| x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| For the fiscal year ended December 31, 2012 | |
| o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| For the transition period from ___________ to ___________ | |
|
Nevada
|
26-3853855
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(IRS Employer Identification No.)
|
|
Room 2801, East Tower of Hui Hao Building
No. 519 Machang Road, Pearl River New City
Guangzhou, People’s Republic of China
|
510627
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Securities registered under Section 12(b) of the Exchange Act:
None
|
|
Securities registered under Section 12(g) of the Exchange Act:
|
|
Common Stock, $0.001 par value
(Title of class)
|
|
Large accelerated filer
|
o
|
Accelerated filer
|
o
|
|
Non-accelerated filer
(Do not check if a smaller reporting company)
|
o
|
Smaller reporting company
|
x
|
|
Page
|
||||
|
PART I
|
||||
|
Item 1.
|
Business.
|
1
|
||
|
Item 1A.
|
Risk Factors.
|
19
|
||
|
Item 1B.
|
Unresolved Staff Comments.
|
34
|
||
|
Item 2.
|
Properties.
|
34
|
||
|
Item 3.
|
Legal Proceedings.
|
34
|
||
|
Item 4.
|
Mine Safety Disclosures.
|
34
|
||
|
PART II
|
||||
|
Item 5.
|
Market for Registrant’s Common Equity, and Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
35
|
||
|
Item 6.
|
Selected Financial Data.
|
35
|
||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
|
36
|
||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
46
|
||
|
Item 8.
|
Financial Statements and Supplementary Data.
|
F-1
|
||
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
|
47
|
||
|
Item 9A.
|
Controls and Procedures.
|
47
|
||
|
Item 9B.
|
Other Information.
|
48
|
||
|
PART III
|
||||
|
Item 10.
|
Directors, Executive Officers, Promoters and Corporate Governance.
|
49
|
||
|
Item 11.
|
Executive Compensation.
|
50
|
||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
52
|
||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence.
|
53
|
||
|
Item 14.
|
Principal Accountant Fees and Services.
|
54
|
||
|
PART IV
|
||||
|
Item 15.
|
Exhibits, Financial Statement Schedules.
|
55
|
||
|
SIGNATURES
|
56
|
|||
|
●
|
Organic Trace Mineral Additives
, which accounted for approximately 79% of our revenue in 2011 and 86% of our revenue in 2012;
|
|
●
|
Feed Acidifiers, Seasonings and Flavor Enhancers
, which accounted for approximately 17% of our revenue in 2011 and 11% of our revenue in 2012; and
|
|
●
|
Herbal Medicinal Additives
, which accounted for approximately 1% of our revenue in both 2011 and 2012.
|
|
●
|
Equity Interest Pledge Agreement. The WFOE and the Tanke Shareholders have entered into Equity Interest Pledge Agreements, pursuant to which each shareholder pledges all of his shares of Guangzhou Tanke to the WFOE in order to guarantee cash-flow payments under the applicable Consulting Services Agreement. The Equity Pledge Agreement further entitles the WFOE to collect dividends from Guangzhou Tanke during the term of the pledge.
|
|
●
|
Consulting Services Agreement. Guangzhou Tanke and the WFOE has entered into a Consulting Services Agreement, which provides that the WFOE will be the exclusive provider of consulting and management services to Guangzhou Tanke and Guangzhou Tanke will pay all of its net income based on the quarterly financial statements to the WFOE for such services. Any such payment from the WFOE to the Company would need to comply with applicable Chinese laws affecting payments from Chinese companies to non-Chinese companies. See “Risk Factors – Risks Associated With Doing Business in China – ‘
Due to various restrictions under Chinese laws on the distribution of dividends by our Chinese operating companies, we may not be able to pay dividends to our stockholders
’ and ‘
The State Administration of Foreign Exchange restrictions or changes in foreign exchange regulations in China may affect our ability to pay dividends in foreign currency or conduct other foreign exchange business
’.”
|
|
●
|
Operating Agreement. Pursuant to the operating agreement among the WFOE, Guangzhou Tanke and each of Tanke Shareholders, the WFOE provides guidance and instructions on Guangzhou Tanke’s daily operations and financial affairs. The Tanke Shareholders must designate the candidates recommended by the WFOE as their representatives on their respective boards of directors. The WFOE has the right to appoint senior executives of Guangzhou Tanke. In addition, the WFOE agrees to guarantee Guangzhou Tanke’s performance under any agreements or arrangements relating to Guangzhou Tanke’s business arrangements with any third party. Guangzhou Tanke, in return, agrees to pledge its accounts receivable and all of its assets to the WFOE. Moreover, Guangzhou Tanke agrees that without the prior consent of the WFOE, Guangzhou Tanke will not engage in any transactions that could materially affect its assets, liabilities, rights or operations, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party.
|
|
●
|
Option Agreement. Pursuant to the option agreement among the WFOE, Guangzhou Tanke and each of Tanke Shareholders, the Tanke Shareholders have granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Guangzhou Tanke upon an event of default.
|
|
●
|
Voting Right Proxy Agreement. Pursuant to the voting right proxy agreement among the WFOE, Guangzhou Tanke and its shareholders, the Tanke Shareholders have granted the WFOE a voting and proxy right to vote their equity interest on Guangzhou Tanke.
|

|
●
|
Changsha Xingjia Bio Tech Co., Ltd., which is engaged in developing, marketing and producing safe, environmental friendly trace mineral feed additives. Changsha offers compound acidifier, amino acid chelated trace elements, copper chloride and other products. Changsha has sales office nationwide and subsidiaries in Thailand and Singapore.
|
|
●
|
Debon Bio Tech Co., Ltd., which was established in 2004 and is a Sino-German joint venture engaged in feed additive development and raw material trading. Debon has a long term partnership with its German partner and imports piglet nutrition and feed additives from overseas.
|
|
●
|
Zinpro Corporation, a manufacturer of trace minerals. Zinpro offers iron, copper, manganese, zinc and cobalt products used in the dairy, beef, poultry, swine, and equine industries. Headquartered in Eden Prairie, Minnesota, Zinpro has sales offices in the United States, Canada, Mexico, the Netherlands, China, Japan, Thailand, Brazil, Australia and New Zealand.
|
|
●
|
Alltech Inc., an animal health and nutrition company. Alltech manufactures nutritional products and solutions for the feed industry. It provides natural feed ingredients and Sel-Plex organic selenium for use in animal species with selenium deficiencies for feed and food manufactures in North America, Latin America, the Asia-Pacific, Europe, the Middle East, and Africa. Alltech is headquartered in Nicholasville, Kentucky and has bioscience centers in the United States, Ireland, and Thailand.
|
|
●
|
Iron glycine chelate (G/Fe-140);
|
|
|
●
|
Iron glycine chelate (G/Fe-185);
|
|
|
●
|
Zinc glycine chelate (G/Zn-220);
|
|
|
●
|
Manganese glycine chelate (G/Mn-220);
|
|
●
|
Copper glycine chelate (G/Cu-210);
|
|
|
●
|
Chromium glycine chelate (G/Cr-001);
|
|
|
●
|
Iron methionine chelate (M/Fe-155);
|
|
|
●
|
Zinc methionline chelate (M/Zn-190);
|
|
●
|
Manganese methionine chelate (M/Mn-155);
|
|
|
●
|
Copper methionine chelate (M/Cr-001);
|
|
|
●
|
Zinc lysine chelate (L/Zn-105);
|
|
|
●
|
Zinc lysine chelate (L/Zn-145); and
|
|
●
|
Copper lysine chelates (L/Cu-100).
