4.3
|
May 10, 2016
|
|
|
4.7
|
10-Q
|
10.2
|
May 10, 2016
|
|
|
4.8
|
10-Q
|
10.4
|
May 10, 2016
|
|
|
4.9
|
8-K
|
4.1
|
August 23, 2016
|
|
|
4.10
|
8-K
|
4.1
|
July 11, 2017
|
|
|
4.11
|
8-K
|
10.3
|
August 8, 2017
|
|
|
4.12
|
10-Q
|
10.7
|
May 9, 2013
|
|
|
4.13
|
8-K
|
4.1
|
September 18, 2013
|
|
|
4.14
|
10-Q
|
4.1
|
August 6, 2015
|
|
|
Exhibit
Number
|
Description
|
Form
|
Exhibit
|
Filing Date
|
|
4.15
|
8-K
|
4.1
|
January 7, 2016
|
|
|
4.16
|
8-K
|
4.1
|
July 8, 2016
|
|
|
4.17
|
8-K
|
4.1
|
February 10, 2017
|
|
|
4.23
|
8-K
|
10.5
|
January 5, 2018
|
|
|
4.18
|
10-K
|
10.73
|
April 13, 2018
|
|
|
4.19
|
10-K
|
10.74
|
April 13, 2018
|
|
|
4.20
|
10-K
|
10.75
|
April 13, 2018
|
|
|
4.21
|
10-K
|
10.76
|
April 13, 2018
|
|
|
4.22
|
10-K
|
10.77
|
April 13, 2018
|
|
|
4.23
|
10-K
|
10.78
|
April 13, 2018
|
|
|
4.24
|
10-K
|
10.79
|
April 13, 2018
|
|
|
4.25
|
10-K
|
10.80
|
April 13, 2018
|
|
|
4.26
|
10-K
|
10.81
|
April 13, 2018
|
|
|
4.27
|
10-K
|
10.82
|
April 13, 2018
|
|
|
4.28
|
10-K
|
10.83
|
April 13, 2018
|
|
|
4.29
|
10-K
|
10.84
|
April 13, 2018
|
|
|
4.30
|
10-K
|
10.85
|
April 13, 2018
|
|
|
4.31
|
10-K
|
10.86
|
April 13, 2018
|
|
|
4.32
|
10-K
|
10.87
|
April 13, 2018
|
|
|
4.33
|
8-K
|
10.1
|
July 18, 2018
|
|
|
4.34
|
8-K
|
10.2
|
July 9, 2019
|
|
|
4.35
|
8-K
|
10.2
|
July 18, 2018
|
|
|
Exhibit
Number
|
Description
|
Form
|
Exhibit
|
Filing Date
|
|
4.36
|
8-K
|
10.3
|
July 18, 2018
|
|
|
4.37
|
8-K
|
10.1
|
June 1, 2018
|
|
|
4.38
|
8-K
|
4.1
|
March 6, 2019
|
|
|
4.39
|
8-K
|
10.1
|
June 18, 2019
|
|
|
4.40
|
8-K
|
10.2
|
June 18, 2019
|
|
|
4.41
|
||||
|
9.1
|
8-K
|
10.1
|
March 12, 2018
|
|
|
10.1*
|
8-K
|
10.3
|
August 22, 2016
|
|
|
10.2*
|
10-Q
|
10.1
|
August 8, 2015
|
|
|
10.3*
|
10-Q
|
10.2
|
August 8, 2015
|
|
|
10.4*
|
S-4/A
|
10.61
|
October 30, 2017
|
|
|
10.5*
|
S-4/A
|
10.63
|
October 30, 2017
|
|
|
10.6*
|
8-K
|
10.10
|
January 5, 2018
|
|
|
10.7*
|
8-K
|
10.11
|
January 5, 2018
|
|
|
10.8*
|
8-K
|
10.2
|
March 19, 2018
|
|
|
10.9*
|
10-K
|
10.9
|
April 13, 2018
|
|
|
10.10*
|
S-4/A
|
10.53
|
October 30, 2017
|
|
|
10.11*
|
S-4/A
|
10.54
|
October 30, 2017
|
|
|
10.12*
|
S-4/A
|
10.55
|
October 30, 2017
|
|
|
10.13*
|
S-4/A
|
10.56
|
October 30, 2017
|
|
|
10.14*
|
S-4/A
|
10.57
|
October 30, 2017
|
|
|
10.15*
|
S-4/A
|
10.58
|
October 30, 2017
|
|
|
10.16*
|
8-K
|
10.1
|
March 19, 2018
|
|
|
Exhibit
Number
|
Description
|
Form
|
Exhibit
|
Filing Date
|
|
10.17+
|
10-Q
|
10.1
|
August 15, 2011
|
|
|
10.18
|
10-Q
|
10.2
|
August 15, 2011
|
|
|
10.19
|
10-Q
|
10.3
|
August 15, 2011
|
|
|
10.20
|
10-Q
|
10.4
|
August 15, 2011
|
|
|
10.21+
|
10-Q
|
10.5
|
August 15, 2011
|
|
|
10.22+
|
10-Q
|
10.6
|
August 15, 2011
|
|
|
10.23
|
10-Q
|
10.10
|
August 15, 2011
|
|
|
10.24+
|
10-Q
|
10.12
|
August 15, 2011
|
|
|
10.25+
|
8-K
|
10.1
|
September 21, 2011
|
|
|
10.26+
|
10-K
|
10.45
|
March 28, 2012
|
|
|
10.27
|
10-K
|
10.46
|
March 28, 2012
|
|
|
10.28+
|
10-K
|
10.43
|
March 12, 2013
|
|
|
Exhibit
Number
|
Description
|
Form
|
Exhibit
|
Filing Date
|
|
10.29+
|
10-K
|
10.36
|
March 17, 2014
|
|
|
10.30+
|
10-K
|
10.37
