|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
|
|
For the fiscal year ended December 31, 2013.
|
|
|
OR
|
|
|
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
|
|
For the transition period from ________ to ________.
|
|
|
Delaware
|
36-3898269
|
|
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
|
|
incorporation or organization)
|
Identification No.)
|
|
|
|
|
|
|
|
|
3 Columbus Circle
|
|
|
|
|
15
th
Floor
|
10019
|
|
|
|
New York, New York
|
(Zip Code)
|
|
|
|
(Address of principal executive offices)
|
|
|
|
Common Stock, Par Value $0.001 Per Share
|
NASDAQ Capital Market
|
|
(Title of Class)
|
(Name of Each Exchange on Which Registered)
|
|
Large accelerated filer
£
|
Accelerated filer
£
|
|
Non-accelerated filer
£
|
Smaller reporting company
x
|
|
|
|
Page
|
|
|
|
|
|
SPECIAL CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
|
|
|
|
|
|
|
|
|
PART I
|
|
|
ITEM 1
|
Business
|
2
|
|
ITEM 1A
|
Risk Factors
|
17
|
|
ITEM 2
|
Properties
|
32
|
|
ITEM 3
|
Legal Proceedings
|
33
|
|
ITEM 4
|
Mine Safety Disclosures
|
33
|
|
|
|
|
|
|
PART II
|
|
|
|
|
|
|
ITEM 5
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
33
|
|
ITEM 7
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
34
|
|
ITEM 8
|
Financial Statements and Supplementary Data
|
41
|
|
ITEM 9
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosures
|
41
|
|
ITEM 9A
|
Controls and Procedures
|
41
|
|
ITEM 9B
|
Other Information
|
42
|
|
|
|
|
|
|
PART III
|
|
|
|
|
|
|
ITEM 10
|
Directors, Executive Officers and Corporate Governance
|
42
|
|
ITEM 11
|
Executive Compensation
|
42
|
|
ITEM 12
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
42
|
|
ITEM 13
|
Certain Relationships and Related Transactions, and Director Independence
|
42
|
|
ITEM 14
|
Principal Accountant Fees and Services
|
42
|
|
|
|
|
|
|
PART IV
|
|
|
|
|
|
|
ITEM 15
|
Exhibits and Financial Statement Schedules
|
43
|
| · | expectations for increases or decreases in expenses; |
| · | expectations for the clinical and pre-clinical development, manufacturing, regulatory approval, and commercialization of our pharmaceutical product candidates or any other products we may acquire or in-license; |
| · | use of clinical research centers and other contractors; |
| · | expectations as to the timing of commencing or completing pre-clinical and clinical trials and the expected outcomes of those trials; |
| · | expectations for incurring capital expenditures to expand our research and development and manufacturing capabilities; |
| · | expectations for generating revenue or becoming profitable on a sustained basis; |
| · | expectations or ability to enter into marketing and other partnership agreements; |
| · | expectations or ability to enter into product acquisition and in-licensing transactions; |
| · | expectations or ability to build our own commercial infrastructure to manufacture, market and sell our drug candidates; |
| · | acceptance of our products by doctors, patients or payors; |
| · | ability to compete against other companies and research institutions; |
| · | ability to secure adequate protection for our intellectual property; |
| · | ability to attract and retain key personnel; |
| · | availability of reimbursement for our products; |
| · | estimates of the sufficiency of our existing cash and cash equivalents and investments to finance our operating requirements, including expectations regarding the value and liquidity of our investments; |
| · | stock price and its volatility; |
| · | expected losses; and |
| · | expectations for future capital requirements. |
| 1 | ||
|
|
| 2 | ||
|
|
| · | TG-1101 in combination with lenalidomide (trade name Revlimid ® ), an immunomodulatory agent, for patients with NHL and CLL; |
| · | TG-1101 in combination with ibrutinib (trade name IMBRUVICA™), a BTK inhibitor, for patients with CLL and Mantle Cell Lymphoma (MCL); and |
| · | TG-1101 in combination with TGR-1202, the Company’s development stage PI3Kδ inhibitor, for patients with CLL and NHL. |
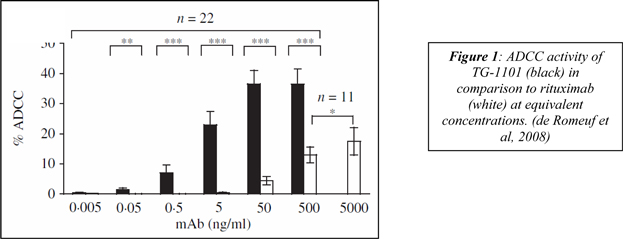
| 3 | ||
|
|
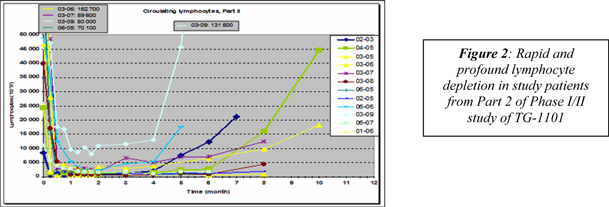
| 4 | ||
|
|
| · | Complete Response (“CR”) : 3 patients – 1 rituximab-refractory MZL, 1 rituximab-refractory FL, and 1 rituximab-relapsed MZL |
| · | Partial Response (“PR”) : 2 patients – 1 rituximab-relapsed MZL, 1 rituximab-relapsed FL |
| 5 | ||
|
|
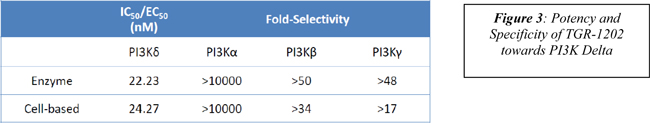
| · | Potent synergy observed with the combination of TGR-1202 and brentuximab vedotin (ADCETRIS ® ) in Hodgkin’s Lymphoma cell lines. Poster 1835. |
| · | TGR-1202 demonstrates enhanced Myeloma cell apoptosis in combination with the proteasome inhibitor carfilzomib (KYPROLIS™) in in-vitro cell lines. Poster 3224. |
| · | The combination of TGR-1202 and proteasome inhibitor carfilzomib demonstrate marked synergy in the killing of various B- and T-Cell lymphoma cell lines. Poster 4421. |
| 6 | ||
|
|
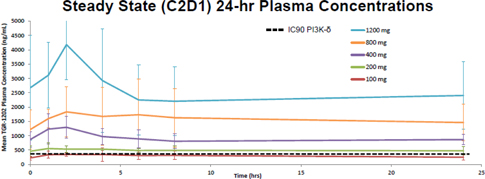
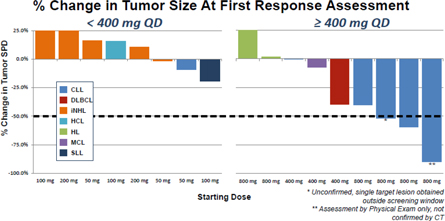
| 7 | ||
|
|
| 8 | ||
|
|
|
|
|
|
|
|
|
|
|
|
Estimated cost
|
|
|
Product
|
|
|
|
Development
|
|
Completion
|
|
|
to
|
|
|
candidate
|
|
Target indication
|
|
Status
|
|
of phase
|
|
|
complete phase
|
|
|
TG-1101 (ublituximab)
|
|
As a single agent and in combination for multiple forms of cancer (includes Phase 1b combination with TGR-1202)
|
|
Phase Ib/II
|
|
End of 2014
|
|
|
Approximately $6 million
|
|
|
TGR-1202
|
|
As a single agent in multiple forms of cancer
|
|
Phase I
|
|
Mid-2014
|
|
|
Approximately $1.5 million
|
|
| 9 | ||
|
|
| 10 | ||
|
|
| 11 | ||
|
|
| 12 | ||
|
|
| 13 | ||
|
|
| · | that the drug is intended to treat a serious or life-threatening condition; |
| · | that the drug is intended to treat a serious aspect of the condition; and |
| · | that the drug has the potential to address unmet medical needs, and this potential is being evaluated in the planned drug development program. |
| 14 | ||
|
|
| · | Phase 1 : The drug is administered to a small group of humans, either healthy volunteers or patients, to test for safety, dosage tolerance, absorption, metabolism, excretion, and clinical pharmacology. |
| · | Phase 2 : Studies are conducted on a larger number of patients to assess the efficacy of the product, to ascertain dose tolerance and the optimal dose range, and to gather additional data relating to safety and potential adverse events. |
| · | Phase 3 : Studies establish safety and efficacy in an expanded patient population. |
| · | Phase 4 : The FDA may require Phase 4 post-marketing studies to find out more about the drug’s long-term risks, benefits, and optimal use, or to test the drug in different populations. |
| · | slow patient enrollment due to the nature of the clinical trial plan, the proximity of patients to clinical sites, the eligibility criteria for participation in the study or other factors; |
| · | inadequately trained or insufficient personnel at the study site to assist in overseeing and monitoring clinical trials or delays in approvals from a study site’s review board; |
| · | longer treatment time required to demonstrate efficacy or determine the appropriate product dose; |
| · | insufficient supply of the drug candidates; |
| · | adverse medical events or side effects in treated patients; and |
| · | ineffectiveness of the drug candidates. |
| 15 | ||
|
|
| 16 | ||
|
|
| 17 | ||
|
|
| · | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| · | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for any indication; |
| 18 | ||
|
|
| · | the FDA may not accept clinical data from trials which are conducted by individual investigators or in countries where the standard of care is potentially different from the United States; |
