|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Form 10-K
|
|
Digirad Corporation
|
|
(Exact Name of Registrant as Specified in its Charter)
|
|
Delaware
|
|
33-0145723
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
13950 Stowe Drive, Poway, CA
|
|
92064
|
|
(Address of Principal Executive Offices)
|
|
(Zip Code)
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
Common Stock, par value $0.0001 per share
|
|
NASDAQ Global Market
|
|
|
|
Page
|
|
Item 1
|
||
|
Item 1A
|
||
|
Item 1B
|
||
|
Item 2
|
||
|
Item 3
|
||
|
Item 4
|
||
|
Item 5
|
||
|
Item 6
|
||
|
Item 7
|
||
|
Item 7A
|
||
|
Item 8
|
||
|
Item 9
|
||
|
Item 9A
|
||
|
Item 9B
|
||
|
Item 10
|
||
|
Item 11
|
||
|
Item 12
|
||
|
Item 13
|
||
|
Item 14
|
||
|
Item 15
|
||
|
ITEM 1.
|
BUSINESS
|
|
•
|
Leading Solid-State Technology.
Our solid-state gamma cameras utilize proprietary photo-detector modules which enable us to build smaller and lighter cameras that are portable, with a degree of ruggedness that can withstand the vibration associated with transportation. We have continued to invest in technology advancements that enhance the performance of our solid-state photodiode detectors over traditional photomultiplier tube-based systems for both cardiac and general purpose nuclear medicine applications. We now offer a more geometric-efficient design for cardiology and introduced our ergo
TM
imaging system in mid-2010, our first large field-of-view solid-state detector system for use in general nuclear medicine, pediatrics, women’s health and surgery. We see expanded opportunities for such systems worldwide as departments replace aged single-head systems and migrate towards more modern solid-state systems offering higher performance, greater clinical flexibility and the ability to be used portably to image patients at their bedsides.
|
|
•
|
Portable Applications through Reduced Size and Weight
. Our cameras, depending on the model, weigh anywhere from 600 to 1,000 pounds. Competitive anger photomultiplier tube-based technology cameras generally weigh 2 to 5 times as much. Our dedicated cardiac imagers require a floor space of as little as seven feet by eight feet and generally can be installed without facility renovations and use standard power (20 Amps @ 120 VAC). Our portable cameras are ideal for mobile operators or practices desiring to service multiple office locations or imaging facilities, and for use in our DIS in-office service business. We bring nuclear technology to the patient. Our systems do not require the patient to be taken to the camera, a significant competitive advantage.
|
|
•
|
Speed and Image Quality.
We believe our Cardius
®
3 XPO and X-ACT rapid imaging dedicated cardiac cameras, equipped with our proprietary nSPEED 3DOSEM software, can acquire images up to four times faster than conventional fixed 90 or variable dual-head photomultiplier vacuum tube camera designs with equivalent image quality. Increased imaging speed optimizes workflow and resource utilization and allows for reduction of the administered dose of radiation to patients or the use of low dose imaging protocols, which we believe is increasingly of interest to our physician customers. Use of rapid imaging systems, combined with nSPEED, gives us an efficiency advantage over other mobile service providers.
|
|
•
|
Fully-Integrated low dose SPECT/CT Technology.
Our Cardius
®
XACT rapid imaging system (triple-head) equipped with a low dose volume CT attenuation correction system allows studies to be performed faster using less radiation than competitive techniques, with improved diagnostic confidence in interpreted results. The competitive advantages of our Cardius
®
XACT system include its ability to deliver higher productivity and lower radiation exposure to patients.
|
|
•
|
Improved Patient Comfort and Utilization.
We believe the upright and open architecture of our patient chair reduces patient claustrophobia and increases patient comfort when compared to traditional vacuum tube-based imaging systems, the majority of which require the patient to lie flat and have detector heads rotate around the patient. Upright imaging positioning also reduces false indications that can result from organs pushing-up against the heart while patients are on their backs. Our Cardius
®
XPO camera series allows for the imaging of patients weighing up to 500 pounds.
|
|
•
|
Broad Portfolio of Cardiovascular Imaging Services.
Another competitive advantage is our ability to offer nuclear cardiology, echocardiography and complete vascular imaging services. Our ability to offer multiple services strengthens our competitive position and expands our revenue potential. The depth of services offered varies depending on the local market opportunity, availability of personnel and credentialing requirements in the individual markets.
|
|
•
|
Unique Dual Sales and Leasing Service Offering.
We sell imaging systems to physicians who wish to perform nuclear imaging in their facilities and manage the related service logistics. Through DIS, we offer both nuclear and ultrasound services in which we lease our systems and certified personnel to physicians on an annual basis in flexible increments, ranging from one day per month to several days per week without requiring them to make a capital investment, hire
|
|
•
|
Intellectual Property Portfolio.
We have developed an intellectual property portfolio that includes product, component and process patents covering various aspects of our imaging systems. As of December 31,
2011
, we had 36 issued U.S. patents and an additional 9 pending U.S. patent applications. We also license patents from third parties to enhance our product offering. In addition to our patent portfolio, we have developed proprietary manufacturing, business know-how, and trade secrets. This portfolio of intellectual property combined with our ability to design, manufacture, sell and service our own equipment provides us with a distinct competitive advantage.
|
|
•
|
Imaging Services (DIS).
After a difficult 2010 with headwinds in radiopharmaceutical shortages, healthcare reform uncertainties and reimbursement declines, 2011 showed signs of stabilization. The supply of radiopharmaceuticals stabilized, the impact of healthcare reform is being absorbed and Medicare reimbursements in nuclear codes increased in 2011 over 2010. We expect to continue supporting our physician customers by working with them to adjust our DIS business model; for example, with regulatory changes in 2011, we developed a process to assist them in obtaining direct accreditation. This initiative added value to our customers and will provide an additional revenue stream to our DIS business. We continue to focus on aligning our labor and other costs with the variable nature of our revenue streams. Also, we expect to provide greater value in our service channel via strategic and technological initiatives designed to increase revenue per day for us and our physician customers, as well as expand our service model offerings.
|
|
•
|
Product Equipment Sales.
In order to overcome the market decline of cardiac specific cameras and the general downturn in the economy that has limited the amount of healthcare capital spending, we intend to increase our market share by expanding beyond the cardiac-specific nuclear market. Our Cardius
®
XACT camera is particularly geared toward hospitals and large physician practices. Our new ergo
TM
imaging system also addresses the larger market of general nuclear imaging and provides us with a new untapped market opportunity within the hospital. Our ergo
TM
imaging system is not just part of a hospital nuclear suite. It is a camera that enables the imaging to be performed wherever the patient is located and has great promise in areas of the hospital where previously no nuclear imaging has been performed, such as the emergency room and the surgical suite. Although the selling cycle in hospitals can be long, we anticipate increased sales of our ergo™ imaging system in 2012 and beyond.
|
|
•
|
Anti-Kickback Laws.
The Medicare/Medicaid Patient Protection Act of 1987, as amended, which is commonly referred to as the Anti-Kickback Statute, prohibits us from knowingly and willingly offering, paying, soliciting, or receiving any form of remuneration in return for the referral of items or services, or to purchase, lease, order or arrange for or recommend purchasing, leasing, or ordering any good, facility service or item, for which payment may be made under a federal healthcare program. Violation of the federal anti-kickback law is a felony, punishable by criminal fines and imprisonment, or both, and can result in civil penalties and exclusion from participation in healthcare programs such as Medicare and Medicaid. Many states have adopted similar statutes prohibiting payments intended to induce referrals of products or services paid by Medicaid or other nongovernmental third-party payors.
|
|
•
|
Physician Self-Referral Laws.
Federal regulations commonly referred to as the “Stark Law” prohibit physician referrals of Medicare or Medicaid patients to an entity for certain designated health services if the physician or an immediate family member has an indirect or direct financial relationship with the entity, unless a statutory exception applies. We believe that referrals made by our physician customers are eligible to qualify for the “in-office ancillary services” exception to the Stark Law, provided that the services are provided or supervised by the physician or a member of his or her “Group Practice,” as that term is defined under the law, the services are performed in the same building in which the physicians regularly practice medicine, and the services are billed by or for the supervising physician or Group Practice. Violations of the Stark Law may lead to the imposition of penalties and fines, the exclusion from participation in federal healthcare programs, and liability under the federal False Claims Act and its whistleblower provisions. Many states have adopted similar statutes prohibiting self-referral arrangements that cover all patients and not just Medicare and Medicaid patients.
|
|
•
|
Federal False Claims Act.