|
|
●
|
Tanksweet ST (a mixed sweetener designed to improve the palatability and acceptability of all pig feed);
|
|
●
|
Tankarom ST (a feed flavor enhancer and functional physiological regulator that assists animals in overcoming the negative effects of weaning, stress, disease, medications or mal-flavored feedstuffs);
|
|
●
|
Tankmix SA (a co-mixed product with sweetener and flavoring that makes feed more attractive);
|
|
●
|
Tankebaal sweet (a mixed sweetener to improve the palatability and acceptability of all pig feed);
|
|
●
|
Tankarom (a functional physiological regulator);
|
|
●
|
Fishy Spicy (an aroma agent added to fishmeal to enhance fishy taste, cover-up mal-flavors in feeds and improve the palatability of feed products);
|
|
●
|
Aquatic Lives’ Attractants (consisting of concentrated extracts from natural seafood and high efficient attractants rich in amino acids that improve the feed intake of fish); and
|
|
●
|
Kimyso™ (a micro-granulated solid dispersion Kitasamycin premix).
|
|
●
|
Extra-Health™ (improves animal immune system and functions);
|
|
●
|
Qilimix™ (a natural feed additive for livestock and poultry designed to improve the reproductive and growth performance of farm animals); and
|
|
●
|
Recoccider™ (a highly efficient anticoccidial premix containing Ethopabate and Diclazuril designed to inhibit DHSS and DHRS).
|
|
% of Consolidated
|
% of Consolidated
|
||
|
Customers
|
Revenue in 2012
|
Customers
|
Revenue in 2011
|
|
Guangzhou Tienhe Lianhua
Agriculture Technology Co., Ltd.
|
8.8%
|
Guangzhou Tienhe Lianhua
Agriculture Technology Co., Ltd.
|
14.0%
|
|
Jin Yin Ke Bio-Tech Co., Ltd.
|
8.6%
|
Jin Yin Ke Bio-Tech Co., Ltd.
|
12.0%
|
|
Nanning Baihe Bio-Tech Co., Ltd.
|
6.3%
|
||
|
Guangzhou Hai Hong Chuang
Aquaculture Co., Ltd.
|
6.1%
|
||
|
Weifeng Zhong Ji Feed Co., Ltd.
|
6.0%
|
||
|
Henan Guangan Bio-Tech Co., Ltd.
|
5.5%
|
|
Suppliers
|
% Supplied in 2012
|
Suppliers
|
% Supplied in 2011
|
|
Zhengzhou Tuoyang Industrial Co.,
Ltd.
|
16.1%
|
Guangzhou Tienhe Lianhua Agriculture Technology Co., Ltd.
|
23.0%
|
|
Shijiazhuang Gu Qiao Chemical
Engineering Co., Ltd.
|
15.2%
|
Shijiazhuang Gu Qiao Chemical
Engineering Co., Ltd.
|
10.0%
|
|
Guangzhou Tienhe Lianhua
Agriculture Technology Co., Ltd.
|
14.5%
|
||
|
Guangzhou Zhongxin Specialty Feed
Co., Ltd.
|
10.2%
|
||
|
Shijiazhuang Donghua Jinlong
Chemical Engineering Co., Ltd.
|
5.8%
|
Research and Development
|
●
|
A Chinese utility model patent (ZL200620154038.5) the Company owns for a mixed drier of feed additives. The patent was issued in November 2007 and will expire on March 2017.
|
|
●
|
A Chinese patent (ZL200710030121.0), that the Company jointly owns with the Guangdong University of Technology for the methodology of preparation of copper and zinc glycine complexes by ball milling and solid-static doping. The patent was issued in July 2010 and will expire in July 2030.
|
|
●
|
A Chinese patent (ZL200810198628.1), that the Company owns for a method of detecting and determining the rate of chelation in amino acid trace mineral chelation. The patent was issued in 2011.
|
|
●
|
price;
|
|
●
|
product quality;
|
|
●
|
brand identification;
|
|
●
|
breadth of product line; and
|
|
●
|
customer service.
|
|
●
|
imposing economic penalties;
|
|
|
●
|
discontinuing or restricting the operations of China Flying, Kanghui Agricultural or Guangzhou Tanke;
|
|
|
●
|
imposing conditions or requirements in respect of the VIE Agreements with which Kanghui Agricultural or Guangzhou Tanke may not be able to comply;
|
|
|
●
|
requiring us to restructure the relevant ownership structure or operations;
|
|
|
●
|
taking other regulatory or enforcement actions that could adversely affect our business; and
|
|
|
●
|
revoking the business license and/or the licenses or certificates of China Flying or Kanghui Agricultural, Guangzhou Tanke, and/or voiding the VIE Agreements.
|
|
●
|
Investors may have difficulty buying and selling or obtaining market quotations;
|
|
●
|
Market visibility for shares of our common stock may be limited; and
|
|
●
|
A lack of visibility for shares of our common stock may have a depressive effect on the market price for shares of our common stock.
|
|
●
|
actual or anticipated fluctuations in our quarterly operating results;
|
|
●
|
changes in financial estimates by securities research analysts;
|
|
●
|
conditions in commodities markets;
|
|
|
|
●
|
changes in the economic performance or market valuations of other feed additive technology companies;
|
|
|
●
|
announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
|
●
|
addition or departure of key personnel;
|
|
|
●
|
fluctuations of exchange rates between RMB and the U.S. dollar;
|
|
|
●
|
intellectual property litigation; and
|
|
|
●
|
general economic or political conditions in China.
|
|
TNBI
|
||||||||
|
High
|
Low
|
|||||||
|
Fiscal Year 2011
|
||||||||
|
First Quarter
|
N/A
|
N/A
|
||||||
|
Second Quarter
|
1.29
|
1.01
|
||||||
|
Third Quarter
|
N/A
|
N/A
|
||||||
|
Fourth Quarter
|
N/A
|
N/A
|
||||||
|
Fiscal Year 2012
|
||||||||
|
First Quarter
|
$
|
0.35
|
$
|
0.15
|
||||
|
Second Quarter
|
$
|
0.40
|
$
|
0.30
|
||||
|
Third Quarter
|
$
|
0.60
|
$
|
0.32
|
||||
|
Fourth Quarter
|
$
|
0.55
|
$
|
0.21
|
||||
|
1.
|
We would not be able to pay our debts as they become due in the usual course of business, or
|
|
2.
|
Our total assets would be less than the sum of our total liabilities plus the amount that would be needed to satisfy the rights of shareholders who have preferential rights superior to those receiving the distribution.
|
|
December 31,
2012
|
December 31,
2011
|
|||||||
|
Current assets and long-term notes receivables
|
$
|
20,457,548
|
$
|
13,929,777
|
||||
|
Property, plant and equipment
|
5,102,735
|
4,801,723
|
||||||
|
Intangible assets
|
1,237,740
|
837,525
|
||||||
|
Total assets
|
$
|
26,798,024
|
$
|
19,569,025
|
||||
|
Total liabilities
|
(6,788,948
|
)
|
(1,572,020
|
)
|
||||
|
Net assets
|
$
|
20,009,076
|
$
|
17,997,005
|
||||
|
Approximately 98% of the Company’s revenue is generated from customers in mainland China. All the Company’s suppliers are located in mainland China.
|
|
Cash and Cash Equivalents
|
|
The Company considers all highly liquid investments with initial maturities of three months or less to be cash equivalents.
|
|
Restricted Cash
|
|
Deposits that are restricted in use are classified as restricted cash.
|
|
Trade Receivables
|
|
The Company periodically assesses its accounts receivable for collectability on a specific identification basis. If collectability of an account becomes unlikely, an allowance is recorded for that doubtful account. Once collection efforts have been exhausted, the account receivable is written off against the allowance. The Company does not require collateral for trade or other accounts receivable.
|
|
Inventories
|
|
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the weighted average method. The cost of inventories includes the purchase cost and other costs incurred in bringing the inventories to their present location and condition. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and the estimated costs necessary to make the sale.
|
|
Prepayments
Prepayments are cash deposits paid to major raw material suppliers to secure the procurement of key raw materials for the upcoming year. Contracts are signed with these suppliers to lock in the prices of raw materials.