| · | the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| · | we may be unable to demonstrate that a product candidate's clinical and other benefits outweigh its safety risks; |
| · | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| · | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a BLA, NDA or other submission or to obtain regulatory approval in the United States or elsewhere; |
| · | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we or our collaborators contract for clinical and commercial supplies; or |
| · | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
| · | regulatory authorities may withdraw their approval of the affected product; |
| · | regulatory authorities may require a more significant clinical benefit for approval to offset the risk; |
| · | regulatory authorities may require the addition of labeling statements that could diminish the usage of the product or otherwise limit the commercial success of the affected product; |
| · | we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product; |
| · | we may choose to discontinue sale of the product; |
| · | we could be sued and held liable for harm caused to patients; |
| 19 | ||
|
|
| · | we may not be able to enter into collaboration agreements on acceptable terms and execute on our business model; and |
| · | our reputation may suffer. |
| · | obtaining regulatory clearance to commence a clinical trial; |
| · | identifying, recruiting and training suitable clinical investigators; |
| · | reaching agreement on acceptable terms with prospective contract research organizations (“CROs”) and trial sites, the terms of which can be subject to extensive negotiation, may be subject to modification from time to time and may vary significantly among different CROs and trial sites; |
| · | obtaining sufficient quantities of a product candidate for use in clinical trials; |
| · | obtaining institutional review board (“IRB”) or ethics committee approval to conduct a clinical trial at a prospective site; |
| · | identifying, recruiting and enrolling patients to participate in a clinical trial; |
| · | retaining patients who have initiated a clinical trial but may withdraw due to adverse events from the therapy, insufficient efficacy, fatigue with the clinical trial process or personal issues; and |
| · | unexpected safety findings. |
| 20 | ||
|
|
| · | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| · | inspection of the clinical trial operations or clinical trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold; |
| · | unforeseen safety issues or any determination that the clinical trial presents unacceptable health risks; and |
| · | lack of adequate funding to continue the clinical trial. |
| 21 | ||
|
|
| · | research and development resources, including personnel and technology; |
| · | regulatory experience; |
| · | pharmaceutical development, clinical trial and pharmaceutical commercialization experience; |
| · | experience and expertise in exploitation of intellectual property rights; and |
| · | capital resources. |
| 22 | ||
|
|
| 23 | ||
|
|
|
|
·
|
the efficacy and safety as demonstrated in clinical trials;
|
|
|
·
|
the clinical indications for which the product is approved;
|
|
|
·
|
acceptance by physicians, major operators of cancer clinics and patients of the product as a safe and effective treatment;
|
|
|
·
|
the potential and perceived advantages of product candidates over alternative treatments;
|
|
|
·
|
the safety of product candidates seen in a broader patient group, including its use outside the approved indications;
|
|
|
·
|
the cost of treatment in relation to alternative treatments;
|
|
|
·
|
the availability of adequate reimbursement and pricing by third parties and government authorities;
|
|
|
·
|
relative convenience and ease of administration;
|
|
|
·
|
the prevalence and severity of adverse events; and
|
|
|
·
|
the effectiveness of our sales and marketing efforts.
|
| · | decreased demand for our product candidates; |
| · | impairment to our business reputation; |
| · | withdrawal of clinical trial participants; |
| · | costs of related litigation; |
| · | distraction of management’s attention from our primary business; |
| · | substantial monetary awards to patients or other claimants; |
| · | the inability to commercialize our product candidates; and |
| · | loss of revenues. |
| 24 | ||
|
|
| · | a covered benefit under its health plan; |
| · | safe, effective and medically necessary; |
| · | appropriate for the specific patient; |
| · | cost-effective; and |
| · | neither experimental nor investigational. |
| · | the demand for any products for which we may obtain regulatory approval; |
| · | our ability to set a price that we believe is fair for our products; |
| · | our ability to generate revenues and achieve or maintain profitability; |
| · | the level of taxes that we are required to pay; and |
| · | the availability of capital. |
| 25 | ||
|
|
| · | manage our clinical trials effectively; |
| · | manage our internal development efforts effectively while carrying out our contractual obligations to licensors, contractors and other third parties; |
| · | continue to improve our operational, financial and management controls and reporting systems and procedures; and |
| · | attract and retain sufficient numbers of talented employees. |
| 26 | ||
|
|
| · | difficulty integrating acquired technologies, products, services, operations and personnel with the existing businesses; |
| · | diversion of management’s attention in connection with both negotiating the acquisitions and integrating the businesses; |
| · | strain on managerial and operational resources as management tries to oversee larger operations; |
| · | difficulty implementing and maintaining effective internal control over financial reporting at businesses that we acquire, particularly if they are not located near our existing operations; |
| · | exposure to unforeseen liabilities of acquired companies; |
| · | potential costly and time-consuming litigation, including stockholder lawsuits; |
| · | potential issuance of securities to equity holders of the company being acquired with rights that are superior to the rights of holders of our common stock, par value $0.001 (“Common Stock”), or which may have a dilutive effect on our stockholders; |
| · | risk of loss of invested capital; |
| · | the need to incur additional debt or use cash; and |
| · | the requirement to record potentially significant additional future operating costs for the amortization of intangible assets. |
| 27 | ||
|
|
| · | the patent applications that we or our partners file may not result in any patents being issued; |
| · | patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked or circumvented, or otherwise may not provide any competitive advantage; |
| · | as of March 16, 2013, the U.S. converted from a “first to invent” to a “first to file” system. If we do not win the filing race, we will not be entitled to inventive priority; |
| · | our competitors, many of which have substantially greater resources than we do, and many of which have made significant investments in competing technologies, may seek, or may already have obtained, patents that will limit, interfere with, or eliminate its ability to make, use, and sell our potential products either in the United States or in international markets; |
| · | there may be significant pressure on the U.S. government and other international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful as a matter of public policy regarding worldwide health concerns; and |