The federal False Claims Act imposes civil and criminal liability on individuals or entities for the submission of false or fraudulent claims for payment to the government. Violations of the federal False Claims Act may result in civil penalties and exclusion from participation in federal healthcare programs. The federal False Claims Act also allows a private individual to bring a qui tam suit on behalf of the government against an individual or entity for violations of the False Claims Act. In a qui tam suit, a private plaintiff initiates a lawsuit for money of which the government was defrauded. If successful, the private plaintiff is entitled to receive up to 30% of the recovered amount plus reasonable expenses and attorney fees. A number of states have enacted laws modeled after the False Claims Act.
|
|
•
|
HIPAA
. The Health Insurance Portability and Accountability Act of 1996, or HIPAA, prohibits schemes to defraud healthcare benefit programs and fraudulent conduct in connection with the delivery of, or payment for, healthcare benefits, items or services. HIPAA also establishes standards governing electronic healthcare transactions and protecting the security and privacy of individually identifiable health information. Some states have also enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA.
|
|
•
|
Medical Device Regulation.
The FDA classifies medical devices, such as our cameras, into one of three classes, depending on the degree of risk associated with the device and the extent of control needed to ensure safety and effectiveness. Devices deemed to pose lower risk are placed in either class I or II, which generally requires the manufacturer to submit to the FDA a pre-market notification requesting permission for commercial distribution. This process is known as 510(k) clearance. Devices deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in Class III, requiring an approved Premarket Approval Application (PMA). Our cameras are Class II medical devices which have been cleared for marketing by the FDA. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness or that would constitute a major change in its intended use requires a new 510(k) clearance. The FDA requires each device manufacturer to determine whether a modification requires a new clearance or approval, but the FDA can disagree with a manufacturer’s determination. If so, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval is obtained. We are also subject to post-market regulatory requirements relating to our manufacturing process, marketing and sales activities, product performance and medical device reports should there be deaths and serious injuries associated with our products.
|
|
•
|
Pharmaceutical Regulation.
Federal and state agencies, including the FDA and state pharmacy boards, regulate the radiopharmaceuticals used in our DIS business. These agencies administer laws governing the manufacturing, sale,
|
|
•
|
Radioactive Materials Laws.
We must maintain licensure under, and comply with, federal and state radioactive materials laws, or RAM laws. RAM laws require, among other things, that radioactive materials are used by, or that their use be supervised by, individuals with specified training, expertise, and credentials and include specific provisions applicable to the medical use of radioactive materials. In our case, the authorized user must be a physician with training and expertise in the use of radioactive materials for diagnostic purposes. We have entered into contracts with qualified physicians in each of our regions to serve as authorized users. Because our physician customers in our lease services business are not licensees, and in most cases are not qualified to serve as authorized users, they perform nuclear medicine procedures as “supervised persons.”
|
|
ITEM 1A.
|
RISK FACTORS
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Year ended December 31,
|
||||||||||||||||
|
2011
|
2010
|
|||||||||||||||
|
High
|
Low
|
High
|
Low
|
|||||||||||||
|
First Quarter
|
$
|
2.63
|
|
$
|
2.13
|
|
$
|
2.16
|
|
$
|
1.83
|
|
||||
|
Second Quarter
|
3.04
|
|
2.40
|
|
2.49
|
|
2.01
|
|
||||||||
|
Third Quarter
|
2.91
|
|
2.15
|
|
2.13
|
|
1.74
|
|
||||||||
|
Fourth Quarter
|
2.40
|
|
1.78
|
|
2.23
|
|
1.88
|
|
||||||||
|
Total Number of
Shares Purchased
During the Period
|
Average Price
Paid Per Share
for Period
Presented
|
Total Cumulative
Number of
Shares Purchased
as Part of Publicly
Announced Plan (1)
|
Maximum Dollar
Value of Shares
that May Yet
Be Purchased
Under the Plan
|
|||||||||||
|
October 1, 2011 – October 31, 2011
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
||||
|
November 1, 2011 – November 30, 2011
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||
|
December 1, 2011 – December 31, 2011
|
9,607
|
|
2.01
|
|
582,825
|
|
957,261
|
|
||||||
|
As of December 31, 2011
|
9,607
|
|
582,825
|
|
$
|
957,261
|
|
|||||||
|
(1)
|
On February 4, 2009, our Board of Directors approved a stock repurchase program whereby we may, from time to time, purchase up to $2.0 million worth of our common stock in the open market, in privately negotiated transactions or otherwise, at prices that we deem appropriate. The plan has no expiration date. The timing of stock repurchases and the number of shares of common stock to be repurchased has been and will be made in compliance with Rule 10b-18 under the Securities Exchange Act of 1934. The timing and extent of the repurchase will depend upon market conditions, applicable legal and contractual requirements, and other factors.
|
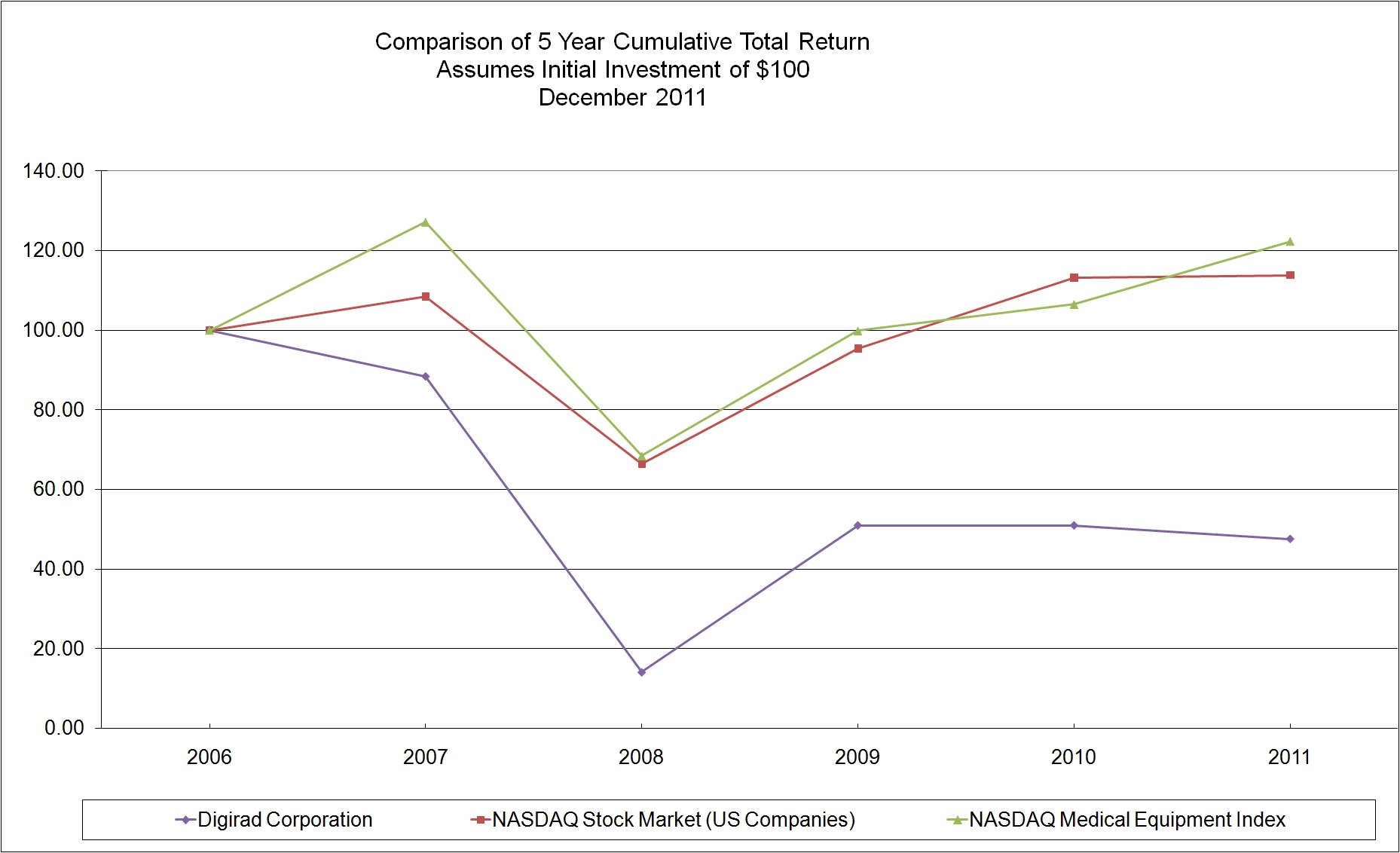
|
12/29/2006
|
12/31/2007
|
12/31/2008
|
12/31/2009
|
12/31/2010
|
12/30/2011
|
|||||||||||||
|
Digirad Corporation
|
$
|
100
|
|
$
|
88.35
|
|
$
|
14.08
|
|
$
|
50.97
|
|
$
|
50.97
|
|
$
|
47.58
|
|
|
NASDAQ Stock Market (US Companies)
|
$
|
100
|
|
$
|
108.47
|
|
$
|
66.35
|
|
$
|
95.38
|
|
$
|
113.2
|
|
$
|
113.81
|
|
|
NASDAQ Medical Equipment Index
|
$
|
100
|
|
$
|
127.15
|
|
$
|
68.47
|
|
$
|
99.85
|
|
$
|
106.48
|
|
$
|
122.33
|
|
|
ITEM 6.