Property and Equipment
|
|
Property and equipment are stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
|
|
Depreciation of property and equipment is calculated using the straight-line method over their estimated useful lives. The estimated useful lives are as follows:
|
|
Buildings
|
15-20 years
|
|
Plant and machinery
|
3-20 years
|
|
Motor vehicle
|
10 years
|
|
Office equipment
|
3-10 years
|
|
Expenditures for additions, major renewals and betterments are capitalized and expenditures for maintenance and repairs are charged to expense as incurred.
|
|
Intangible Assets
|
|
Intangible assets consist of two land use rights, which are recorded at cost less accumulated amortization and a new production technology. Amortization of the land use rights is provided over the term of the land use right agreements on a straight-line basis. The new production technology was acquired from a research institute that gave us the right to commercialize the technology and to apply for patent protection. Because this technology transfer will benefit the Company in the form of sales and profit growth for a longer period of time and two patent applications have been filed with the assistance of this research institute that will create intellectual property, we decided to amortize the cost over three years, beginning in the third quarter of 2012. After we applied the technology developed by this research institute to our manufacturing process, the sales of this product type – antioxidant has already increased substantially in the second half of 2012.
|
|
Impairment of Long-lived Assets
|
|
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cash flows attributable to such assets.
|
|
Revenue Recognition
|
|
The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) 605-10, Revenue Recognition, and SEC Staff Accounting Bulletin No. 104. Pursuant to these pronouncements, revenue is recognized when all of the following criteria are met:
|
|
- Persuasive evidence of an arrangement exists;
|
|
- Delivery has occurred or services have been rendered;
|
|
- The seller's price to the buyer is fixed or determinable; and
|
|
- Collectability is reasonably assured.
|
|
The Company’s revenue is generated through the wholesale and retail sale of livestock feed additives including organic trace mineral additives, functional regulation additives, herbal medicinal additives and raw materials. Before the Company recognizes revenue on these product sales, written purchase orders and contracts are received in advance of all shipments of goods to customers. For sales within the Company’s own province, delivery is made by Company employees. Such delivery occurs on the same day as shipment. For delivery outside the province, shipment is made through a separate logistics company that assumes the risk of loss. Revenue is recognized upon shipment of goods to the customers. The Company typically does not incur bad debt losses because this type of loss is deducted from the salesperson’s compensation, thereby partially mitigating the loss to the Company. Therefore, collectability is reasonably assured.
|
|
Revenue is presented net of sales returns, which are not significant. However, the Company continually performs analyses of returns and records a provision at the time of sale if necessary. As of December 31, 2012 and 2011, it was determined that potential returns and allowances were not material so the Company did not record a provision for returns. The Company revisits this estimate regularly and adjusts it if conditions change.
|
|
Cost of Goods Sold
|
|
Cost of revenue consists primarily of material cost, labor cost, overhead associated with the manufacturing process and directly related expenses.
|
|
Research and Development Costs
|
|
Value Added Tax (VAT)
|
|
In accordance with the relevant tax laws in the PRC, VAT is levied on the invoiced value of sales and is payable by the purchaser. The Company is required to remit the VAT it collects to the tax authority, but may deduct the VAT it has paid on eligible purchases. The difference between the amounts collected and paid is presented as VAT recoverable or payable balance on the balance sheet.
Income Taxes
|
|
The Company uses the asset and liability method of accounting for income taxes pursuant to ASC Topic 740, ”Income Tax”. Under the asset and liability method of ASC Topic 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
|
|
Comprehensive Income (Loss)
|
|
The Company reports comprehensive income (loss) in accordance with the Financial Accounting Standards Board (FASB) issued authoritative guidance which establishes standards for reporting comprehensive income (loss) and its component in financial statements. Comprehensive income (loss), as defined, includes all changes in equity during a period from non-owner sources.
|
|
Level 1 -
|
Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access. Valuation adjustments and block discounts are not being applied. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these securities does not entail a significant degree of judgment.
|
|
Level 2 -
|
Valuations based on (i) quoted prices in active markets for similar assets and liabilities, (ii) quoted prices in markets that are not active for identical or similar assets, (iii) inputs other than quoted prices for the assets or liabilities, or (iv) inputs that are derived principally from or corroborated by market through correlation or other means.
|
|
Level 3 -
|
Valuations based on inputs that are unobservable and significant to the overall fair value measurement.
|
|
Year Ended
December 31,
|
||||||||||||||||
|
2012
|
2011
|
$ Change
|
% Change
|
|||||||||||||
|
Segment revenues
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 24,588,201 | $ | 18,855,958 | $ | 5,732,243 | 30.4 | % | ||||||||
|
Functional Regulation Additives
|
3,056,847 | 4,163,823 | (1,106,976 | ) | (26.6 | %) | ||||||||||
|
Herbal Medicinal Additives
|
293,454 | 281,036 | 12,418 | 4.4 | % | |||||||||||
|
Other
|
623,327 | 531,910 | 91,417 | 17.2 | % | |||||||||||
| $ | 28,561,829 | $ | 23,832,727 | $ | 4,729,102 | 19.8 | % | |||||||||
|
Segment costs of sales
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 15,719,425 | $ | 11,907,294 | $ | 3,812,131 | 32.0 | % | ||||||||
|
Functional Regulation Additives
|
2,046,917 | 2,571,920 | (525,003 | ) | (20.4 | %) | ||||||||||
|
Herbal Medicinal Additives
|
269,844 | 219,856 | 49,988 | 22.7 | % | |||||||||||
|
Other
|
581,178 | 363,449 | 217,729 | 59.9 | % | |||||||||||
| $ | 18,617,364 | $ | 15,062,519 | $ | 3,554,845 | 23.6 | % | |||||||||
|
Segment gross profit
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 8,868,775 | $ | 6,948,664 | $ | 1,920,111 | 27.6 | % | ||||||||
|
Functional Regulation Additives
|
1,009,931 | 1,591,903 | (581,972 | ) | (36.6 | %) | ||||||||||
|