|
March 17, 2014
|
|
|
10.31
|
8-K
|
10.1
|
September 11, 2017
|
|
|
10.32
|
S-4/A
|
10.64
|
October 30, 2017
|
|
|
10.33+
|
S-4/A
|
10.65
|
October 30, 2017
|
|
|
10.34
|
8-K
|
10.8
|
January 5, 2018
|
|
|
10.35
|
8-K
|
10.1
|
May 24, 2018
|
|
|
10.36
|
8-K
|
10.2
|
May 24, 2018
|
|
|
10.37
|
8-K
|
10.1
|
November 9, 2018
|
|
|
10.38
|
10-Q
|
10.1
|
May 10, 2016
|
|
|
10.39
|
8-K
|
10-1
|
August 23, 2016
|
|
|
10.40
|
8-K
|
10.3
|
February 7, 2017
|
|
|
10.41
|
8-K
|
10.1
|
April 3, 2017
|
|
|
10.42
|
8-K
|
10.2
|
March 12, 2018
|
|
|
10.43
|
8-K
|
4.1
|
March 12, 2018
|
|
|
10.44
|
8-K
|
10.1
|
March 6, 2019
|
|
|
10.45
|
8-K
|
10.1
|
May 2, 2017
|
|
|
10.46
|
8-K
|
10.1
|
October 31, 2019
|
|
|
10.47
|
8-K
|
10.1
|
July 9, 2019
|
|
|
10.48
|
||||
|
10.49
|
||||
|
14.1
|
8-K
|
14.1
|
January 5, 2018
|
|
|
21.1
|
10-K
|
21.1
|
April 13, 2018
|
|
|
23.1
|
||||
|
Exhibit
Number
|
Description
|
Form
|
Exhibit
|
Filing Date
|
|
24.1
|
Powers of Attorney (included on signature page hereto)
|
|||
|
31.1
|
||||
|
31.2
|
||||
|
32.1**
|
||||
|
101.INS***
|
XBRL Instance Document.
|
|||
|
101.SCH***
|
XBRL Taxonomy Extension Schema.
|
|||
|
101.CAL***
|
XBRL Taxonomy Extension Calculation Linkbase.
|
|||
|
101.DEF***
|
XBRL Taxonomy Extension Definition Linkbase.
|
|||
|
101.LAB***
|
XBRL Taxonomy Extension Label Linkbase.
|
|||
|
101.PRE***
|
XBRL Taxonomy Extension Presentation Linkbase.
|
|||
|
*
|
Indicates management contract or compensatory plans or arrangements.
|
|
**
|
The certification attached as Exhibit 32.1 pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the Company for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing of the registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
|
|
^
|
The schedules and exhibits to this exhibit have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request.
|
|
+
|
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC.
|
|
***
|
In accordance with Rule 406T of Regulation S-T, the Interactive Data Files in Exhibit 101 are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Exchange Act of 1934, as amended, and otherwise are not subject to liability under these sections.
|
|
SELLAS Life Sciences Group, Inc.
|
|||
|
Date: March 13, 2020
|
By:
|
/s/ Angelos M. Stergiou
|
|
|
Angelos M. Stergiou, MD, ScD h.c.
|
|||
|
President and Chief Executive Officer
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Angelos M. Stergiou
|
President, Chief Executive Officer and Director
(Principal Executive Officer and Principal Financial Officer)
|
March 13, 2020
|
||
|
Angelos M. Stergiou, M.D., ScD h.c.
|
||||
|
/s/ John T. Burns
|
Vice President, Finance and Corporate Controller
(Interim Principal Accounting Officer)
|
March 13, 2020
|
||
|
John T. Burns, CPA
|
||||
|
/s/ Jane Wasman
|
Chair of the Board of Directors
|
March 13, 2020
|
||
|
Jane Wasman
|
||||
|
/s/ David Scheinberg
|
Director
|
March 13, 2020
|
||
|
David Scheinberg, M.D., PhD.
|
||||
|
/s/ Robert Van Nostrand
|
Director
|
March 13, 2020
|
||
|
Robert Van Nostrand
|
||||
|
/s/ John Varian
|
Director
|
March 13, 2020
|
||
|
John Varian
|
||||