|
SELECTED CONSOLIDATED FINANCIAL DATA
|
|
|
Years Ended December 31,
|
|||||||||||||||||||
|
|
2011
|
2010
|
2009
|
2008
|
2007
|
|||||||||||||||
|
Statement of Operations Data:
|
||||||||||||||||||||
|
Revenues:
|
||||||||||||||||||||
|
DIS
|
$
|
37,794
|
|
$
|
39,542
|
|
$
|
52,318
|
|
$
|
56,204
|
|
$
|
52,440
|
|
|||||
|
Product
|
15,951
|
|
16,641
|
|
17,278
|
|
24,154
|
|
21,507
|
|
||||||||||
|
Total revenues
|
53,745
|
|
56,183
|
|
69,596
|
|
80,358
|
|
73,947
|
|
||||||||||
|
Cost of revenues:
|
||||||||||||||||||||
|
DIS
|
29,672
|
|
32,561
|
|
38,476
|
|
44,697
|
|
39,520
|
|
||||||||||
|
Product
|
9,315
|
|
11,618
|
|
10,895
|
|
15,590
|
|
13,909
|
|
||||||||||
|
Total cost of revenues
|
38,987
|
|
44,179
|
|
49,371
|
|
60,287
|
|
53,429
|
|
||||||||||
|
Gross profit
|
14,758
|
|
12,004
|
|
20,225
|
|
20,071
|
|
20,518
|
|
||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
2,738
|
|
2,875
|
|
3,360
|
|
2,764
|
|
3,072
|
|
||||||||||
|
Marketing and sales
|
7,622
|
|
5,922
|
|
6,977
|
|
8,554
|
|
7,670
|
|
||||||||||
|
General and administrative
|
7,741
|
|
9,007
|
|
8,921
|
|
11,805
|
|
11,920
|
|
||||||||||
|
Amortization and impairment of intangible assets
|
331
|
|
435
|
|
590
|
|
798
|
|
697
|
|
||||||||||
|
Restructuring loss
|
(164
|
)
|
355
|
|
319
|
|
1,308
|
|
—
|
|
||||||||||
|
Goodwill impairment loss
|
—
|
|
—
|
|
—
|
|
2,466
|
|
—
|
|
||||||||||
|
Total operating expenses
|
18,268
|
|
18,594
|
|
20,167
|
|
27,695
|
|
23,359
|
|
||||||||||
|
Income (loss) from operations
|
(3,510
|
)
|
(6,590
|
)
|
58
|
|
(7,624
|
)
|
(2,841
|
)
|
||||||||||
|
Other income, net
|
168
|
|
376
|
|
550
|
|
759
|
|
1,465
|
|
||||||||||
|
Net income (loss)
|
$
|
(3,342
|
)
|
$
|
(6,214
|
)
|
$
|
608
|
|
$
|
(6,865
|
)
|
$
|
(1,376
|
)
|
|||||
|
Net income (loss) per share:
|
||||||||||||||||||||
|
Basic and diluted
|
$
|
(0.18
|
)
|
$
|
(0.33
|
)
|
$
|
0.03
|
|
$
|
(0.36
|
)
|
$
|
(0.07
|
)
|
|||||
|
Shares used in per share calculations:
|
||||||||||||||||||||
|
Basic
|
19,052
|
|
18,774
|
|
18,836
|
|
18,955
|
|
18,845
|
|
||||||||||
|
Diluted
|
19,052
|
|
18,774
|
|
19,320
|
|
18,955
|
|
18,845
|
|
||||||||||
|
|
As of December 31,
|
|||||||||||||||||||
|
|
2011
|
2010
|
2009
|
2008
|
2007
|
|||||||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Cash, cash equivalents and securities
|
$
|
30,452
|
|
$
|
30,247
|
|
$
|
31,810
|
|
$
|
28,284
|
|
$
|
31,662
|
|
|||||
|
Working capital
|
35,585
|
|
35,920
|
|
37,826
|
|
33,650
|
|
33,905
|
|
||||||||||
|
Total assets
|
50,027
|
|
52,244
|
|
58,689
|
|
61,195
|
|
69,015
|
|
||||||||||
|
Total debt
|
—
|
|
—
|
|
—
|
|
106
|
|
213
|
|
||||||||||
|
Total stockholders’ equity
|
41,487
|
|
43,959
|
|
49,389
|
|
48,959
|
|
55,247
|
|
||||||||||
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
|
Years ended December 31,
|
Change from
Prior Year
|
|||||||||||||||||||
|
|
2011
|
% of 2011
Revenues
|
2010
|
% of 2010
Revenues
|
Dollars
|
Percent
|
|||||||||||||||
|
Revenues:
|
|||||||||||||||||||||
|
DIS
|
$
|
37,794
|
|
70.3
|
%
|
$
|
39,542
|
|
70.4
|
%
|
$
|
(1,748
|
)
|
(4.4
|
)%
|
||||||
|
Product
|
15,951
|
|
29.7
|
%
|
16,641
|
|
29.6
|
%
|
(690
|
)
|
(4.1
|
)%
|
|||||||||
|
Total revenues
|
53,745
|
|
100.0
|
%
|
56,183
|
|
100.0
|
%
|
(2,438
|
)
|
(4.3
|
)%
|
|||||||||
|
Total cost of revenues
|
38,987
|
|
72.5
|
%
|
44,179
|
|
78.6
|
%
|
(5,192
|
)
|
(11.8
|
)%
|
|||||||||
|
Gross profit
|
14,758
|
|
27.5
|
%
|
12,004
|
|
21.4
|
%
|
2,754
|
|
22.9
|
%
|
|||||||||
|
Operating expenses:
|
|||||||||||||||||||||
|
Research and development
|
2,738
|
|
5.1
|
%
|
2,875
|
|
5.1
|
%
|
(137
|
)
|
(4.8
|
)%
|
|||||||||
|
Marketing and sales
|
7,622
|
|
14.2
|
%
|
5,922
|
|
10.5
|
%
|
1,700
|
|
28.7
|
%
|
|||||||||
|
General and administrative
|
7,741
|
|
14.4
|
%
|
9,007
|
|
16.0
|
%
|
(1,266
|
)
|
(14.1
|
)%
|
|||||||||
|
Amortization of intangible assets
|
331
|
|
0.6
|
%
|
435
|
|
0.8
|
%
|
(104
|
)
|
(23.9
|
)%
|
|||||||||
|
Restructuring loss (gain)
|
(164
|
)
|
(0.3
|
)%
|
355
|
|
0.6
|
%
|
(519
|
)
|
(146.2
|
)%
|
|||||||||
|
Total operating expenses
|
18,268
|
|
34.0
|
%
|
18,594
|
|
33.1
|
%
|
(326
|
)
|
(1.8
|
)%
|
|||||||||
|
Income (loss) from operations
|
(3,510
|
)
|
(6.5
|
)%
|
(6,590
|
)
|
(11.7
|
)%
|
3,080
|
|
(46.7
|
)%
|
|||||||||
|
Other income
|
168
|
|
0.3
|
%
|
376
|
|
0.7
|
%
|
(208
|
)
|
(55.3
|
)%
|
|||||||||
|
Net income (loss)
|
$
|
(3,342
|
)
|
(6.2
|
)%
|
$
|
(6,214
|
)
|
(11.1
|
)%
|
$
|
2,872
|
|
(46.2
|
)%
|
||||||
|
|
Year Ended December 31,
|
Change from
Prior Year
|
|||||||||||||||||||
|
|
2010
|
% of 2010
Revenues
|
2009
|
% of 2009
Revenues
|
Dollars
|
Percent
|
|||||||||||||||
|
Revenues:
|
|||||||||||||||||||||
|
DIS
|
$
|
39,542
|
|
70.4
|
%
|
$
|
52,318
|
|
75.2
|
%
|
$
|
(12,776
|
)
|
(24.4
|
)%
|
||||||
|
Product
|
16,641
|
|
29.6
|
%
|
17,278
|
|
24.8
|
%
|
(637
|
)
|
(3.7
|
)%
|
|||||||||
|
Total revenues
|
56,183
|
|
100
|
%
|
69,596
|
|
100
|
%
|
(13,413
|
)
|
(19.3
|
)%
|
|||||||||
|
Total cost of revenues
|
44,179
|
|
78.6
|
%
|
49,371
|
|
70.9
|
%
|
(5,192
|
)
|
(10.5
|
)%
|
|||||||||
|
Gross profit
|
12,004
|
|
21.4
|
%
|
20,225
|
|
29.1
|
%
|
(8,221
|
)
|
(40.6
|
)%
|
|||||||||
|
Operating expenses:
|
|||||||||||||||||||||
|
Research and development
|
2,875
|
|
5.1
|
%
|
3,360
|
|
4.8
|
%
|
(485
|
)
|
(14.4
|
)%
|
|||||||||
|
Marketing and sales
|
5,922
|
|
10.5
|
%
|
6,977
|
|
10.0
|
%
|
(1,055
|
)
|
(15.1
|
)%
|
|||||||||
|
General and administrative
|
9,007
|
|
16.0
|
%
|
8,921
|
|
12.8
|
%
|
86
|
|
1.0
|
%
|
|||||||||
|
Amortization of intangible assets
|
435
|
|
0.8
|
%
|
590
|
|
0.8
|
%
|
(155
|
)
|
(26.3
|
)%
|
|||||||||
|
Restructuring loss
|
355
|
|
0.6
|
%
|
319
|
|
0.5
|
%
|
36
|