Herbal Medicinal Additives
|
23,611 | 61,180 | (37,569 | ) | (61.4 | %) | ||||||||||
|
Other
|
42,149 | 168,461 | (126,312 | ) | (75.0 | %) | ||||||||||
| $ | 9,944,466 | $ | 8,770,208 | $ | 1,174,258 | 13.4 | % | |||||||||
|
Year Ended
December 31,
|
||||||||||||||||
|
2012
|
2011
|
$ Change
|
% Change
|
|||||||||||||
|
Gross profit
|
$ | 9,944,465 | $ | 8,770,208 | $ | 1,174,257 | 13.4 | % | ||||||||
|
Selling expenses
|
(2,679,714 | ) | (2,463,901 | ) | 215,813 | 8.8 | % | |||||||||
|
Administrative expenses
|
(2,571,671 | ) | (4,486,490 | ) | (1,914,819 | ) | (42.7 | %) | ||||||||
|
Depreciation and amortization
|
(161,460 | ) | (81,004 | ) | 80,456 | 99.3 | % | |||||||||
|
Income from operations
|
4,531,619 | 1,738,813 | 2,792,806 | (160.6 | %) | |||||||||||
|
Other income/expense
|
||||||||||||||||
|
Interest income
|
295,556 | 95,834 | 199,722 | 208.4 | % | |||||||||||
|
Interest expense
|
(1,445,787 | ) | (1,361,703 | ) | 84,084 | 6.2 | % | |||||||||
|
Amortization of discount on notes
|
(2,778,796 | ) | (2,467,511 | ) | 311,285 | 12.6 | % | |||||||||
|
Registration rights agreement expense
|
- | (460,206 | ) | (460,206 | ) | (100.0 | %) | |||||||||
|
Income (loss) before income taxes
|
602,591 | (2,454,773 | ) | 3,057,364 | 124.5 | % | ||||||||||
|
Income tax expense
|
(831,597 | ) | (681,321 | ) | 150,276 | 22.1 | % | |||||||||
|
Net loss
|
$ | (229,006 | ) | $ | (3,136,094 | ) | $ | 2,907,088 | 92.7 | % | ||||||
|
Year Ended
December 31,
|
$ | % | ||||||||||||||
|
2012
|
2011
|
Change
|
Change
|
|||||||||||||
|
Net cash provided by (used in) operating activities
|
$ | (1,184,097 | ) | $ | 3,653,141 | $ | (4,837,238 | ) | (132.4 | %) | ||||||
|
Net cash used in investing activities
|
(1,526,860 | ) | (3,668,345 | ) | 2,141,485 | (58.4 | %) | |||||||||
|
Net cash provided by financing activities
|
1,121,816 | 5,815,761 | (4,693,945 | ) | (80.7 | %) | ||||||||||
|
Effect of foreign currency conversion on cash
|
57,739 | (322,427 | ) | 380,166 | (117.9 | %) | ||||||||||
|
Net (decease) increase in cash
|
$ | (1,531,402 | ) | $ | 5,478,130 | $ | (7,009,532 | ) | (128.0 | %) | ||||||
Operating Activities
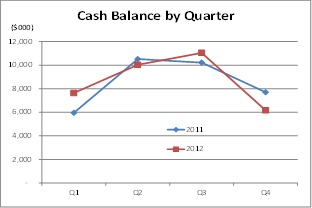
|
●
|
a form which describes the amount and type of the proposed transfer in accordance with China’s currency exchange control regulations;
|
|
|
●
|
documentation that all required approvals or filings for the product, service or license to be purchased (where applicable)
|
|
●
|
have been obtained (for example, if a China domiciled entity desires to purchase imported technology, the purchase requires approval from the Ministry of Commerce);
|
|
|
●
|
relevant agreements or contracts (e.g., sales contracts, service agreements or license agreements, etc.);
|
|
|
●
|
corresponding invoices or payment notices; and
|
|
●
|
tax payment receipts.
|
|
●
|
a form which describes the amount and type of the proposed transfer in accordance with China’s currency exchange control regulations;
|
|
|
●
|
a foreign-invested enterprise foreign exchange registration certificate;
|
|
●
|
a board resolution or shareholder’s resolution approving the repatriation of profits or dividends;
|
|
●
|
a copy of its statutory accounts for the most recently completed fiscal year (financial statements prepared in accordance with Chinese governmental regulations and audited by an approved independent auditor); and
|
|
●
|
corresponding tax payment receipts.
|
|
Report of Independent Registered Public Accounting Firm - UHY LLP
|
F-2
|
|||
|
Report of Independent Registered Public Accounting Firm - EFP Rotenberg LLP
|
F-3
|
|||
|
Consolidated Balance Sheets As of December 31, 2012 and 2011
|
F-4
|
|||
|
Consolidated Statements of Operations and Comprehensive Loss For the Years ended December 31, 2012 and 2011
|
F-5
|
|||
|
Consolidated Statements of Changes in Equity For the Years Ended December 31, 2012 and 2011
|
F-6
|
|||
|
Consolidated Statements of Cash Flows For the Years ended December 31, 2012 and 2011
|
F-7
|
|||
|
Notes to Consolidated Financial Statements
|
F-8 to F-21
|
|||

|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 6,168,754 | $ | 7,700,156 | ||||
|
Restricted cash
|
231,838 | 706,802 | ||||||
|
Accounts receivable, net
|
1,986,663 | 1,917,699 | ||||||
|
Inventories, net
|
1,414,600 | 1,187,895 | ||||||
|
Note receivable - related parties
|
- | 239,476 | ||||||
|
Loans to customer and supplier
|
2,892,868 | 2,513,460 | ||||||
|
Other receivables
|
483,884 | 53,936 | ||||||
|
Prepayments
|
9,029,524 | 3,633,674 | ||||||
|
Other current assets
|
132,746 | 914,594 | ||||||
|
Deferred tax assets
|
27,042 | 46,042 | ||||||
|
Total current assets
|
22,367,919 | 18,913,734 | ||||||
|
Property, plant and equipment, net
|
4,813,232 | 4,771,299 | ||||||
|
Construction in progress
|
295,248 | 35,878 | ||||||
|
Intangible assets, net
|
1,238,025 | 838,089 | ||||||
|
Other non-current assets
|
134,736 | 328,006 | ||||||
|
Total assets
|
$ | 28,849,160 | $ | 24,887,006 | ||||
|
LIABILITIES AND EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 646,649 | $ | 784,777 | ||||
|
Advance from customers
|
128,321 | - | ||||||
|
Other payables and accrued liabilities
|
942,793 | 758,907 | ||||||
|
Income tax payable
|
1,842,139 | 1,216,841 | ||||||
|
Convertible notes, net of discount
|
7,267,677 | - | ||||||
|
Current portion of long-term borrowing
|
1,268,106 | 785,456 | ||||||
|
Total current liabilities
|
12,095,685 | 3,545,981 | ||||||
|
Convertible notes, net of discount
|
- | 4,488,881 | ||||||
|
Note payable - related party
|
- | 13,722 | ||||||
|
Advance from government grant
|
37,948 | 355,754 | ||||||
|
Long-term borrowing, net of current portion
|
792,567 | 628,365 | ||||||
|
Total liabilities
|
12,926,200 | 9,032,703 | ||||||
|
Commitments and contingencies
|
||||||||
|
EQUITY
|
||||||||
|
Common stock, $0.001 par value, 50,000,000 shares authorized, 13,324,083 issued and outstanding as of December 31, 2012 and December 31, 2011
|
13,324 | 13,324 | ||||||
|
Additional paid-in capital
|
12,220,181 | 12,220,181 | ||||||
|
Retained earnings
|
2,485,736 | 2,695,983 | ||||||
|
Statutory reserve
|
373,406 | 373,406 | ||||||
|
Accumulated other comprehensive income
|
658,870 | 551,409 | ||||||
|
Total Tanke Biosciences Corporation stockholders' equity
|
15,751,517 | 15,854,303 | ||||||
|
Non-controlling interest
|
171,443 | - | ||||||
|
Total Equity
|
15,922,960 | 15,854,303 | ||||||
|
Total Liabilities and Equity
|
$ | 28,849,160 | $ | 24,887,006 | ||||
|
Year Ended
December 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Net sales
|
$ | 28,561,829 | $ | 23,832,727 | ||||
|
Costs of sales
|
(18,617,364 | ) | (15,062,519 | ) | ||||
|
Gross profit
|
9,944,465 | 8,770,208 | ||||||
|
Selling expenses
|
(2,679,714 | ) | (2,463,901 | ) | ||||
|
Administrative expenses
|
(2,571,671 | ) | (4,486,490 | ) | ||||