| · | countries other than the United States may have less restrictive patent laws than those upheld by United States courts, allowing foreign competitors the ability to exploit these laws to create, develop, and market competing products. |
| 28 | ||
|
|
| · | obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| · | abandon an infringing product candidate or redesign its products or processes to avoid infringement; |
| · | pay substantial damages, including treble damages and attorneys’ fees, which we may have to pay if a court decides that the product or proprietary technology at issue infringes on or violates the third party’s rights; |
| · | pay substantial royalties, fees and/or grant cross licenses to our technology; and/or |
| · | defend litigation or administrative proceedings which may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
| 29 | ||
|
|
| 30 | ||
|
|
| · | successful completion of preclinical studies of its product candidates; |
| · | successful commencement and completion of clinical trials of its product candidates and any future product candidates we advance into clinical trials; |
| · | achievement of regulatory approval for our product candidates and any future product candidates we advance into clinical trials (unless we successfully utilize early access programs which allow for revenue generation prior to approval); |
| · | manufacturing commercial quantities of our products at acceptable cost levels if regulatory approvals are obtained; |
| · | successful sales, distribution and marketing of our future products, if any; and |
| · | our entry into collaborative arrangements or co-promotion agreements to market and sell our products. |
| · | the progress of our clinical trials, including expenses to support the trials and milestone payments that may become payable under our license agreements; |
| · | the costs and timing of regulatory approvals; |
| · | the costs and timing of clinical and commercial manufacturing supply arrangements for each product candidate; |
| · | the costs of establishing sales or distribution capabilities; |
| · | the success of the commercialization of our products; |
| · | our ability to establish and maintain strategic collaborations, including licensing and other arrangements; |
| · | the costs involved in enforcing or defending patent claims or other intellectual property rights; and |
| · | the extent to which we in-license or invest in other indications or product candidates. |
| 31 | ||
|
|
| · | the global economic crisis, which affected stock prices of many companies, and particularly many small pharmaceutical companies like ours; |
| · | publicity regarding actual or potential clinical results relating to products under development by our competitors or us; |
| · | delay or failure in initiating, completing or analyzing nonclinical or clinical trials or the unsatisfactory design or results of these trials; |
| · | achievement or rejection of regulatory approvals by our competitors or us; |
| · | announcements of technological innovations or new commercial products by our competitors or us; |
| · | developments concerning proprietary rights, including patents; |
| · | developments concerning our collaborations; |
| · | regulatory developments in the United States and foreign countries; |
| · | economic or other crises and other external factors; |
| · | period-to-period fluctuations in our revenues and other results of operations; |
| · | changes in financial estimates by securities analysts; and |
| · | sales of our common stock. |
| 32 | ||
|
|
|
|
|
High
|
|
Low
|
|
||
|
Fiscal Year Ended December 31, 2013
|
|
|
|
|
|
|
|
|
Fourth Quarter
|
|
$
|
5.16
|
|
$
|
3.17
|
|
|
Third Quarter
|
|
$
|
6.87
|
|
$
|
5.04
|
|
|
Second Quarter
|
|
$
|
7.49
|
|
$
|
5.26
|
|
|
First Quarter
|
|
$
|
6.40
|
|
$
|
3.50
|
|
|
|
|
High
|
|
Low
|
|
||
|
Fiscal Year Ended December 31, 2012
|
|
|
|
|
|
|
|
|
Fourth Quarter
|
|
$
|
3.64
|
|
$
|
1.90
|
|
|
Third Quarter
|
|
$
|
6.25
|
|
$
|
2.24
|
|
|
Second Quarter
|
|
$
|
7.31
|
|
$
|
5.63
|
|
|
First Quarter
|
|
$
|
33.19
|
|
$
|
1.13
|
|
| 33 | ||
|
|
|
Equity Compensation Plan Information
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
|
|
|
|
securities
|
|
|
|
|
|
|
|
|
|
|
|
remaining
|
|
|
|
|
|
|
|
|
|
|
|
available for
|
|
|
|
|
|
|
|
|
|
|
|
future issuance
|
|
|
|
|
|
Number of
|
|
|
|
|
under equity
|
|
||
|
|
|
securities to be
|
|
Weighted-average
|
|
compensation
|
|
|||
|
|
|
issued upon
|
|
exercise price of
|
|
plans (excluding
|
|
|||
|
|
|
exercise of
|
|
outstanding
|
|
securities reflected in
|
|
|||
|
Plan Category
|
|
outstanding options
|
|
options
|
|
column (a))
|
|
|||
|
|
|
(a)
|
|
(b)
|
|
(c)
|
|
|||
|
Equity compensation plans approved by
security holders |
|
|
46,591
|
|
$
|
46.37
|
|
|
1,107,793
|
|
|
Equity compensation plans not approved by
security holders |
|
|
—
|
|
|
—
|
|
|
—
|
|
|
Total
|
|
|
46,591
|
|
$
|
46.37
|
|
|
1,107,793
|
|
| 34 | ||
|
|
|
|
|
2013
|
|
2012
|
|
||
|
TG-1101
|
|
$
|
8,281,028
|
|
$
|
19,140,621
|
|
|
TGR-1202
|
|
|
4,340,133
|
|
|
1,259,289
|
|
|
AST-726
|
|
|
—
|
|
|
72,272
|
|
|
Terminated programs
|
|
|
—
|
|
|
100,000
|
|
|
Total
|
|
$
|
12,621,161
|
|
$
|
20,572,182
|
|
| 35 | ||
|
|
|
|
|
Years Ended December 31,
|
|
||||
|
|
|
2013
|
|
2012
|
|
||
|
|
|
|
|
|
|
|
|
|
License revenue
|
|
$
|
152,381
|
|
$
|
19,048
|
|
|
|
|
|
|
|
|
|
|
|
Costs and expenses:
|
|
|
|
|
|
|
|
|
Research and development:
|
|
|
|
|
|
|
|
|
Noncash stock expense associated with in-licensing agreement
|
|
|
—
|
|
|
16,578,000
|
|
|
Noncash compensation
|
|
|
1,041,519
|
|
|
455,809
|
|
|
Other research and development
|
|
|
12,621,161
|
|
|
3,994,182
|
|
|
Total research and development
|
|
|
13,662,680
|
|
|
21,027,991
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative:
|
|
|
|
|
|
|
|
|
Noncash compensation
|
|
|
4,161,629
|
|
|
2,966,373
|
|
|
Other general and administrative
|
|
|
2,496,461
|
|
|
1,815,083
|
|
|
Total general and administrative
|
|
|
6,658,090
|
|
|
4,781,456
|
|
|
|
|
|
|
|
|
|
|
|
Impairment of in-process research and development
|
|
|
2,797,600
|
|
|
1,104,700
|
|
|
|
|
|
|
|
|
|
|
|
Total costs and expenses
|
|
|
23,118,370
|
|
|
26,914,147
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(22,965,989)
|
|
|
(26,895,099)
|
|
|
|
|
|
|
|
|
|
|
|
Other income, net
|
|
|
(2,487,779)
|
|
|
(1,042,147)
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income taxes
|
|
|
(20,478,210)
|
|
|
(25,852,952)
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes
|
|
|
—
|
|
|
330,000
|
|
|
Consolidated net loss
|
|
$
|
(20,478,210)
|
|
$
|
(26,182,952)
|
|
| 36 | ||
|
|
| 37 | ||
|
|
| 38 | ||
|
|
| 39 | ||
|
|
| 40 | ||
|
|
| 41 | ||
|
|
| 42 | ||
|
|
|
Contents
|
Page
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
|
|
|
Consolidated Balance Sheets as of December 31, 2013 and 2012
|
F-3
|
|
|
|
|
Consolidated Statements of Operations for the years ended December 31, 2013 and 2012 and cumulative period ended December 31, 2013
|
F-4
|
|
|
|
|
Consolidated Statements of Equity for the years ended December 31, 2013, 2012 and 2011 and cumulative period ended December 31, 2013
|
F-5
|
|
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2013 and 2012 and cumulative period ended December 31, 2013
|
F-7
|
|
|
|
|
Notes to Consolidated Financial Statements
|
F-8
|
|
Exhibit
|
|
|
Number
|
Exhibit Description
|
|
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation, of TG Therapeutics, Inc. dated April 26, 2012 (incorporated by reference to Exhibit 3.2 to the Registrant’s Form 10-Q for the quarter ended June 30, 2012).