|
11.3
|
%
|
|||||||||
|
Total operating expenses
|
18,594
|
|
33.1
|
%
|
20,167
|
|
29.0
|
%
|
(1,573
|
)
|
(7.8
|
)%
|
|||||||||
|
Income (loss) from operations
|
(6,590
|
)
|
(11.7
|
)%
|
58
|
|
0.1
|
%
|
(6,648
|
)
|
(11,462.1
|
)%
|
|||||||||
|
Other income
|
376
|
|
0.7
|
%
|
550
|
|
0.8
|
%
|
(174
|
)
|
(31.6
|
)%
|
|||||||||
|
Net income (loss)
|
$
|
(6,214
|
)
|
(11.1
|
)%
|
$
|
608
|
|
0.9
|
%
|
$
|
(6,822
|
)
|
(1,122.0
|
)%
|
||||||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Net cash provided by operating activities
|
$
|
965
|
|
$
|
229
|
|
$
|
4,806
|
|
|||
|
Net cash provided by (used in) investing activities
|
2,515
|
|
6,710
|
|
(3,764
|
)
|
||||||
|
Net cash provided by (used in) financing activities
|
$
|
100
|
|
$
|
(40
|
)
|
$
|
(1,007
|
)
|
|||
|
|
Payments Due by Period
|
|||||||||||||||||||
|
Contractual obligations
|
Total
|
Less than 1
year
|
1-3 years
|
3-5 years
|
More than 5
years
|
|||||||||||||||
|
Operating lease obligations
|
$
|
3,323
|
|
$
|
1,231
|
|
$
|
1,408
|
|
$
|
684
|
|
$
|
—
|
|
|||||
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
/s/ Ernst & Young LLP
|
|
|
|
|
As of December 31,
|
|||||||
|
|
2011
|
2010
|
||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
24,039
|
|
$
|
20,459
|
|
||
|
Securities available-for-sale
|
6,413
|
|
9,788
|
|
||||
|
Accounts receivable, net
|
6,320
|
|
7,527
|
|
||||
|
Inventories, net
|
6,178
|
|
5,432
|
|
||||
|
Other current assets
|
855
|
|
861
|
|
||||
|
Restricted cash
|
194
|
|
—
|
|
||||
|
Total current assets
|
43,999
|
|
44,067
|
|
||||
|
Property and equipment, net
|
5,367
|
|
7,185
|
|
||||
|
Intangible assets, net
|
477
|
|
808
|
|
||||
|
Goodwill
|
184
|
|
184
|
|
||||
|
Total assets
|
$
|
50,027
|
|
$
|
52,244
|
|
||
|
Liabilities and stockholders’ equity
|
||||||||
|
Accounts payable
|
$
|
1,330
|
|
$
|
1,694
|
|
||
|
Accrued compensation
|
2,291
|
|
1,600
|
|
||||
|
Accrued warranty
|
297
|
|
378
|
|
||||
|
Deferred revenue
|
2,099
|
|
2,379
|
|
||||
|
Other accrued liabilities
|
2,397
|
|
2,096
|
|
||||
|
Total current liabilities
|
8,414
|
|
8,147
|
|
||||
|
Deferred rent
|
126
|
|
138
|
|
||||
|
Total liabilities
|
8,540
|
|
8,285
|
|
||||
|
Commitments and contingencies (Note 6)
|
|
|
||||||
|
Stockholders’ equity:
|
||||||||
|
Preferred stock, $0.0001 par value: 10,000,000 shares authorized; no shares issued or outstanding
|
—
|
|
—
|
|
||||
|
Common stock, $0.0001 par value: 80,000,000 shares authorized; 18,901,160 and 18,597,311 shares issued and outstanding (net of treasury shares) at December 31, 2011 and 2010, respectively
|
2
|
|
2
|
|
||||
|
Treasury stock, at cost; 582,825 shares and 573,218 shares at December 31, 2011 and 2010, respectively
|
(1,058
|
)
|
(1,039
|
)
|
||||
|
Additional paid-in capital
|
155,704
|
|
154,785
|
|
||||
|
Accumulated other comprehensive income
|
33
|
|
63
|
|
||||
|
Accumulated deficit
|
(113,194
|
)
|
(109,852
|
)
|
||||
|
Total stockholders’ equity
|
41,487
|
|
43,959
|
|
||||
|
Total liabilities and stockholders’ equity
|
$
|
50,027
|
|
$
|
52,244
|
|
||
|
|
Years ended December 31,
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Revenues:
|
||||||||||||
|
DIS
|
$
|
37,794
|
|
$
|
39,542
|
|
$
|
52,318
|
|
|||
|
Product
|
15,951
|
|
16,641
|
|
17,278
|
|
||||||
|
Total revenues
|
53,745
|
|
56,183
|
|
69,596
|
|
||||||
|
Cost of revenues:
|
||||||||||||
|
DIS
|
29,672
|
|
32,561
|
|
38,476
|
|
||||||
|
Product
|
9,315
|
|
11,618
|
|
10,895
|
|
||||||
|
Total cost of revenues
|
38,987
|
|
44,179
|
|
49,371
|
|
||||||
|
Gross profit
|
14,758
|
|
12,004
|
|
20,225
|
|
||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
2,738
|
|
2,875
|
|
3,360
|
|
||||||
|
Marketing and sales
|
7,622
|
|
5,922
|
|
6,977
|
|
||||||
|
General and administrative
|
7,741
|
|
9,007
|
|
8,921
|
|
||||||
|
Amortization and impairment of intangible assets
|
331
|
|
435
|
|
590
|
|
||||||
|
Restructuring loss (gain)
|
(164
|
)
|
355
|
|
319
|
|
||||||
|
Total operating expenses
|
18,268
|
|
18,594
|
|
20,167
|
|
||||||
|
Income (loss) from operations
|
(3,510
|
)
|
(6,590
|
)
|
58
|
|
||||||
|
Other income (expense):
|
||||||||||||
|
Interest income
|
165
|
|
378
|
|
499
|
|
||||||
|
Other income (expense)
|
3
|
|
(2
|
)
|
51
|
|
||||||
|
Total other income
|
168
|
|
376
|
|
550
|
|
||||||
|
Net income (loss)
|
$
|
(3,342
|
)
|
$
|
(6,214
|
)
|
$
|
608
|
|
|||
|
Net income (loss) per share:
|
||||||||||||
|
Basic and diluted
|
$
|
(0.18
|
)
|
$
|
(0.33
|
)
|
$
|
0.03
|
|
|||
|
Shares used in per share computations:
|
||||||||||||
|
Weighted average shares outstanding—basic
|
19,052
|
|
18,774
|
|
18,836
|
|
||||||
|
Weighted average shares outstanding—diluted
|
19,052
|
|
18,774
|
|
19,320
|
|
||||||
|
|
Years ended December 31,
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Operating activities
|
||||||||||||
|
Net income (loss)
|
$
|
(3,342
|
)
|
$
|
(6,214
|
)
|
$
|
608
|
|
|||
|
Adjustments to reconcile net income (loss) to cash provided by operating activities:
|
||||||||||||
|
Depreciation
|
2,765
|
|
3,815
|
|
4,588
|
|
||||||
|
Amortization and impairment of intangible assets
|
331
|
|
435
|
|
590
|
|
||||||
|
Provision for bad debts
|
237
|
|
832
|
|
58
|
|
||||||
|
Stock-based compensation
|
800
|
|
891
|
|
606
|
|
||||||
|
Restructuring loss
|
—
|
|
355
|
|
319
|
|
||||||
|
(Gain) loss on disposal of assets
|
(103
|
)
|
154
|
|
(26
|
)
|
||||||
|
Amortization of premium on securities available-for-sale
|
286
|
|
285
|
|
454
|
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable
|
970
|
|
(806
|
)
|
1,713
|
|
||||||
|
Inventories
|
(1,046
|
)
|
1,280
|
|
(1,565
|
)
|
||||||
|
Other assets
|
6
|
|
196
|
|
809
|
|
||||||
|
Accounts payable
|
(364