|
Depreciation and amortization
|
(161,460 | ) | (81,004 | ) | ||||
|
Income from operations
|
4,531,619 | 1,738,813 | ||||||
|
Other income (expense)
|
||||||||
|
Interest income
|
295,556 | 95,834 | ||||||
|
Interest expense
|
(1,445,787 | ) | (1,361,703 | ) | ||||
|
Amortization of discount on notes
|
(2,778,796 | ) | (2,467,511 | ) | ||||
|
Registration rights agreement expense
|
- | (460,206 | ) | |||||
|
Income (loss) before income taxes
|
602,591 | (2,454,773 | ) | |||||
|
Income tax expense
|
(831,597 | ) | (681,321 | ) | ||||
|
Net loss
|
$ | (229,006 | ) | $ | (3,136,094 | ) | ||
|
Net loss attributable to non-controlling interest
|
(18,759 | ) | - | |||||
|
Net loss attributable to Tanke Biosciences Corporation
|
$ | (210,247 | ) | $ | (3,136,094 | ) | ||
|
Net loss
|
$ | (229,006 | ) | $ | (3,136,094 | ) | ||
|
Other comprehensive income, net of tax:
|
||||||||
|
Foreign currency translation adjustments
|
107,589 | 56,629 | ||||||
|
Comprehensive loss
|
$ | (121,417 | ) | $ | (3,079,465 | ) | ||
|
Comprehensive loss attributable to non-controlling interest
|
(18,631 | ) | - | |||||
|
Comprehensive loss attributable to Tanke Biosciences Corporation
|
$ | (102,786 | ) | $ | (3,079,465 | ) | ||
|
Loss per common share:
|
||||||||
|
Basic and diluted
|
$ | (0.02 | ) | $ | (0.24 | ) | ||
|
Weighted average number of common shares used in computation
|
||||||||
|
Basic and diluted
|
13,324,083 | 13,083,333 | ||||||
|
Accumulated
|
|
|||||||||||||||||||||||||||||||||||
|
Common Stock
|
Additional
Paid-in
|
Retained
|
Statuory
|
Other
Comprehensive
|
Total
Stockholders'
|
Non-
Controlling
|
Total
|
|||||||||||||||||||||||||||||
|
Shares
|
Amount
|
capital
|
Earnings
|
Reserve
|
Income
|
Equity
|
Interest
|
Equity
|
||||||||||||||||||||||||||||
|
Balance at December 31, 2010, restated in terms of the Share Exchange Agreement
|
10,758,000 | $ | 10,758 | $ | 1,417,098 | $ | 5,832,077 | $ | 373,406 | $ | 530,070 | $ | 8,163,409 | $ | 2,170,737 | $ | 10,334,146 | |||||||||||||||||||
|
Effect of Acquisition of China Flying
|
2,133,917 | (35,290 | ) | 2,098,627 | (2,170,737 | ) | (72,110 | ) | ||||||||||||||||||||||||||||
|
Effect of Share Exchange Agreement
|
399,180 | 399 | 54,200 | 54,599 | 54,599 | |||||||||||||||||||||||||||||||
|
Effect of Private Placement
|
6,125,195 | 6,125,195 | 6,125,195 | |||||||||||||||||||||||||||||||||
|
Shares issued for consulting services
|
2,166,903 | 2,167 | 2,489,771 | 2,491,938 | 2,491,938 | |||||||||||||||||||||||||||||||
|
Net loss
|
(3,136,094 | ) | (3,136,094 | ) | (3,136,094 | ) | ||||||||||||||||||||||||||||||
|
Foreign currency translation
|
56,629 | 56,629 | 56,629 | |||||||||||||||||||||||||||||||||
|
Balance at December 31, 2011
|
13,324,083 | 13,324 | 12,220,181 | 2,695,983 | 373,406 | 551,409 | 15,854,303 | - | 15,854,303 | |||||||||||||||||||||||||||
|
Net loss
|
(210,247 | ) | (210,247 | ) | (18,759 | ) | (229,006 | ) | ||||||||||||||||||||||||||||
|
Foreign currency translation
|
107,461 | 107,461 | 128 | 107,589 | ||||||||||||||||||||||||||||||||
|
60% investment in Qingyuan Tanke Bio-Technology
|
- | 190,074 | 190,074 | |||||||||||||||||||||||||||||||||
|
Balance at December 31, 2012
|
13,324,083 | $ | 13,324 | $ | 12,220,181 | $ | 2,485,736 | $ | 373,406 | $ | 658,870 | $ | 15,751,517 | $ | 171,443 | $ | 15,922,960 | |||||||||||||||||||
|
Year Ended
December 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Operating activities:
|
||||||||
|
Net loss
|
$ | (229,006 | ) | $ | (3,136,094 | ) | ||
|
Adjustments to reconcile net loss to net cash (used in)
|
||||||||
|
provided by operating activities:
|
||||||||
|
Depreciation and amortization
|
636,286 | 400,013 | ||||||
|
Common stock issued for services
|
- | 2,491,938 | ||||||
|
Amortization of discount on convertible notes payable
|
2,778,796 | 2,467,511 | ||||||
|
Amortization of capitalized offering costs
|
798,906 | 709,409 | ||||||
|
Provision for bad debt
|
103,425 | 90,185 | ||||||
|
Inventory provision
|
377 | 41,585 | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
(172,389 | ) | (169,664 | ) | ||||
|
Inventories
|
(227,082 | ) | 173,356 | |||||
|
Note receivables and payables - related parties
|
225,754 | 2,584,892 | ||||||
|
Other receivables
|
(426,459 | ) | 108,862 | |||||
|
Deferred tax asset
|
19,000 | (26,946 | ) | |||||
|
Prepayments
|
(5,352,068 | ) | (3,518,597 | ) | ||||
|
Other current assets
|
(17,058 | ) | 138,079 | |||||
|
Other non-current assets
|
193,270 | (61,660 | ) | |||||
|
Advance from government grant
|
(315,227 | ) | 274,171 | |||||
|
Accounts payable
|
(138,128 | ) | 153,420 | |||||
|
Other payables and accrued liabilities
|
183,886 | 457,109 | ||||||
|
Income tax payable
|
625,298 | 478,810 | ||||||
|
Advance from customer
|
128,321 | (3,238 | ) | |||||
|
Net cash (used in) provided by operating activities
|
(1,184,097 | ) | 3,653,141 | |||||
|
|
||||||||
|
Investing activities:
|
||||||||
|
Increase in loans to customer and supplier
|
(379,408 | ) | (2,513,460 | ) | ||||
|
Purchase of property and equipment
|
(856,361 | ) | (701,022 | ) | ||||
|
Acquisition of new production technology
|
(481,165 | ) | (529,938 | ) | ||||
|
60% investment in Qingyuan Tanke Bio-Technology
|
190,074 | - | ||||||
|
Increase in cash due to acquisition of China Flying
|
- | 76,075 | ||||||
|
Net cash used in investing activities
|
(1,526,860 | ) | (3,668,345 | ) | ||||
|
Financing activities:
|
||||||||
|
Change in restricted cash
|
474,964 | (706,802 | ) | |||||
|
Net proceeds from issuance of convertible notes
|
- | 6,522,563 | ||||||
|
Increase in bank borrowings
|
646,852 | - | ||||||
|
Net cash provided by financing activities
|
1,121,816 | 5,815,761 | ||||||
|
Effect of exchange rate fluctuations on cash and cash equivalents
|
57,739 | (322,427 | ) | |||||
|
Net increase (decrease) in cash
|
(1,531,402 | ) | 5,478,130 | |||||
|
Cash and cash equivalents, beginning of period
|
7,700,156 | 2,222,025 | ||||||
|
Cash and cash equivalents, end of period
|
$ | 6,168,754 | $ | 7,700,155 | ||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Cash paid for interest
|
$ | 634,463 | $ | 652,294 | ||||
|
Cash paid for income taxes
|
$ | 346,836 | $ | 249,916 | ||||
|
December 31,
2012
|
December 31,
2011
|
|||||||
|
Current assets and long-term notes receivables
|
$
|
20,457,548
|
$
|
13,929,777
|
||||
|
Property, plant and equipment
|
5,102,735
|
4,801,723
|
||||||
|
Intangible assets
|
1,237,740
|
837,525
|
||||||
|
Total assets
|
$
|
26,798,024
|
$
|
19,569,025
|
||||
|
Total liabilities
|
(6,788,948
|
)
|
(1,572,020
|
)
|
||||
|
Net assets
|
$
|
20,009,076
|
$
|
17,997,005
|
||||
|
Percentage Interest
|
||||||||
|
Name of Entity
|
December 31,
2012
|
December 31,
2011
|
||||||
|
China Flying Development Limited
|
100 | % | 100 | % | ||||
|
Guangzhou Kanghui Agricultural Technology Co., Ltd.