|
|
|
|
|
3.2
|
Restated Bylaws of TG Therapeutics, Inc. dated May 14, 2012 (incorporated by reference to Exhibit 3.2 to the Registrant’s Form 10-Q for the quarter ended March 31, 2012).
|
|
|
|
|
4.1
|
Specimen common stock certificate (incorporated by reference to Exhibit 4.1 to the Registrant’s Form 10-K for the year ended December 31, 2011).
|
|
|
|
|
4.2
|
Form of Warrant issued to Noteholders on September 11, 2008 (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on September 15, 2008).
|
|
|
|
|
4.3
|
Form of Warrant issued to Noteholders on November 19, 2008 (incorporated by reference to Exhibit 10.6 to the Registrant’s Current Report on Form 8-K filed on November 25, 2008).
|
|
|
|
|
4.4
|
Form of warrant to purchase common stock of TG Therapeutics, Inc. (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed on November 13, 2012).
|
|
|
|
|
4.5
|
Form of Placement Agent Warrant (incorporated by reference to Exhibit 10.9 to the Registrant’s Current Report on Form 8-K filed on November 25, 2008).
|
|
|
|
|
4.6
|
Warrant, dated October 28, 2009 (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed on November 3, 2009).
|
| 43 | ||
|
|
|
4.7
|
Form of Placement Agent Warrant (incorporated by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K filed on November 3, 2009).
|
|
|
|
|
10.1
|
2003 Stock Option Plan (incorporated by reference to Exhibit 4.1 to Registrant’s Registration Statement on Form S-8 filed February 17, 2004).
|
|
|
|
|
10.2
|
Form of Stock Option Agreement issued under the Registrant’s 2003 Stock Option Plan (incorporated by reference to Exhibit 10.15 to the Registrant's Form 10-KSB filed April 2, 2007).
|
|
|
|
|
10.3
|
Amended and Restated Convertible Promissory Note, dated March 1, 2011 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on March 7, 2011).
|
|
|
|
|
10.4
|
Exchange Transaction Agreement dated December 29, 2011, by and among the Registrant, Opus Point Partners, LLC and TG Therapeutics, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on January 5, 2012).
|
|
|
|
|
10.5
|
Amendment No. 1 to Exchange Transaction Agreement, dated as of December 29, 2011, by and among Opus Point Partners, LLC, TG Biologics, Inc. and TG Therapeutics, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K filed on August 8, 2012).
|
|
|
|
|
10.6
|
Employment Agreement, effective December 29, 2011, between the Registrant and Michael Weiss (incorporated by reference to Exhibit 10.30 to the Registrant’s Form 10-K for the fiscal year ended December 31, 2011). †
|
|
|
|
|
10.7
|
Restricted Stock Subscription Agreement, effective December 29, 2011, between the Registrant and Michael Weiss (incorporated by reference to Exhibit 10.31 to the Registrant’s Form 10-K for the fiscal year ended December 31, 2011). †
|
|
|
|
|
10.8
|
Employment Agreement, effective December 29, 2011, between the Registrant and Sean Power (incorporated by reference to Exhibit 10.32 to the Registrant’s Form 10-K for the fiscal year ended December 31, 2011). †
|
|
|
|
|
10.9
|
Restricted Stock Subscription Agreement, effective December 29, 2011 between the Registrant and Sean Power (incorporated by reference to Exhibit 10.33 to the Registrant’s Form 10-K for the fiscal year ended December 31, 2011).
†
|
|
|
|
|
10.10
|
Form of Warrant issued to stockholders on December 29, 2011, January 31, 2012 and February 24, 2012 (incorporated by reference to Exhibit 10.34 to the Registrant’s Form 10-K for the fiscal year ended December 31, 2011).
|
|
|
|
|
10.11
|
License Agreement, dated January 30, 2012, by and among the Registrant, GTC Biotherapeutics, Inc., LFB Biotechnologies S.A.S. and LFB/GTC LLC (incorporated by reference to Exhibit 10.35 to the Registrant’s Form 10-K for the fiscal year ended December 31, 2011). *
|
|
|
|
|
10.12
|
TG Therapeutics, Inc. Amended and Restated 2012 Incentive Plan, dated May 14, 2012 (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q/A for the quarter ended March 31, 2012).
|
|
|
|
|
10.13
|
Securities Exchange Agreement, dated November 9, 2012, by and between the Company and LFB Biotechnologies S.A.S. (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on November 13, 2012).
|
|
|
|
|
10.14
|
Joint Venture Agreement between TG Therapeutics, Inc. and Rhizen Pharmaceuticals SA, dated August 15, 2012 (incorporated by reference to Exhibit 10.1 to the Registrant’s Form 10-Q for the quarter ended September 30, 2012). *
|
|
|
|
|
10.15
|
Sublicense Agreement between TG Therapeutics, Inc. and Ildong Pharmaceutical Co. Ltd., dated November 13, 2012 (incorporated by reference to Exhibit 10.37 to the Registrant’s Form 10-K for the fiscal year ended December 31, 2012). *
|
| 44 | ||
|
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm
|
|
|
|
|
31.1
|
Certification of Principal Executive Officer
|
|
|
|
|
31.2
|
Certification of Principal Financial Officer
|
|
|
|
|
32.1
|
Certifications pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
| 45 | ||
|
|
TG Therapeutics, Inc. |
|
Consolidated Financial Statements as of December 31, 2013
|
|
|
|
Contents
|
Page
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
|
|
|
Consolidated Balance Sheets as of December 31, 2013 and 2012
|
F-3
|
|
|
|
|
Consolidated Statements of Operations for the years ended December 31, 2013 and 2012 and cumulative period ended December 31, 2013
|
F-4
|
|
|
|
|
Consolidated Statements of Equity for the years ended December 31, 2013, 2012 and 2011 and cumulative period ended December 31, 2013
|
F-5
|
|
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2013 and 2012 and cumulative period ended December 31, 2013
|
F-7
|
|
|
|
|
Notes to Consolidated Financial Statements
|
F-8
|
| F-1 | ||
|
|
| F-2 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
|
|
|
|
|
|
2013
|
|
2012
|
|
||
|
Assets
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
40,485,466
|
|
$
|
16,455,995
|
|
|
Interest receivable
|
|
|
27,169
|
|
|
—
|
|
|
Prepaid research and development
|
|
|
1,742,824
|
|
|
1,990,759
|
|
|
Other current assets
|
|
|
47,804
|
|
|
29,128
|
|
|
Total current assets
|
|
|
42,303,263
|
|
|
18,475,882
|
|
|
Long-term investment securities