|
)
|
74
|
|
(400
|
)
|
||||||
|
Accrued compensation
|
691
|
|
(912
|
)
|
(1,295
|
)
|
||||||
|
Deferred revenue
|
(280
|
)
|
(215
|
)
|
(129
|
)
|
||||||
|
Other accrued liabilities
|
208
|
|
59
|
|
(1,524
|
)
|
||||||
|
Restricted cash
|
(194
|
)
|
—
|
|
—
|
|
||||||
|
Net cash provided by operating activities
|
965
|
|
229
|
|
4,806
|
|
||||||
|
Investing activities
|
||||||||||||
|
Purchases of property and equipment
|
(709
|
)
|
(1,437
|
)
|
(1,014
|
)
|
||||||
|
Proceeds from sale of property and equipment
|
165
|
|
55
|
|
1,024
|
|
||||||
|
Purchases of securities available-for-sale
|
(13,086
|
)
|
(5,477
|
)
|
(20,360
|
)
|
||||||
|
Sales and maturities of securities available-for-sale
|
16,145
|
|
13,569
|
|
16,586
|
|
||||||
|
Net cash provided by (used in) investing activities
|
2,515
|
|
6,710
|
|
(3,764
|
)
|
||||||
|
Financing activities
|
||||||||||||
|
Issuances of common stock
|
119
|
|
44
|
|
36
|
|
||||||
|
Repurchases of common stock
|
(19
|
)
|
(48
|
)
|
(991
|
)
|
||||||
|
Repayment of obligations under capital leases
|
—
|
|
(36
|
)
|
(52
|
)
|
||||||
|
Net cash provided by (used in) financing activities
|
100
|
|
(40
|
)
|
(1,007
|
)
|
||||||
|
Net increase in cash and cash equivalents
|
3,580
|
|
6,899
|
|
35
|
|
||||||
|
Cash and cash equivalents at beginning of year
|
20,459
|
|
13,560
|
|
13,525
|
|
||||||
|
Cash and cash equivalents at end of year
|
$
|
24,039
|
|
$
|
20,459
|
|
$
|
13,560
|
|
|||
|
Supplemental information:
|
||||||||||||
|
Cash paid during the period for interest
|
$
|
—
|
|
$
|
6
|
|
$
|
9
|
|
|||
|
Non-cash investing and financing activities:
|
||||||||||||
|
Purchase of assets under capital leases
|
$
|
—
|
|
$
|
—
|
|
$
|
113
|
|
|||
|
|
Common stock
|
Treasury Stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
income (loss)
|
Accumulated
deficit
|
Total
stockholders’
equity
|
||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||
|
Balance at December 31, 2008
|
18,944
|
|
$
|
2
|
|
—
|
|
$
|
—
|
|
$
|
153,225
|
|
$
|
(22
|
)
|
$
|
(104,246
|
)
|
$
|
48,959
|
|
||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
606
|
|
—
|
|
—
|
|
606
|
|
||||||||||||||
|
Exercise of stock options
|
80
|
|
—
|
|
—
|
|
—
|
|
36
|
|
—
|
|
—
|
|
36
|
|
||||||||||||||
|
Repurchases of common stock
|
—
|
|
—
|
|
547
|
|
(991
|
)
|
—
|
|
—
|
|
—
|
|
(991
|
)
|
||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
608
|
|
608
|
|
||||||||||||||
|
Unrealized gain on securities available-for-sale
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
171
|
|
—
|
|
171
|
|
||||||||||||||
|
Total comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
779
|
|
||||||||||||||
|
Balance at December 31, 2009
|
19,024
|
|
2
|
|
547
|
|
(991
|
)
|
153,867
|
|
149
|
|
(103,638
|
)
|
49,389
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
874
|
|
—
|
|
—
|
|
874
|
|
||||||||||||||
|
Exercise of stock options and settlement of restricted stock awards
|
147
|
|
—
|
|
—
|
|
—
|
|
44
|
|
—
|
|
—
|
|
44
|
|
||||||||||||||
|
Repurchases of common stock
|
—
|
|
—
|
|
26
|
|
(48
|
)
|
—
|
|
—
|
|
—
|
|
(48
|
)
|
||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6,214
|
)
|
(6,214
|
)
|
||||||||||||||
|
Unrealized loss on securities available-for-sale
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(86
|
)
|
—
|
|
(86
|
)
|
||||||||||||||
|
Total comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6,300
|
)
|
||||||||||||||
|
Balance at December 31, 2010
|
19,171
|
|
2
|
|
573
|
|
(1,039
|
)
|
154,785
|
|
63
|
|
(109,852
|
)
|
43,959
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
800
|
|
—
|
|
—
|
|
800
|
|
||||||||||||||
|
Exercise of stock options and settlement of restricted stock awards
|
313
|
|
—
|
|
—
|
|
—
|
|
119
|
|
—
|
|
—
|
|
119
|
|
||||||||||||||
|
Repurchases of common stock
|
—
|
|
—
|
|
10
|
|
(19
|
)
|
—
|
|
—
|
|
—
|
|
(19
|
)
|
||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(3,342
|
)
|
(3,342
|
)
|
||||||||||||||
|
Unrealized loss on securities available-for-sale
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(30
|
)
|
—
|
|
(30
|
)
|
||||||||||||||
|
Total comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(3,372
|
)
|
||||||||||||||
|
Balance at December 31, 2011
|
19,484
|
|
$
|
2
|
|
583
|
|
$
|
(1,058
|
)
|
$
|
155,704
|
|
$
|
33
|
|
$
|
(113,194
|
)
|
$
|
41,487
|
|
||||||||
|
NOTE 1.
|
The Company
|
|
NOTE 2.
|
Basis of Presentation and Significant Accounting Policies
|
|
As of December 31, 2011
|
Maturity in
Years
|
Amortized Cost
|
Unrealized
|
Fair Value
|
||||||||||||||
|
Gains
|
Losses
|
|||||||||||||||||
|
Corporate debt securities
|
2 or less
|
$
|
6,380
|
|
$
|
33
|
|
$
|
—
|
|
$
|
6,413
|
|
|||||
|
As of December 31, 2010
|
Maturity in
Years
|
Amortized Cost
|
Unrealized
|
Fair Value
|
||||||||||||||
|
Gains
|
Losses
|
|||||||||||||||||
|
Corporate debt securities
|
3 or less
|
$
|
9,725
|
|
$
|
91
|
|
$
|
(28
|
)
|
$
|
9,788
|
|
|||||
|
Allowance for Doubtful Accounts (1)
|
Reserves for Billing
Adjustments and
Contractual Allowances (2)
|
|||||||
|
Balance at December 31, 2008
|
$
|
837
|
|
$
|
408
|
|
||
|
Provision
|
58
|
|
1,280
|
|
||||
|
Write-offs and recoveries, net
|
(18
|
)
|
(1,275
|
)
|
||||
|
Balance at December 31, 2009
|
877
|
|
413
|
|
||||
|
Provision
|
832
|
|
1,127
|
|
||||
|
Write-offs and recoveries, net
|
(522
|
)
|
(1,128
|
)
|
||||
|
Balance at December 31, 2010
|
1,187
|
|
412
|
|
||||
|
Provision
|
237
|
|
868
|
|
||||
|
Write-offs and recoveries, net
|
(676
|
)
|
(924
|
)
|
||||
|
Balance at December 31, 2011
|
$
|
748
|
|
$
|
356
|
|
||
|
(1)
|
The provision was charged against general and administrative expenses.
|
|
(2)
|
The provision was charged against revenue.