|
100 | % | 100 | % | ||||
|
Guangzhou Tanke Industry Co., Ltd.
|
100 | % | 100 | % | ||||
|
Guangzhou Tanke Bio-Tech Co., Ltd.
|
100 | % | 100 | % | ||||
|
Guangzhou Jenyi Bio-Tech Co., Ltd.
|
100 | % | 100 | % | ||||
|
Guangzhou Tanke Animal Health Co., Ltd.
|
100 | % | 100 | % | ||||
|
Qingyuan Tanke Bio-Tech Co., Ltd.
|
60 | % | 60 | % | ||||
|
Cash and Cash Equivalents
|
|
The Company considers all highly liquid investments with initial maturities of three months or less to be cash equivalents.
|
|
Restricted Cash
|
|
Deposits that are restricted in use are classified as restricted cash. As of December 31, 2011, the Company had restricted cash in an escrow account which represented six months of interest on the convertible notes payable. When the notes mature or are converted into stock, the cash in this account will be released from restriction. On December 31, 2012, the Company utilized the escrow fund to pay for the last accrued interest payment on the convertible notes payable. The Company has a second escrow account of approximately $232,000 as of December 31, 2012 to be utilized for Investor Relations activities.
|
|
Trade and Other Receivables
|
|
The Company periodically assesses its accounts receivable for collectability on a specific identification basis. If collectability of an account becomes unlikely, an allowance is recorded for that doubtful account. After all collection efforts have been exhausted, the account receivable is written off against the allowance. The Company generally does not require collateral for trade or other accounts receivable.
|
|
Inventories
|
|
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the weighted average method. The cost of inventories includes the purchase cost and other costs incurred in bringing the inventories to their present location and condition. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and the estimated costs necessary to make the sale.
|
|
As of December 31, 2012 and 2011, the Company’s provision for slow-moving or defective inventories amounted to $41,962 and $41,585, respectively.
|
|
Property, Plant and Equipment
|
|
Property and equipment are stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
|
|
Depreciation of property and equipment is calculated using the straight-line method over their estimated useful lives. The estimated useful lives are as follows:
|
|
Buildings
|
15-20 years
|
|
Plant and machinery
|
3-20 years
|
|
Motor vehicle
|
10 years
|
|
Office equipment
|
3-10 years
|
|
Expenditures for additions, major renewals and betterments are capitalized and expenditures for maintenance and repairs are charged to expense as incurred.
|
|
Upon sale or disposition, the applicable amounts of asset cost and accumulated depreciation are removed from the accounts and the net amount less the proceeds from disposal is charged or credited to income.
|
|
Intangible Assets
|
|
Amortization of intangible assets is calculated using the straight-line method over their estimated useful lives. The estimated useful lives are as follows:
|
|
Land use rights
|
50 years
|
|
New Production Technology
|
3 – 5 years
|
|
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cash flows attributable to such assets. There were no impairments of long-lived assets for the periods presented.
|
|
Revenue Recognition
|
|
- Persuasive evidence of an arrangement exists;
|
|
- Delivery has occurred or services have been rendered;
|
|
- The seller's price to the buyer is fixed or determinable; and
|
|
- Collectability is reasonably assured.
|
|
The Company’s revenue is generated through the wholesale and retail sale of livestock feed additives including organic trace mineral additives, functional regulation additives, herbal medicinal additives and raw materials. Before the Company recognizes revenue on these product sales, written purchase orders and contracts are received in advance of all shipments of goods to customers. For sales within the Company’s own province, delivery is made by Company employees. Such delivery occurs on the same day as shipment. For delivery outside the province, shipment is made through a separate logistics company that assumes the risk of loss. Revenue is recognized upon shipment of goods to the customers. The Company typically does not incur bad debt losses because this type of loss is deducted from the salesperson’s compensation, thereby partially mitigating the loss to the Company. Therefore, collectability is reasonably assured.
|
|
Revenue is presented net of sales returns, which are not significant. However, the Company continually performs analysis of returns and records a provision at the time of sale if necessary. As of December 31, 2012 and 2011, it was determined that potential returns and allowances were not material so the Company did not record a provision for returns. The Company revisits this estimate regularly and adjusts it if conditions change.
|
|
Cost of Goods Sold
|
|
Cost of revenue consists primarily of material cost, labor cost, rent of land allocated to production, overhead associated with the manufacturing process and directly related expenses.
|
|
Research and Development Costs
|
|
Value Added Tax (VAT)
|
|
In accordance with the relevant tax laws in the PRC, VAT is levied on the invoiced value of sales and is payable by the purchaser. The Company is required to remit the VAT it collects to the tax authority, but may deduct the VAT it has paid on eligible purchases. The difference between the amounts collected and paid is presented as VAT recoverable or payable balance on the balance sheet.
|
|
The Company uses the asset and liability method of accounting for income taxes pursuant to ASC 740, ”Income Tax”. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
The Company is not subject to United States income tax. Furthermore, the Company is audited every year by an agency of the Chinese tax authority. Consequently, there are no uncertain tax positions requiring additional accrual or disclosure in accordance with ASC 740-10,
Income Taxes.
|
|
Comprehensive Income (Loss)
|
|
Comprehensive income (loss) is defined to include all changes in equity except those resulting from net income or loss, investments by owners and distributions to owners. The Company’s only component of other comprehensive income (loss) is the foreign currency translation adjustment.