|
|
|
4,918,897
|
|
|
—
|
|
|
Equipment, net
|
|
|
5,718
|
|
|
1,164
|
|
|
In-process research and development
|
|
|
—
|
|
|
2,797,600
|
|
|
Goodwill
|
|
|
799,391
|
|
|
799,391
|
|
|
Other assets
|
|
|
85,121
|
|
|
—
|
|
|
Total assets
|
|
$
|
48,112,390
|
|
$
|
22,074,037
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and equity
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
Notes payable, current portion
|
|
$
|
677,778
|
|
$
|
677,778
|
|
|
Accounts payable and accrued expenses
|
|
|
4,764,502
|
|
|
1,117,397
|
|
|
Accrued compensation
|
|
|
532,500
|
|
|
145,000
|
|
|
Current portion of deferred revenue
|
|
|
152,381
|
|
|
152,381
|
|
|
Interest payable
|
|
|
190,017
|
|
|
123,511
|
|
|
Total current liabilities
|
|
|
6,317,178
|
|
|
2,216,067
|
|
|
Deferred revenue, net of current portion
|
|
|
1,676,191
|
|
|
1,828,571
|
|
|
Notes payable, less current portion, at fair value
|
|
|
64,529
|
|
|
2,479,098
|
|
|
Total liabilities
|
|
|
8,057,898
|
|
|
6,523,736
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
|
Equity:
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value per share (10,000,000 shares
authorized, 0 issued and outstanding as of December 31, 2013 and 2012) |
|
|
—
|
|
|
—
|
|
|
Common stock, $0.001 par value per share (500,000,000 shares
authorized, 34,336,235 and 25,820,738 shares issued, 34,294,926 and 25,807,212 shares outstanding at December 31, 2013 and 2012, respectively) |
|
|
34,336
|
|
|
25,821
|
|
|
Contingently issuable shares
|
|
|
6
|
|
|
6
|
|
|
Additional paid-in capital
|
|
|
79,658,490
|
|
|
34,534,805
|
|
|
Treasury stock, at cost, 41,309 and 13,526 shares at December 31,
2013 and 2012, respectively |
|
|
(234,337)
|
|
|
(84,538)
|
|
|
Deficit accumulated in the development stage
|
|
|
(39,404,003)
|
|
|
(18,925,793)
|
|
|
Total equity
|
|
|
40,054,492
|
|
|
15,550,301
|
|
|
Total liabilities and equity
|
|
$
|
48,112,390
|
|
$
|
22,074,037
|
|
| F-3 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Consolidated Statements of Operations for the Years Ended December 31, 2013 and 2012 and Cumulative Period Ended December 31, 2013
|
|
|
|
|
|
2013
|
|
2012
|
|
Amounts accumulated
during the development stage |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
License revenue
|
|
$
|
152,381
|
|
$
|
19,048
|
|
$
|
171,429
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Costs and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
Research and development:
|
|
|
|
|
|
|
|
|
|
|
|
Noncash stock expense associated with in-licensing agreement
|
|
|
—
|
|
|
16,578,000
|
|
|
16,875,000
|
|
|
Noncash compensation
|
|
|
1,041,519
|
|
|
455,809
|
|
|
1,497,328
|
|
|
Other research and development
|
|
|
12,621,161
|
|
|
3,994,182
|
|
|
16,645,626
|
|
|
Total research and development
|
|
|
13,662,680
|
|
|
21,027,991
|
|
|
35,017,954
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative:
|
|
|
|
|
|
|
|
|
|
|
|
Noncash compensation
|
|
|
4,161,629
|
|
|
2,966,373
|
|
|
7,214,496
|
|
|
Other general and administrative
|
|
|
2,496,461
|
|
|
1,815,083
|
|
|
4,779,741
|
|
|
Total general and administrative
|
|
|
6,658,090
|
|
|
4,781,456
|
|
|
11,994,237
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Impairment of in-process research and development
|
|
|
2,797,600
|
|
|
1,104,700
|
|
|
3,902,300
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total costs and expenses
|
|
|
23,118,370
|
|
|
26,914,147
|
|
|
50,914,491
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(22,965,989)
|
|
|
(26,895,099)
|
|
|
(50,743,062)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense:
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
(30,822)
|
|
|
(15,787)
|
|
|
(46,609)
|
|
|
Other income
|
|
|
(108,894)
|
|
|
(272,232)
|
|
|
(381,126)
|
|
|
Interest expense
|
|
|
952,888
|
|
|
905,744
|
|
|
1,865,729
|
|
|
Change in fair value of notes payable
|
|
|
(3,300,951)
|
|
|
(1,659,872)
|
|
|
(4,960,823)
|
|
|
Total other income, net
|
|
|
(2,487,779)
|
|
|
(1,042,147)
|
|
|
(3,522,829)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income taxes
|
|
|
(20,478,210)
|
|
|
(25,852,952)
|
|
|
(47,220,233)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes
|
|
|
—
|
|
|
330,000
|
|
|
330,000
|
|
|
Consolidated net loss
|
|
|
(20,478,210)
|
|
|
(26,182,952)
|
|
|
(47,550,233)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to non-controlling interest
|
|
|
—
|
|
|
(8,110,233)
|
|
|
(8,146,230)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to TG Therapeutics, Inc. and
Subsidiaries |
|
$
|
(20,478,210)
|
|
$
|
(18,072,719)
|
|
$
|
(39,404,003)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per common share
|
|
$
|
(0.81)
|
|
$
|
(1.38)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares used in computing basic and diluted net loss
per common share |
|
|
25,413,964
|
|
|
13,113,758
|
|
|
|
|
| F-4 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Consolidated Statements of Equity for the Years Ended December 31, 2013, 2012 and 2011 and Cumulative Period Ended December 31, 2013
|
|
|
|
|
|
Preferred stock
|
|
Common stock
|
|
Contingently
issuable |
|
Additional
paid-in |
|
Treasury Stock
|
|
Deficit
accumulated in the development |
|
Non- controlling
interest in |
|
|
|
|
|||||||||||||
|
|
|
Shares
|
|
|
Amount
|
|
Shares
|
|
|
Amount
|
|
shares
|
|
capital
|
|
Shares
|
|
Amount
|
|
stage
|
|
subsidiary
|
|
|
Total
|
|
|||||
|
Common stock issued
to founders in exchange for seed capital in April 2011 |
|
|
|
|
|
|
2,500,000
|
|
$
|
2,500
|
|
|
|
|
$
|
104,078
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
106,578
|
|
|
Stock issued at $2.25
per share in exchange for license option |
|
|
|
|
|
|
132,000
|
|
|
132
|
|
|
|
|
|
296,868
|
|
|
|
|
|
|
|
|
|
|
|
|
|
297,000
|
|
|
Issuance of restricted
stock to employees |
|
|
|
|
|
|
1,150,000
|
|
|
1,150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,150
|
|
|
Effect of reverse
acquisition |
|
281,250
|
|
$
|
281
|
|
(2,500,124)
|
|
|
(2,501)
|
|
$
|
6
|
|
|
277,500
|
|
|
|
|
|
|
|
|
|
$
|
47,678
|
|
|
322,964
|
|
|
Conversion of note
payable to preferred stock |
|
2,763
|
|
|
3
|
|
|
|
|
|
|
|
|
|