|
|
|
Reserve for Excess and
Obsolete Inventories (1)
|
||
|
Balance at December 31, 2008
|
$
|
595
|
|
|
Provision
|
538
|
|
|
|
Write-offs and scrap
|
(336
|
)
|
|
|
Balance at December 31, 2009
|
797
|
|
|
|
Provision
|
1,411
|
|
|
|
Write-offs and scrap
|
(317
|
)
|
|
|
Balance at December 31, 2010
|
1,891
|
|
|
|
Provision
|
82
|
|
|
|
Write-offs and scrap
|
(379
|
)
|
|
|
Balance at December 31, 2011
|
$
|
1,594
|
|
|
(1)
|
The provision was charged against Product cost of revenues.
|
|
|
Years Ended December 31,
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Balance at beginning of year
|
$
|
378
|
|
$
|
332
|
|
$
|
906
|
|
|||
|
Charges to Product cost of revenues
|
708
|
|
670
|
|
406
|
|
||||||
|
Applied to liability
|
(789
|
)
|
(624
|
)
|
(980
|
)
|
||||||
|
Balance at end of year
|
$
|
297
|
|
$
|
378
|
|
$
|
332
|
|
|||
|
|
Years Ended December 31,
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Net income (loss)
|
$
|
(3,342
|
)
|
$
|
(6,214
|
)
|
$
|
608
|
|
|||
|
Shares used to compute basic net income (loss) per share
|
19,052
|
|
18,774
|
|
18,836
|
|
||||||
|
Dilutive potential common shares:
|
||||||||||||
|
Stock options
|
—
|
|
—
|
|
408
|
|
||||||
|
Restricted stock units
|
—
|
|
—
|
|
76
|
|
||||||
|
Shares used to compute diluted net income (loss) per share
|
19,052
|
|
18,774
|
|
19,320
|
|
||||||
|
Basic and diluted net income (loss) per share
|
$
|
(0.18
|
)
|
$
|
(0.33
|
)
|
$
|
0.03
|
|
|||
|
NOTE 3.
|
Supplementary Balance Sheet Information (in thousands):
|
|
December 31,
2011 |
December 31,
2010 |
|||||||
|
Inventories, net:
|
||||||||
|
Raw materials
|
$
|
2,899
|
|
$
|
3,050
|
|
||
|
Work-in-process
|
2,665
|
|
2,641
|
|
||||
|
Finished goods
|
2,207
|
|
1,632
|
|
||||
|
7,771
|
|
7,323
|
|
|||||
|
Less reserve for excess and obsolete inventories
|
(1,593
|
)
|
(1,891
|
)
|
||||
|
$
|
6,178
|
|
$
|
5,432
|
|
|||
|
December 31,
2011 |
December 31,
2010 |
|||||||
|
Property and equipment, net:
|
||||||||
|
Machinery and equipment
|
$
|
21,684
|
|
$
|
21,627
|
|
||
|
Computer hardware and software
|
2,712
|
|
2,417
|
|
||||
|
Leasehold improvements
|
813
|
|
807
|
|
||||
|
25,209
|
|
24,851
|
|
|||||
|
Accumulated depreciation
|
(19,842
|
)
|
(17,666
|
)
|
||||
|
$
|
5,367
|
|
$
|
7,185
|
|
|||
|
December 31,
2011 |
December 31,
2010 |
|||||||
|
Intangible assets, net (1):
|
||||||||
|
Customer relationships
|
$
|
2,600
|
|
$
|
2,600
|
|
||
|
Covenants not to compete
|
300
|
|
300
|
|
||||
|
Patents
|
141
|
|
141
|
|
||||
|
3,041
|
|
3,041
|
|
|||||
|
Accumulated amortization of customer relationships
|
(2,201
|
)
|
(1,942
|
)
|
||||
|
Accumulated amortization of covenants not to compete
|
(280
|
)
|
(220
|
)
|
||||
|
Accumulated amortization of patents
|
(83
|
)
|
(71
|
)
|
||||
|
$
|
477
|
|
$
|
808
|
|
|||
|
(1)
|
Amortization expense for intangible assets, net for the years ended
December 31, 2011
,
2010
and
2009
was $0.3 million, $0.4 million and $0.6 million, respectively. Estimated amortization expense for intangible assets for 2012 is $0.2 million, for 2013 is $0.2 million, for 2014 is $0.1 million, for 2015 and thereafter less than $0.1 million.
|
|
December 31,
2011 |
December 31,
2010 |
|||||||
|
Other accrued liabilities:
|
||||||||
|
Outside services and consulting
|
$
|
836
|
|
$
|
318
|
|
||
|
Sales and property taxes payable
|
473
|
|
464
|
|
||||
|
Professional fees
|
293
|
|
284
|
|
||||
|
Radiopharmaceuticals and consumable medical supplies
|
243
|
|
365
|
|
||||
|
Facilities and related costs
|
129
|
|
210
|
|
||||
|
Travel expenses
|
110
|
|
101
|
|
||||
|
Other accrued liabilities
|
313
|
|
354
|
|
||||
|
$
|
2,397
|
|
$
|
2,096
|
|
|||
|
NOTE 4.
|
Fair Value Measurements
|
|
Level 1:
|
Quoted prices in active markets for identical assets or liabilities.
|
|
Level 2:
|
Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
Level 3:
|
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Such assets and liabilities may have values determined using pricing models,
|
|
|
At Fair Value as of December 31, 2011
|
|||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||
|
Assets:
|
||||||||||||||||
|
Corporate debt securities
|
$
|
—
|
|
$
|
6,413
|
|
$
|
—
|
|
$
|
6,413
|
|
||||
|
|
At Fair Value as of December 31, 2010
|
|||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||
|
Assets:
|
||||||||||||||||
|
Corporate debt securities
|
$
|
—
|
|
$
|
9,788
|
|
$
|
—
|
|
$
|
9,788
|
|
||||
|
NOTE 5.
|
Goodwill
|
|
NOTE 6.
|
Commitments and Contingencies
|
|
|
Operating
Leases
|
||
|
2012
|
$
|
1,231
|
|
|
2013
|
809
|
|
|
|
2014
|
599
|
|
|
|
2015
|
586
|
|
|
|
2016
|
98
|
|
|
|
Thereafter
|
—
|
|
|
|
Total minimum lease payments
|
$
|
3,323
|
|
|
NOTE 7.
|
Share-Based Compensation
|
|
|
Years Ended December 31,
|
||||||||
|
|
2011
|
2010
|
2009
|
||||||
|
Expected volatility
|
62
|
%
|
65
|
%
|
65
|
%
|
|||
|
Expected term (in years)
|
6.5
|
|
6.1
|
|
6.0
|
|
|||
|
Risk-free interest rate
|
1.9
|
%
|
2.9
|
%
|
3.0
|
%
|
|||
|
Expected dividend yield
|
—
|
|
—
|
|
—
|
|
|||
|
Number of
Shares
|
Weighted-
Average
Exercise
Price per
Share
|
Weighted-
Average
Remaining
Contractual
Term (In Years)
|
Aggregate
Intrinsic Value
|
|||||||||||
|
Options outstanding at December 31, 2010
|
1,997
|
|
$
|
2.11
|
|
|||||||||
|
Options exercisable at December 31, 2010
|
1,221
|
|
$
|
2.59
|
|
|||||||||
|
Options granted
|
70
|
|
$
|
2.82
|
|
|||||||||
|
Options forfeited
|
(57
|
)
|
1.63
|
|
||||||||||
|
Options expired
|
(8
|
)
|
47.82
|
|
||||||||||
|
Options exercised
|
(100
|
)
|
1.20
|
|
||||||||||
|
Options outstanding at December 31, 2011
|
1,902
|
|
$
|
2.01
|
|
4.90
|
|
$
|
1,259
|
|
||||
|
Options exercisable at December 31, 2011
|
1,468
|
|
$
|
2.13
|
|
4.53
|
|
$
|
1,054
|
|
||||
|
Number of
Shares
|
Weighted-
Average
Grant Date
Fair Value
Per Share
|
||||||
|
Non-vested restricted stock units outstanding at December 31, 2010
|
455
|
|
$
|
2.00
|
|
||
|
Issued
|
95
|
|
$
|
2.80
|
|
||
|
Forfeited
|
(30
|
)
|
$
|
1.99
|
|
||
|
Vested
|
(236
|
)
|
$
|
2.15
|
|
||
|
Non-vested restricted stock units outstanding at December 31, 2011
|
284
|
|
$
|
2.15
|
|
||
|
|
Years Ended December 31,
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Fair value on vesting date of vested restricted stock units
|
$
|
507
|
|
$
|
362
|
|
$
|
225
|
|
|||
|
|
Years Ended December 31,
|
|||||||||||
|
Cost of revenues:
|
2011
|
2010
|
2009
|
|||||||||
|
DIS
|
$
|
13
|
|
$
|
26
|
|
$
|
27
|
|
|||
|
Product
|
99
|
|
60
|
|
56
|
|
||||||
|
Research and development
|
84
|
|
61
|
|
37
|
|
||||||
|
Marketing and sales
|
110
|
|
113
|
|
93
|
|
||||||
|
General and administrative
|
494
|
|
614
|
|
393
|
|
||||||
|
Share-based compensation expense
|
$
|
800
|
|
$
|
874
|
|
$
|
606
|
|
|||
|
NOTE 8.