|
|
Exchange rates used (RMB to USD)
|
2011
|
2012
|
|
|
Assets and liabilities
|
Balance sheet date (December 31)
|
0.1573
|
0.1585
|
|
Revenue and expenses
|
Period average (Twelve months ended December 31)
|
0.1545
|
0.1584
|
|
Level 1 -
|
Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access. Valuation adjustments and block discounts are not being applied. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these securities does not entail a significant degree of judgment.
|
|
Level 2 -
|
Valuations based on (i) quoted prices in active markets for similar assets and liabilities, (ii) quoted prices in markets that are not active for identical or similar assets, (iii) inputs other than quoted prices for the assets or liabilities, or (iv) inputs that are derived principally from or corroborated by market through correlation or other means.
|
|
Level 3 -
|
Valuations based on inputs that are unobservable and significant to the overall fair value measurement.
|
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Raw materials
|
$ | 636,319 | $ | 538,465 | ||||
|
Finished goods
|
576,509 | 402,458 | ||||||
|
Work in pogress
|
210,625 | 242,748 | ||||||
|
Packaging material
|
33,109 | 45,809 | ||||||
|
Less: Inventory allowance
|
(41,962 | ) | (41,585 | ) | ||||
| $ | 1,414,600 | $ | 1,187,895 | |||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Account receivables
|
$ | 2,323,370 | $ | 2,150,981 | ||||
|
Less: Allowance for doubtful accounts
|
(336,707 | ) | (233,282 | ) | ||||
| $ | 1,986,663 | $ | 1,917,699 | |||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Business advance to staff
|
$ | 166,451 | $ | 40,511 | ||||
|
Business advance to directors
|
287,032 | - | ||||||
|
Deposits
|
30,401 | 13,425 | ||||||
| $ | 483,884 | $ | 53,936 | |||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Loans to customer and supplier
|
$ | 2,892,868 | $ | 2,513,460 | ||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Prepayments to suppliers
|
$ |
9,029,524
|
$ | 3,633,674 | ||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Offering costs, net
|
$ | 115,688 | $ | 914,594 | ||||
|
Deferred expenses and Other
|
17,058 | - | ||||||
| $ | 132,746 | $ | 914,594 | |||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Buildings
|
$ | 4,596,527 | $ | 4,518,397 | ||||
|
Plant and equipment
|
1,365,043 | 1,024,245 | ||||||
|
Motor vehicles
|
168,984 | 166,884 | ||||||
|
Office equipment
|
340,870 | 164,907 | ||||||
|
Total property, plant and equipment
|
6,471,423 | 5,874,433 | ||||||
|
Less: accumulated depreciation
|
(1,658,192 | ) | (1,103,134 | ) | ||||
|
Property, plant and equipment, net
|
$ | 4,813,232 | $ | 4,771,299 | ||||
|
Construction in progress
|
$ | 295,248 | $ | 35,878 | ||||
|
Years Ended December 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Depreciation expense charged to:
|
||||||||
|
Cost of Goods Sold
|
$ | 473,584 | $ | 350,927 | ||||
|
Selling, general and administrative expense
|
81,474 | 49,086 | ||||||
|
Total Depreciation Expense
|
$ | 555,058 | $ | 400,013 | ||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Deposits for land use right
|
$
|
840,514
|
$
|
835,985
|
||||
|
New production technology, net
|
396,315
|
-
|
||||||
|
Other
|
1,196
|
2,104
|
||||||
|
$
|
1,238,025
|
$
|
838,089
|
|||||
|
Balance at
|
Foreign
|
Balance at
|
||||||||||||||||||||||
|
January 1,
|
Amortization
|
Impairment
|
Currency
|
December 31,
|
||||||||||||||||||||
|
2012
|
Additions
|
Expense
|
Charge
|
Translation
|
2012
|
|||||||||||||||||||
|
Amortized intangible assets:
|
||||||||||||||||||||||||
|
Deposits for land use right
|
$ | 835,985 | $ | $ | (1,082 | ) | $ | - | $ | 5,611 | $ | 840,514 | ||||||||||||
|
New production technology, net
|
475,540 | (80,146 | ) | 921 | 396,315 | |||||||||||||||||||
|
Other
|
2,104 | 5,625 | (6,321 | ) | (212 | ) | 1,196 | |||||||||||||||||
|
Total amortized intangible assets
|
$ | 838,089 | $ | 481,165 | $ | (81,228 | ) | $ | (6,321 | ) | $ | 6,320 | $ | 1,238,025 | ||||||||||
|
Balance at
|
Foreign
|
Balance at
|
||||||||||||||||||||||
|
January 1,
|
Amortization
|
Impairment
|
Currency
|
December 31,
|
||||||||||||||||||||
| 2011 |
Additions
|
Expense
|
Charge
|
Translation
|
2011 | |||||||||||||||||||
|
Amortized intangible assets:
|
||||||||||||||||||||||||
|
Deposits for land use right
|
$ | 239,610 | $ | 580,573 | $ | (1,899 | ) | $ | - | $ | 17,701 | $ | 835,985 | |||||||||||
|
New production technology, net
|
- | |||||||||||||||||||||||
|
Other
|
2,807 | 1,178 | (885 | ) | (996 | ) | 2,104 | |||||||||||||||||
|
Total amortized intangible assets
|
$ | 242,417 | 581,751 | $ | (1,899 | ) | $ | (885 | ) | $ | 16,705 | $ | 838,089 | |||||||||||
|
Estimated amortization expense of intangibles for the years ending December 31, 2013 through 2017 and thereafter is as follows:
|
||||||||||||||||||||||||
|
Year Ended December 31,
|
Amounts
|
||||||||||||||||||||||
| 2013 | $ | 173,200 | |||||||||||||||||||||
| 2014 | 173,200 | ||||||||||||||||||||||
| 2015 | 93,200 | ||||||||||||||||||||||
| 2016 | 13,200 | ||||||||||||||||||||||
| 2017 | 13,200 | ||||||||||||||||||||||
|
Thereafter
|
772,000 | ||||||||||||||||||||||
| $ | 1,238,000 | ||||||||||||||||||||||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Other payables
|
$ | 22,930 | $ | 67,751 | ||||
|
Staff welfare payable
|
68,578 | 68,057 | ||||||
|
Accrued payroll
|
306,174 | 46,294 | ||||||
|
Value added tax payable
|
62,401 | 93,140 | ||||||
|
Registration rights penalties
|
460,206 | 460,206 | ||||||
|
Other tax payable
|
22,504 | 23,459 | ||||||
| $ | 942,793 | $ | 758,907 | |||||
|
2012
|
2011
|
||||||||||||
|
Number of
|
Number of
|
||||||||||||
|
Warrants
|
Warrants
|
Exercise
|
Expiration
|
||||||||||
|
Warrants Outstanding
|
Outstanding
|
Outstanding
|
Price
|
Date
|
|||||||||
|
2011 Private Placement Investor Warrants
|
6,669,627 | 6,669,627 | $ | 1.40 |
2/19/2014
|
||||||||
|
2011 Private Placement Agent Warrants
|
666,963 | 666,963 | $ | 1.15 |
2/20/2014
|
||||||||
| 7,336,590 | 7,336,590 | ||||||||||||
|
Year Ended
|
||||||||
|
December 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Current provision
|
||||||||
|
PRC
|
$ | 812,597 | $ | 709,476 | ||||
|
Deferred provision
|
||||||||
| PRC | 19,000 | (28,155 | ) | |||||
| Total Income tax provision | $ | 831,597 | $ | 681,321 | ||||
|
Year Ended
|
||||||||
|
December 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Income tax at US Federal statutory rate
|
$ | 204,881 | $ | (834,624 | ) | |||
|
Difference in Chinese rate versus US rate
|
(504,102 | ) | (441,937 | ) | ||||
|
Tax holiday for Chinese subsidiary
|
(601,261 | ) | (617,714 | ) | ||||
|
Difference in Hong Kong tax rate
|
102,666 | 204,827 | ||||||
|
Other
|
(80,489 | ) | 4,008 | |||||
|
Change in valuation allowance
|
1,709,901 | 2,366,760 | ||||||
|
Total
|
$ | 831,597 | $ | 681,321 | ||||
|
December 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Allowance for doubtful accounts in China
|
$ | 61,373 | $ | 46,042 | ||||
|
Net operating loss carryforward
|
2,995,201 | 1,873,030 | ||||||
|
Share based payment
|
1,047,130 | 493,730 | ||||||
|
Valuation allowance
|
(4,076,661 | ) | (2,366,760 | ) | ||||
|
Net deferred tax asset
|
$ | 27,042 | $ | 46,042 | ||||
|
For The Years Ended Dec. 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Numerator for basic/diluted loss per share attributable to the
|
||||||||
|
Company's common stockholders
|
||||||||
|
Net loss used in computing basic/diluted loss per share
|
$ | (229,006 | ) | $ | (3,136,094 | ) | ||
|
Basic/diluted weighted average shares outstanding
|
13,324,083 | 13,083,333 | ||||||
|
Basic/diluted loss per share
|
$ | (0.02 | ) | $ | (0.24 | ) | ||
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Bank loans bearing interest at 5.85% and 7.36% per
|
||||||||
|
annum, maturing on January 30, 2013 and June 20, 2014.