|
55,268
|
|
|
|
|
|
|
|
|
|
|
|
|
|
55,271
|
|
|
Issuance of
replacement restricted preferred stock to employees |
|
129,375
|
|
|
129
|
|
(1,150,000)
|
|
|
(1,150)
|
|
|
|
|
|
1,021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Common stock issued
at $2.25 per share, net of expenses |
|
|
|
|
|
|
4,929,523
|
|
|
4,930
|
|
|
|
|
|
9,650,886
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9,655,816
|
|
|
Compensation in
respect of restricted stock preferred stock granted to employees |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
86,494
|
|
|
|
|
|
|
|
|
|
|
|
|
|
86,494
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
(853,074)
|
|
|
(35,997)
|
|
|
(889,071)
|
|
|
Balance at December
31, 2011 |
|
413,388
|
|
|
413
|
|
5,061,399
|
|
|
5,061
|
|
|
6
|
|
|
10,472,115
|
|
|
|
|
|
|
|
(853,074)
|
|
|
11,681
|
|
|
9,636,202
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation in
respect of restricted preferred stock granted to employees |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
188,509
|
|
|
|
|
|
|
|
|
|
|
|
|
|
188,509
|
|
|
Preferred stock issued
at $20.00 per share, net of expenses |
|
695,428
|
|
|
696
|
|
|
|
|
|
|
|
|
|
|
12,180,710
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12,181,406
|
|
|
Shares issued in
subsidiary to non- controlling interest in connection with in-licensing agreement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
16,578,000
|
|
|
16,578,000
|
|
|
Conversion of
preferred stock to common stock in conjunction with reverse stock split |
|
(1,108,816)
|
|
|
(1,109)
|
|
9,857,596
|
|
|
9,858
|
|
|
|
|
|
(8,749)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Issuance of restricted
stock |
|
|
|
|
|
|
5,901,743
|
|
|
5,902
|
|
|
|
|
|
(5,902)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Compensation in
respect of restricted common stock granted to employees, directors and consultants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,233,674
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,233,674
|
|
|
Non-controlling
interest subsidiary shares exchanged for shares in TG Therapeutics, Inc. |
|
|
|
|
|
|
5,000,000
|
|
|
5,000
|
|
|
|
|
|
8,474,448
|
|
|
|
|
|
|
|
|
|
|
(8,479,448)
|
|
|
—
|
|
|
Surrender of common
stock for tax withholding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
13,526
|
|
$
|
(84,538)
|
|
|
|
|
|
|
|
|
(84,538)
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(18,072,719)
|
|
|
(8,110,233)
|
|
|
(26,182,952)
|
|
|
Balance at December
31, 2012 |
|
—
|
|
$
|
—
|
|
25,820,738
|
|
$
|
25,821
|
|
$
|
6
|
|
$
|
34,534,805
|
|
13,526
|
|
$
|
(84,538)
|
|
$
|
(18,925,793)
|
|
$
|
—
|
|
$
|
15,550,301
|
|
| F-5 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Consolidated Statements of Equity for the Years Ended December 31, 2013, 2012 and 2011 and Cumulative Period Ended December 31, 2013
|
|
|
|
|
|
Preferred stock
|
|
Common stock
|
|
|
Contingently
|
|
Additional
paid-in capital |
|
Treasury Stock
|
|
Deficit
accumulated in the |
|
Non-
controlling |
|
|
|
|
||||||||||||
|
|
|
Shares
|
|
|
Amount
|
|
Shares
|
|
|
Amount
|
|
|
issuable shares
|
|
|
Shares
|
|
|
Amount
|
|
development
stage |
|
interest in
subsidiary |
|
Total
|
|
|||||
|
Balance at December31,
2012 |
|
—
|
|
$
|
—
|
|
25,820,738
|
|
$
|
25,821
|
|
$
|
6
|
|
$
|
34,534,805
|
|
13,526
|
|
$
|
(84,538)
|
|
$
|
(18,925,793)
|
|
$
|
—
|
|
$
|
15,550,301
|
|
|
Issuance of common
stock in connection with exercise of warrants |
|
|
|
|
|
|
1,013,009
|
|
|
1,013
|
|
|
|
|
|
2,279,760
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,280,773
|
|
|
Issuance of common
stock in connection with cashless exercise of warrants |
|
|
|
|
|
|
3,024
|
|
|
3
|
|
|
|
|
|
(4)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
|
Issuance of restricted
stock |
|
|
|
|
|
|
944,464
|
|
|
944
|
|
|
|
|
|
(944)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
Issuance of common
stock in public offering (net of offering costs of $2,664,970) |
|
|
|
|
|
|
6,555,000
|
|
|
6,555
|
|
|
|
|
|
37,641,725
|
|
|
|
|
|
|
|
|
|
|
|
|
|
37,648,280
|
|
|
Compensation in respect
of restricted stock and stock options granted to employees, directors and consultants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,203,148
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,203,148
|
|
|
Surrender of common
stock for tax withholding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
27,783
|
|
|
(149,799)
|
|
|
|
|
|
|
|
|
(149,799)
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(20,478,210)
|
|
|
|
|
|
(20,478,210)
|
|
|
Balance at December
31, 2013 |
|
—
|
|
$
|
—
|
|
34,336,235
|
|
$
|
34,336
|
|
$
|
6
|
|
$
|
79,658,490
|
|
41,309
|
|
$
|
(234,337)
|
|
$
|
(39,404,003)
|
|
$
|
—
|
|
$
|
40,054,492
|
|
| F-6 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2013 and 2012 and Cumulative Period Ended December 31, 2013
|
|
|
|
|
|
2013
|
|
2012
|
|
Amounts
accumulated during the development stage |
|
|||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated net loss
|
|
$
|
(20,478,210)
|
|
$
|
(26,182,952)
|
|
$
|
(47,550,233)
|
|
|
Adjustments to reconcile consolidated net loss to net cash used in
operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Stock compensation expense
|
|
|
5,203,148
|
|
|
3,422,182
|
|
|
8,711,824
|
|
|
Noncash stock expense associated with in-licensing
agreement |
|
|
—
|
|
|
16,578,000
|
|
|
16,875,000
|
|
|
Impairment of in-process research and development
|
|
|
2,797,600
|
|
|
1,104,700
|
|
|
3,902,300
|
|
|
Depreciation
|
|
|
1,127
|
|
|
235
|
|
|
1,362
|
|
|
Amortization of premiums on investment securities, net
|
|
|
975
|
|
|
—
|
|
|
975
|
|
|
Change in fair value and accrued interest of notes payable
|
|
|
(2,414,569)
|
|
|
(815,699)
|
|
|
(3,230,268)
|
|
|
Changes in assets and liabilities, net of effects of acquisition:
|
|
|
|
|
|
|
|
|
|
|
|
Decrease (increase) in other current assets
|
|
|
229,258
|
|
|
(1,932,710)
|
|
|
(1,699,859)
|
|
|
Increase in accrued interest receivable
|
|
|
(27,169)
|
|
|
—
|
|
|
(27,169)
|
|
|
Increase in other assets
|
|
|
(85,121)
|
|
|
—
|
|
|
(85,121)
|
|
|
Increase in accounts payable and accrued expenses