|
Income Taxes
|
|
|
As of December 31,
|
|||||||
|
|
2011
|
2010
|
||||||
|
Deferred tax assets:
|
||||||||
|
Net operating loss carry forwards
|
$
|
34,518
|
|
$
|
33,489
|
|
||
|
Research and development and other credits
|
1,878
|
|
1,889
|
|
||||
|
Reserves
|
1,282
|
|
1,744
|
|
||||
|
Intangibles
|
2,206
|
|
2,382
|
|
||||
|
Other, net
|
1,392
|
|
1,084
|
|
||||
|
Total deferred tax assets
|
41,276
|
|
40,588
|
|
||||
|
Deferred tax liabilities—depreciation
|
(391
|
)
|
(374
|
)
|
||||
|
Valuation allowance for deferred tax assets
|
(40,885
|
)
|
(40,214
|
)
|
||||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Years Ended December 31,
|
||||||||
|
|
2011
|
2010
|
2009
|
||||||
|
Income tax at statutory federal rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
|||
|
State income taxes, net of federal benefit
|
2.7
|
%
|
2.5
|
%
|
13.0
|
%
|
|||
|
Permanent differences, tax credits and other
|
2.0
|
%
|
(0.7
|
)%
|
4.9
|
%
|
|||
|
Change in effective state tax rates
|
(10.3
|
)%
|
—
|
%
|
—
|
%
|
|||
|
Expiration of net operating loss carryovers
|
(9.4
|
)%
|
—
|
%
|
—
|
%
|
|||
|
Stock compensation expense
|
0.9
|
%
|
(12.3
|
)%
|
—
|
%
|
|||
|
Reserve for uncertain tax positions and other reserves
|
(3.1
|
)%
|
(0.9
|
)%
|
(1.0
|
)%
|
|||
|
Change in valuation allowance
|
(20.3
|
)%
|
(24.6
|
)%
|
(45.6
|
)%
|
|||
|
Provision (benefit) for income taxes
|
(2.5
|
)%
|
(1.0
|
)%
|
6.3
|
%
|
|||
|
|
December 31,
|
|||||||
|
|
2011
|
2010
|
||||||
|
Balance at beginning of year
|
$
|
1,617
|
|
$
|
1,563
|
|
||
|
Increases related to prior year tax positions
|
30
|
|
50
|
|
||||
|
Increases related to current year tax positions
|
42
|
|
24
|
|
||||
|
Expiration of the statute of limitations for the assessment of taxes
|
(48
|
)
|
(28
|
)
|
||||
|
Change in valuation allowances
|
(20
|
)
|
8
|
|
||||
|
Balance at end of year
|
$
|
1,621
|
|
$
|
1,617
|
|
||
|
NOTE 9.
|
Employee Retirement Plan
|
|
NOTE 10.
|
Segments
|
|
|
Years ended December 31,
|
|||||||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Gross profit by segment:
|
||||||||||||
|
DIS
|
$
|
8,122
|
|
|
$
|
6,981
|
|
|
$
|
13,842
|
|
|
|
Product
|
6,636
|
|
|
5,023
|
|
|
6,383
|
|
||||
|
Consolidated gross profit
|
$
|
14,758
|
|
$
|
12,004
|
|
$
|
20,225
|
|
|||
|
Income (loss) from operations by segment:
|
||||||||||||
|
DIS
|
$
|
(535
|
)
|
$
|
(3,483
|
)
|
$
|
1,290
|
|
|||
|
Product
|
(2,975
|
)
|
(3,107
|
)
|
(1,232
|
)
|
||||||
|
Consolidated income (loss) from operations
|
$
|
(3,510
|
)
|
$
|
(6,590
|
)
|
$
|
58
|
|
|||
|
Depreciation and amortization of tangible and intangible assets by segment:
|
||||||||||||
|
DIS
|
$
|
2,765
|
|
$
|
3,763
|
|
$
|
4,464
|
|
|||
|
Product
|
331
|
|
453
|
|
714
|
|
||||||
|
Consolidated depreciation and amortization
|
$
|
3,096
|
|
$
|
4,216
|
|
$
|
5,178
|
|
|||
|
|
As of December 31,
|
|
||||||||||
|
|
2011
|
2010
|
|
|||||||||
|
Identifiable assets by segment:
|
||||||||||||
|
DIS
|
$
|
12,789
|
|
$
|
13,874
|
|
||||||
|
Product
|
37,238
|
|
38,370
|
|
||||||||
|
Consolidated assets
|
$
|
50,027
|
|
$
|
52,244
|
|
||||||
|
NOTE 11.
|
Quarterly Financial Information (Unaudited)
|
|
1st
Quarter
|
2nd
Quarter
|
3rd
Quarter
|
4th
Quarter
|
|||||||||||||
|
Fiscal 2011
|
||||||||||||||||
|
Revenues
|
$
|
14,175
|
|
$
|
14,249
|
|
$
|
13,439
|
|
$
|
11,882
|
|
||||
|
Gross profit
|
$
|
3,519
|
|
$
|
3,995
|
|
$
|
4,150
|
|
$
|
3,094
|
|
||||
|
Income (loss) from operations
|
$
|
(647
|
)
|
$
|
(284
|
)
|
$
|
(52
|
)
|
$
|
(2,527
|
)
|
||||
|
Net income (loss)
|
$
|
(387
|
)
|
$
|
(227
|
)
|
$
|
99
|
|
$
|
(2,827
|
)
|
||||
|
Net income (loss) per common share—basic and diluted (1)
|
$
|
(0.02
|
)
|
$
|
(0.01
|
)
|
$
|
0.01
|
|
$
|
(0.15
|
)
|
||||
|
Fiscal 2010
|
||||||||||||||||
|
Revenues
|
$
|
15,069
|
|
$
|
13,159
|
|
$
|
13,299
|
|
$
|
14,656
|
|
||||
|
Gross profit
|
$
|
3,372
|
|
$
|
1,908
|
|
$
|
3,114
|
|
$
|
3,610
|
|
||||
|
Loss from operations
|
$
|
(1,377
|
)
|
$
|
(3,111
|
)
|
$
|
(1,469
|
)
|
$
|
(633
|
)
|
||||
|
Net loss
|
$
|
(1,235
|
)
|
$
|
(3,084
|
)
|
$
|
(1,336
|
)
|
$
|
(559
|
)
|
||||
|
Net loss per common share—basic and diluted (1)
|
$
|
(0.06
|
)
|
$
|
(0.16
|
)
|
$
|
(0.07
|
)
|
$
|
(0.03
|
)
|
||||
|
(1)
|
Earnings per share are computed independently for each of the quarters presented. Therefore, the sum of the quarterly net earnings per share will not necessarily equal the total for the year.
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
(a)
|
Evaluation of Disclosure Controls and Procedures
|
|
(b)
|
Management’s Report on Internal Control over Financial Reporting
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
|
(a)
|
Financial Statements and Financial Statement Schedules
|
|
1.
|
Financial Statements:
|
|
2.
|
Financial Statement Schedules:
|
|
Exhibit
Number
|
|
Description
|
|
2.1(11)
|
|
Asset Purchase Agreement, by and between Digirad Corporation, Digirad Imaging Solutions, Inc., Digirad Ultrascan Solutions, Inc. and Ultrascan, Inc. dated May 1, 2007.
|
|
2.2(19)
|
|
Asset Purchase Agreement, dated February 2, 2009, by and among the Company, Digirad Imaging Solutions, Inc. and MD Office Solutions.
|
|
2.3(20)
|
|
Asset Purchase Agreement, dated as of March 2, 2009, by and among Digirad Imaging Solutions, Inc. Daniel D. Rice, Denise Nelson, Greg Nelson and Antigua Medical Services, LLC.
|
|
3.1(1)
|
|
Amended and Restated Certificate of Incorporation.
|
|
3.2(13)
|
|
Amended and Restated Bylaws.
|
|
4.1(2)
|
|
Form of Specimen Stock Certificate.
|
|
4.2(14)
|
|
Preferred Stock Rights Agreement, by and between Digirad Corporation and American Stock Transfer and Trust Company, dated November 22, 2005.
|
|
10.1(2)†
|
|
License Agreement, by and between Digirad Corporation and the Regents of the University of California dated May 19, 1999, as amended.
|
|
10.2(2)†
|
|
Amendment to License Agreement by and between Digirad Corporation and the Regents of the University of California, dated July 28, 2004, as amended.
|
|
10.3(2)†
|
|
License Agreement, by and between Digirad Corporation and Cedars-Sinai Health System, dated May 22, 2001, as amended.
|
|
10.4(2)†
|
|
License Agreement, by and between Digirad Corporation and Cedars-Sinai Health System, dated April 1, 2003, as amended.
|
|
10.7(10)#
|
|
Digirad Corporation 2004 Stock Incentive Plan, as Amended and Restated on August 2, 2007.
|
|
10.8(7)#
|
|
Form of Notice of Stock Option Award and Stock Option Award Agreement for 2004 Stock Incentive Plan.
|
|
Exhibit
Number
|
|
Description
|
|
10.9(2)#
|
|
2004 Non-Employee Director Option Program.
|
|
10.10(7)#
|
|
Form of Notice of Stock Option Award and Stock Option Award Agreement for 2004 Non-Employee Director Option Program.
|
|
10.11(2)#
|
|
Form of Indemnification Agreement.
|
|
10.12(12)+
|
|
Agreement for Services between the Registrant’s wholly-owned subsidiary, Digirad Imaging Solutions, Inc. and MBR and Associates, Inc., dated April 1, 2008.
|
|
10.15(15)#
|
|
Executive Employment Agreement, by and between Digirad Corporation and Todd Clyde, dated October 30, 2008.
|
|
10.16(16)#
|
|
Amendment to Employment Agreement, dated December 31, 2010, by and between the Company and Todd P. Clyde.
|
|
10.17(17)#
|
|
Executive Employment Agreement, by and between the Company and Richard B. Slansky, dated February 9, 2009.
|
|
10.18(16)#
|
|
Amendment to Employment Agreement, dated December 31, 2010, by and between the Company and Richard B. Slansky.
|
|
10.19(16)#
|
|
Severance Agreement, dated December 31, 2010, by and between the Company and Virgil Lott.
|
|
10.21(18)
|
|
Commercial Lease Agreement, dated August 1, 2009, by and between the Company and B. Young Properties, LLC.
|
|
10.23(21)#
|
Form of 2011 Inducement Stock Incentive Plan.
|
|
|
10.24(21)#
|
Form of 2011 Inducement Stock Incentive Plan Stock Option Agreement.
|
|
|
10.25(21)#
|
Form of 2011 Inducement Stock Incentive Plan Restricted Stock Unit Agreement.
|
|
|
10.26#
|
Offer Letter, dated July1, 2011, by and between the Company and Armando Jackson.
|
|
|
21.1(2)
|
|
Subsidiaries of Digirad Corporation.
|
|
23.1
|
|
Consent of Independent Registered Public Accounting Firm.
|
|
24.1
|
|
Power of Attorney (included on the signature page of this Form 10-K).
|
|
31.1
|
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
31.2
|
|
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
32.1(9)
|
|
Certification of Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
32.2(9)
|
|
Certification of Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
101****
|
The following financial information from the Company's Quarterly Report on Form 10-K for the annual period ended December 31, 2011, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Cash Flows, and (iv) the Notes to Consolidated Financial Statements, tagged as blocks of text.
|
|
|
(1)
|
This exhibit was previously filed as an exhibit to the Company’s current report on Form 8-K originally filed with the Commission on May 3, 2006, as amended thereafter, and is incorporated herein by reference.
|
|
(2)
|
This exhibit was previously filed as an exhibit to the Registration Statement on Form S-1 (File No. 333-113760) originally filed with the Securities and Exchange Commission on March 19, 2004, as amended thereafter, and is incorporated herein by reference.
|
|
(3)
|
Reserved.
|
|
(4)
|
Reserved.
|
|
(5)
|
Reserved.
|
|
(6)
|
Reserved.
|
|
(7)
|
This exhibit was previously filed as an exhibit to the Company’s annual report on Form 10-K filed with the Commission on March 3, 2005, and is incorporated herein by reference.
|
|
(8)
|
Reserved.
|
|
(9)
|
The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report on Form 10-K, are not
|
|
(10)
|
The exhibit was previously filed as an exhibit to the Company’s quarterly report on Form 10-Q filed with the Commission on August 7, 2007, and is incorporated herein by reference.
|
|
(11)
|
The exhibit was previously filed as an exhibit to the Company’s quarterly report on Form 10-Q filed with the Commission on May 7, 2007, and is incorporated herein by reference.
|
|
(12)
|
The exhibit was previously filed as an exhibit to the Company’s quarterly report on Form 10-K filed with the Commission on February 20, 2007, and is incorporated herein by reference.
|
|
(13)
|
The exhibit was previously filed as an exhibit to the Company’s quarterly report on Form 8-K filed with the Commission on May 9, 2007, and is incorporated herein by reference.
|
|
(14)
|
The exhibit was previously filed as an exhibit to the Registration Statement on Form 8-A originally filed with the Commission on November 29, 2005, and is incorporated herein by reference.
|
|
(15)
|
This exhibit was previously filed as an exhibit to the Company’s annual report on Form 10-K filed with the Commission on February 13, 2009, and is incorporated herein by reference.
|
|
(16)
|
This exhibit was previously filed as an exhibit to the Company’s current report on Form 8-K filed with the Commission on January 3, 2011, and is incorporated herein by reference.
|
|
(17)
|
This exhibit was previously filed as an exhibit to the Company’s current report on Form 8-K filed with the Commission on February 17, 2009, and is incorporated herein by reference.
|
|
(18)
|
This exhibit was previously filed as an exhibit to the Company’s current report on Form 8-K filed with the Commission September 4, 2009, and is incorporated herein by reference.
|
|
(19)
|
This exhibit was previously filed as an exhibit to the Company’s current report on Form 8-K filed with the Commission on February 6, 2009, and is incorporated herein by reference.
|
|
(20)
|
This exhibit was previously filed as an exhibit to the Company’s current report on Form 8-K filed with the Commission on March 4, 2009, and is incorporated herein by reference.
|
|
(21)
|
This exhibit was previously filed as an exhibit to the Company's current report on Form 8-K filed with the Commission on July 29, 2011, and is incorporated herein by reference.
|
|
†
|
Digirad Corporation has been granted confidential treatment with respect to certain portions of this exhibit (indicated by asterisks), which have been filed separately with the Commission.
|
|
+
|
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment and have been separately filed with the Commission.
|
|
#
|
Indicates management contract or compensatory plan.
|
|
****
|
Users of this data are advised that pursuant to Rule 406T of Regulation S-T, this XBRL information is being furnished and not filed herewith for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and Sections 11 or 12 of the Securities Act of 1933, as amended, and is not to be incorporated by reference into any filing, or part of any registration statement or prospectus, of Digirad Corporation, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
|
|
|
DIGIRAD CORPORATION
|
|||
|
Dated: February 17, 2012
|
|
By:
|
|
/
S
/ T
ODD
P. C
LYDE
|
|
|
Name:
|
|
Todd P. Clyde
|
|
|
|
Title:
|
|
President and Chief Executive Officer
|
|
|
Name
|
|
Title
|
|
Date
|
|
/
S
/ T
ODD
P. C
LYDE
|
|
President and Chief Executive Officer
|
|
February 17, 2012
|
|
Todd P. Clyde
|
(Principal Executive Officer)
|
|||
|
/
S
/ R
ICHARD
B. S
LANSKY
|
|
Chief Financial Officer
|
|
February 17, 2012
|
|
Richard B. Slansky
|
(Principal Financial Officer)
|
|||
|
/
S
/ R. K
ING
N
ELSON
|
|
Director
|
|
February 17, 2012
|
|
R. King Nelson
|
(Chairman of the Board of Directors)
|
|||
|
/
S
/ G
ARY
F. B
URBACH
|
|
Director
|
|
February 17, 2012
|
|
Gary F. Burbach
|
||||
|
/
S
/ S
TEVE
C. M
ENDELL
|
|
Director
|
|
February 17, 2012
|
|
Steve C. Mendell
|
||||
|
/
S
/ J
OHN
W. S
AYWARD
|
|
Director
|
|
February 17, 2012
|
|
John W. Sayward
|
||||
|
/
S
/ K
ENNETH
O
LSON
|
|
Director
|
|
February 17, 2012
|
|
Kenneth Olson
|
||||