|
|
|
4,034,605
|
|
|
595,757
|
|
|
5,038,672
|
|
|
Increase in interest payable
|
|
|
66,506
|
|
|
61,571
|
|
|
135,174
|
|
|
(Decrease) increase in deferred revenue
|
|
|
(152,381)
|
|
|
1,980,952
|
|
|
1,828,571
|
|
|
Net cash used in operating activities
|
|
|
(10,824,231)
|
|
|
(5,187,964)
|
|
|
(16,098,772)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of equipment
|
|
|
(5,681)
|
|
|
(1,399)
|
|
|
(7,080)
|
|
|
Investment in held-to-maturity long-term securities
|
|
|
(4,919,871)
|
|
|
—
|
|
|
(4,919,871)
|
|
|
Cash acquired in connection with acquisition
|
|
|
—
|
|
|
—
|
|
|
10,386
|
|
|
Net cash used in investing activities
|
|
|
(4,925,552)
|
|
|
(1,399)
|
|
|
(4,916,565)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of warrants
|
|
|
2,280,773
|
|
|
—
|
|
|
2,280,773
|
|
|
Payments of short-term loans
|
|
|
—
|
|
|
(200,001)
|
|
|
(200,001)
|
|
|
Proceeds from sale of common stock, net
|
|
|
37,648,280
|
|
|
—
|
|
|
47,472,962
|
|
|
Proceeds from sale of preferred stock, net
|
|
|
—
|
|
|
12,257,309
|
|
|
12,257,309
|
|
|
Offering costs paid
|
|
|
—
|
|
|
(75,903)
|
|
|
(75,903)
|
|
|
Purchase of treasury stock
|
|
|
(149,799)
|
|
|
(84,538)
|
|
|
(234,337)
|
|
|
Net cash provided by financing activities
|
|
|
39,779,254
|
|
|
11,896,867
|
|
|
61,500,803
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCREASE IN CASH AND CASH EQUIVALENTS
|
|
|
24,029,471
|
|
|
6,707,504
|
|
|
40,485,466
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at beginning of period
|
|
|
16,455,995
|
|
|
9,748,491
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH AND CASH EQUIVALENTS AT END OF PERIOD
|
|
$
|
40,485,466
|
|
$
|
16,455,995
|
|
$
|
40,485,466
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NONCASH TRANSACTIONS
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of notes payable to preferred stock
|
|
$
|
—
|
|
$
|
—
|
|
$
|
55,271
|
|
|
Accrued financing costs
|
|
$
|
—
|
|
$
|
—
|
|
$
|
61,138
|
|
| F-7 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
| F-8 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
| F-9 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
| F-10 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
| F-11 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
December 31, 2013
|
|
December 31, 2012
|
|
||
|
|
|
|
|
|
|
|
|
|
Money market funds
|
|
$
|
554,069
|
|
$
|
500,007
|
|
|
Checking and bank deposits
|
|
|
39,931,397
|
|
|
15,955,988
|
|
|
Total
|
|
$
|
40,485,466
|
|
$
|
16,455,995
|
|
|
|
|
December 31, 2013
|
|
||||||||||
|
|
|
Amortized cost,
as adjusted |
|
Gross
unrealized holding gains |
|
Gross
unrealized holding losses |
|
Estimated
fair value |
|
||||
|
Long-term investments:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Obligations of domestic governmental agencies
(maturing between January 2015 and April 2015) (held-to-maturity) |
|
$
|
4,918,897
|
|
$
|
—
|
|
$
|
650
|
|
$
|
4,918,247
|
|
|
Total long-term investment securities
|
|
$
|
4,918,897
|
|
$
|
—
|
|
$
|
650
|
|
$
|
4,918,247
|
|
|
|
⋅
|
Level 1 – quoted prices in active markets for identical assets and liabilities;
|
|
|
⋅
|
Level 2 – inputs other than Level 1 quoted prices that are directly or indirectly observable; and
|
|
|
⋅
|
Level 3 – unobservable inputs that are not corroborated by market data.
|
| F-12 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
|
Financial liabilities at fair value
as of December 31, 2013 |
|
||||||||||
|
|
|
|
Level 1
|
|
Level 2
|
|
Level 3
|
|
Total
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5% Notes
|
|
|
$
|
—
|
|
$
|
—
|
|
$
|
64,529
|
|
$
|
64,529
|
|
|
Totals
|
|
|
$
|
—
|
|
$
|
—
|
|
$
|
64,529
|
|
$
|
64,529
|
|
|
|
|
Financial liabilities at fair value
as of December 31, 2012 |
|||||||||||
|
|
|
Level 1
|
|
Level 2
|
|
Level 3
|
|
Total
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5% Notes
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2,479,098
|
|
$
|
2,479,098
|
|
|
Totals
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2,479,098
|
|
$
|
2,479,098
|
|
| F-13 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
Balance at January 1, 2012
|
|
$
|
3,294,797
|
|
|
Interest accrued on face value of 5% Notes
|
|
|
844,173
|
|
|
Change in fair value of Level 3 liabilities
|
|
|
(1,659,872)
|
|
|
Balance at December 31, 2012
|
|
|
2,479,098
|
|
|
Interest accrued on face value of 5% Notes
|
|
|
886,382
|
|
|
Change in fair value of Level 3 liabilities
|
|
|
(3,300,951)
|
|
|
Balance at December 31, 2013
|
|
$
|
64,529
|
|
|
|
|
Fair value measurements
as of December 31, 2013 |
|
|
Total impairment charge for the year ended
|
|
|||||||
|
|
|
Level 1
|
|
Level 2
|
|
Level 3
|
|
|
December 31, 2013
|
|
|||
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In-process research and development
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(2,797,600)
|
|
|
Total
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(2,797,600)
|
|
|
|
|
Fair value measurements
as of December 31, 2012 |
|
|
Total impairment charge for the year ended
|
|
|||||||
|
|
|
Level 1
|
|
Level 2
|
|
Level 3
|
|
|
December 31, 2012
|
|
|||
|
Assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In-process research and development
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2,797,600
|
|
$
|
(1,104,700)
|
|
|
Total
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2,797,600
|
|
$
|
(1,104,700)
|
|
| F-14 | ||
|
|
|
TG Therapeutics, Inc.
|
| F-15 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
Number
of shares |
|
Weighted-
average exercise price |
|
Weighted-
average Contractual Term |
|
Aggregate Intrinsic
Value |
|
||
|
|
|
|
|
|
|
|
(in years)
|
|
|
|
|
|
Outstanding at January 1, 2012
|
|
3,379
|
|
$
|
1,315.62
|
|
|
|
|
|
|
|
Granted
|
|
46,000
|
|
|
4.40
|
|
|
|
|
|
|
|
Exercised
|
|
—
|
|
|
—
|
|
|
|
|
|
|
|
Forfeited
|
|
(2,475)
|
|
|
720.45
|
|
|
|
|
|
|
|
Expired
|
|
—
|
|
|
—
|
|
|
|
|
|
|
|
Outstanding at December 31, 2012
|
|
46,904
|
|
|
61.08
|
|
9.44
|
|
$
|
—
|
|
|
Granted
|
|
—
|
|
|
—
|
|
|
|
|
|
|
|
Exercised
|
|
—
|
|
|
—
|
|
|
|
|
|
|
|
Forfeited
|
|
(313)
|
|
|
2,249.85
|
|
|
|
|
|
|
|
Expired
|
|
—
|
|
|
—
|
|
|
|
|
|
|
|
Outstanding at December 31, 2013
|
|
46,591
|
|
$
|
46.37
|
|
8.50
|
|
$
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and expected to vest at December 31, 2013
|
|
9,591
|
|
$
|
208.30
|
|
8.16
|
|
$
|
—
|
|
|
Exercisable at December 31, 2013
|
|
9,591
|
|
$
|
208.30
|
|
8.16
|
|
$
|
—
|
|
| F-16 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
Number of Shares
Restricted Series A Preferred Stock (1) |
|
Weighted
Average Grant Date Fair Value |
|
|
|
Outstanding at January 1, 2012
|
|
129,375
|
|
$
|
20.00
|
|
|
Granted
|
|
—
|
|
|
—
|
|
|
Vested
|
|
—
|
|
|
—
|
|
|
Forfeited
|
|
—
|
|
|
—
|
|
|
Conversion to restricted common stock
|
|
(129,375)
|
|
|
20.00
|
|
|
Outstanding at December 31, 2012
|
|
—
|
|
|
—
|
|
|
Granted
|
|
—
|
|
|
—
|
|
|
Vested
|
|
—
|
|
|
—
|
|
|
Forfeited
|
|
—
|
|
|
—
|
|
|
Outstanding at December 31, 2013
|
|
—
|
|
$
|
—
|
|
|
|
|
Number of Shares
|
|
Weighted
Average Grant Date Fair Value |
|
|
|
Outstanding at January 1, 2012
|
|
—
|
|
$
|
—
|
|
|
Converted preferred stock
|
|
1,150,000
|
|
|
2.25
|
|
|
Granted
|
|
5,901,743
|
|
|
4.77
|
|
|
Vested
|
|
(437,500)
|
|
|
2.25
|
|
|
Forfeited
|
|
—
|
|
|
—
|
|
|
Outstanding at December 31, 2012
|
|
6,614,243
|
|
|
4.49
|
|
|
Granted
|
|
944,464
|
|
|
4.13
|
|
|
Vested
|
|
(523,750)
|
|
|
2.48
|
|
|
Forfeited
|
|
—
|
|
|
—
|
|
|
Outstanding at December 31, 2013
|
|
7,034,957
|
|
$
|
4.60
|
|
| F-17 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
Warrants
|
|
Weighted-
average exercise price |
|
Aggregate
Intrinsic Value |
|
||
|
Outstanding at January 1, 2012
|
|
1,625,911
|
|
|
5.27
|
|
$
|
—
|
|
|
Issued
|
|
5,156,599
|
|
|
1.21
|
|
|
|
|
|
Exercised
|
|
—
|
|
|
—
|
|
|
|
|
|
Expired
|
|
(1,503)
|
|
|
2,739.75
|
|
|
|
|
|
Outstanding at December 31, 2012
|
|
6,781,007
|
|
|
1.58
|
|
$
|
14,563,539
|
|
|
Issued
|
|
—
|
|
|
—
|
|
|
|
|
|
Exercised
|
|
(1,018,068)
|
|
|
2.25
|
|
|
|
|
|
Expired
|
|
(43,992)
|
|
|
16.26
|
|
|
|
|
|
Outstanding at December 31, 2013
|
|
5,718,947
|
|
$
|
1.34
|
|
$
|
14,809,030
|
|
|
|
|
2013
|
|
2012
|
|
||
|
Stock-based compensation expense associated with restricted stock
|
|
$
|
5,146,743
|
|
$
|
3,422,182
|
|
|
Stock-based compensation expense associated with option grants
|
|
|
56,405
|
|
|
—
|
|
|
|
|
$
|
5,203,148
|
|
$
|
3,422,182
|
|
| F-18 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
December 31, 2013
|
|
December 31, 2012
|
|
||||||||||||||
|
|
|
Current
portion, net |
|
Non-current
portion, net |
|
Total
|
|
Current
portion, net |
|
Non-current
portion, net |
|
Total
|
|
||||||
|
Convertible 5% Notes Payable
|
|
$
|
-
|
|
$
|
64,529
|
|
$
|
64,529
|
|
$
|
-
|
|
$
|
2,479,098
|
|
$
|
2,479,098
|
|
|
ICON Convertible Note
|
|
|
677,778
|
|
|
-
|
|
|
677,778
|
|
|
677,778
|
|
|
-
|
|
|
677,778
|
|
|
Totals
|
|
$
|
677,778
|
|
$
|
64,529
|
|
$
|
742,307
|
|
$
|
677,778
|
|
$
|
2,479,098
|
|
$
|
3,156,876
|
|
| F-19 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
2013
|
|
2012
|
|
||
|
Deferred tax assets (liabilities):
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards
|
|
$
|
32,399,879
|
|
$
|
32,190,080
|
|
|
Research and development credit
|
|
|
2,266,167
|
|
|
1,882,238
|
|
|
Noncash compensation
|
|
|
10,971,912
|
|
|
3,051,920
|
|
|
Acquired in-process research and development
|
|
|
—
|
|
|
(1,202,556)
|
|
|
Foreign tax credit
|
|
|
—
|
|
|
330,000
|
|
|
Other
|
|
|
644,899
|
|
|
19,241
|
|
|
Deferred tax asset, excluding valuation allowance
|
|
|
46,282,857
|
|
|
36,270,923
|
|
|
|
|
|
|
|
|
|
|
|
Less valuation allowance
|
|
|
(46,282,857)
|
|
|
(36,270,923)
|
|
|
Net deferred tax assets
|
|
$
|
—
|
|
$
|
—
|
|
| F-20 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
|
|
|
For the year ended December 31,
|
|
||||
|
|
|
2013
|
|
2012
|
|
||
|
|
|
|
|
|
|
|
|
|
Loss before income taxes, as reported in the consolidated statements
of operations |
|
$
|
(20,478,210)
|
|
$
|
(25,852,952)
|
|
|
|
|
|
|
|
|
|
|
|
Computed “expected” tax benefit
|
|
$
|
(6,962,592)
|
|
$
|
(8,790,003)
|
|
|
|
|
|
|
|
|
|
|
|
Increase (decrease) in income taxes resulting from:
|
|
|
|
|
|
|
|
|
Expected benefit from state and local taxes
|
|
|
(917,810)
|
|
|
(1,758,001)
|
|
|
Research and development credits
|
|
|
(250,000)
|
|
|
(75,000)
|
|
|
Other
|
|
|
43,026
|
|
|
230,643
|
|
|
Permanent difference related to contingent note payable
|
|
|
(1,924,558)
|
|
|
—
|
|
|
Foreign tax withholding
|
|
|
—
|
|
|
330,000
|
|
|
Change in the balance of the valuation allowance for deferred tax
assets |
|
|
10,011,934
|
|
|
10,392,361
|
|
|
|
|
$
|
—
|
|
$
|
330,000
|
|
| F-21 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
| F-22 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
| F-23 | ||
|
|
|
TG Therapeutics, Inc.
|
|
(a development stage company)
|
|
Notes to the Consolidated Financial Statements
|
|
|
| F-24 | ||
|
|
|
|
TG THERAPEUTICS, INC. |
|
|
|
|
|
|
|
By:
|
/s/ Michael S. Weiss
|
|
|
|
Michael S. Weiss
|
|
|
|
Executive Chairman, Interim Chief Executive Officer and President
|
|
Signatures
|
|
Title
|
|
|
|
|
|
/s/ Michael S. Weiss
|
|
Executive Chairman, Interim Chief Executive Officer and President
|
|
Michael S. Weiss
|
|
(principal executive officer)
|
|
|
|
|
|
/s/ Sean A. Power
|
|
Chief Financial Officer
|
|
Sean A. Power
|
|
(principal financial and accounting officer)
|
|
|
|
|
|
/s/ Laurence N. Charney
|
|
Director
|
|
Laurence N. Charney
|
|
|
|
|
|
|
|
/s/ Yann Echelard
|
|
Director
|
|
Yann Echelard
|
|
|
|
|
|
|
|
/s/ Neil Herskowitz
|
|
Director
|
|
Neil Herskowitz
|
|
|
|
|
|
|
|
/s/ William J. Kennedy
|
|
Director
|
|
William J. Kennedy
|
|
|
|
|
|
|
|
/s/ Mark Schoenebaum, M.D.
|
|
Director
|
|
Mark Schoenebaum, M.D.
|
|
|
| 46 | ||
|
|
|
Exhibit
|
|
|
|
Number
|
|
Exhibit Description
|
|
|
|
|
|
23.1
|
|
Consent of Independent Registered Public Accounting Firm
|
|
|
|
|
|
31.1
|
|
Certification of Principal Executive Officer
|
|
|
|
|
|
31.2
|
|
Certification of Principal Financial Officer
|
|
|
|
|
|
32.1
|
|
Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
32.2
|
|
Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|