|
$
|
2,060,673
|
$
|
1,413,821
|
||||
|
Less: Current portion
|
(1,268,106
|
)
|
(785,456
|
)
|
||||
|
Long-term borrowing
|
$
|
792,567
|
$
|
628,365
|
||||
|
Year Ended
|
||||
|
December 31,
|
||||
|
2013
|
$ | 1,268,106 | ||
|
2014
|
792,567 | |||
| $ | 2,060,673 | |||
|
Year Ended
|
||||||||
|
December 31,
|
||||||||
|
2012
|
2011
|
|||||||
|
Segment revenues
|
||||||||
|
Organic Trace Mineral Additives
|
$ | 24,588,201 | $ | 18,855,958 | ||||
|
Functional Regulation Additives
|
3,056,847 | 4,163,823 | ||||||
|
Herbal Medicinal Additives
|
293,454 | 281,036 | ||||||
|
Other
|
623,327 | 531,910 | ||||||
| $ | 28,561,829 | $ | 23,832,727 | |||||
|
Segment costs of sales
|
||||||||
|
Organic Trace Mineral Additives
|
$ | 15,719,425 | $ | 11,907,294 | ||||
|
Functional Regulation Additives
|
2,046,917 | 2,571,920 | ||||||
|
Herbal Medicinal Additives
|
269,844 | 219,856 | ||||||
|
Other
|
581,178 | 363,449 | ||||||
| $ | 18,617,364 | $ | 15,062,519 | |||||
|
Segment gross profit
|
||||||||
|
Organic Trace Mineral Additives
|
$ | 8,868,775 | $ | 6,948,664 | ||||
|
Functional Regulation Additives
|
1,009,931 | 1,591,903 | ||||||
|
Herbal Medicinal Additives
|
23,610 | 61,180 | ||||||
|
Other
|
42,149 | 168,461 | ||||||
| $ | 9,944,465 | $ | 8,770,208 | |||||
|
Name
|
Age
|
Position
|
Date Appointed
|
|||
|
Guixiong Qiu
|
46
|
Founder, CEO and Chairman of the Board of Directors
|
February 2010
|
|||
|
Xugang Shu
|
35
|
Vice President of Research and Development
|
February 2010
|
|||
|
Bo Jun
|
33
|
Marketing Director
|
February 2010
|
|||
|
Gilbert Lee
|
54
|
Chief Financial Officer
|
August 2011
|
|
Name and Principal
Position
|
Fiscal Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total
($)
|
||||||||||||||||||||||||
|
Guixiong Qiu
|
2012
|
$ |
47,520.00
|
$ |
47,520.00
|
$ |
95,040.00
|
||||||||||||||||||||||||||
|
Founder, CEO and Chairman
|
2011
|
$
|
46,350.00
|
46,350.00
|
-
|
-
|
-
|
-
|
-
|
$
|
92,700.00
|
||||||||||||||||||||||
|
Gilbert Lee (1)
|
2012
|
137,500.00
|
-
|
$ |
137,500.00
|
||||||||||||||||||||||||||||
|
Chief Financial Officer
|
2011
|
$
|
52,083.30
|
-
|
-
|
-
|
-
|
-
|
-
|
$
|
52,083.30
|
||||||||||||||||||||||
|
Xugang Shu
|
|||||||||||||||||||||||||||||||||
|
Vice President of Research
|
2012
|
$ |
31,680.00
|
$ |
39,600.00
|
$ |
71,280.00
|
||||||||||||||||||||||||||
|
And Development
|
2011
|
$
|
23,175.00
|
23,175.00
|
-
|
-
|
-
|
-
|
-
|
$
|
46,350.00
|
||||||||||||||||||||||
|
Bo Jun
|
2012
|
$ |
15,840.00
|
$ |
47,520.00
|
$ |
63,360.00
|
||||||||||||||||||||||||||
|
Marketing Director
|
2011
|
$
|
7,725.00
|
30,900.00
|
-
|
-
|
-
|
-
|
-
|
$
|
38,625.00
|
||||||||||||||||||||||
|
Name and Address
of Beneficial Owner (1)
|
Title
|
Shares of
Common Stock
Beneficially Owned
|
Percent of Class
Beneficially Owned (2)
|
|||||||
|
Directors and Executive Officers
|
||||||||||
|
Guixiong Qiu (3)
Room 401, No. 5 Building, Chashan Street
South China Agricultural University
No. 483 Wushan Road, Tianhe District
Guangzhou City, Guangdong
P.R. China
|
Chief Executive Officer and
Chairman of the Board of Directors
|
4,055,161
|
30.43
|
%
|
||||||
|
Xugang Shu
Room 401, No. 5 Building, Chashan Street
South China Agricultural University
No. 483 Wushan Road, Tianhe District
Guangzhou City, Guangdong
P.R. China
|
Vice President of Research and Development
|
0
|
0
|
%
|
||||||
|
Bo Jun
Room 401, No. 5 Building, Chashan Street
South China Agricultural University
No. 483 Wushan Road, Tianhe District
Guangzhou City, Guangdong
P.R. China
|
Director of Marketing
|
0
|
0
|
%
|
||||||
|
Gilbert Lee
23 Langdale Road
Wayne, New Jersey 07470
|
Chief Financial Officer
|
0
|
0
|
%
|
||||||
|
Officers and Directors as a Group (a total of four persons beneficially owning stock)
|
4,055,161
|
30.43
|
%
|
|||||||
|
5% Owners
|
||||||||||
|
Bi Gao (4)
Room 704, No. 10 Tianhe Guanghe Road
Guangzhou City, Guangdong
P.R. China
|
2,883,670
|
21.64
|
%
|
|||||||
|
Xiuzhen Liang (5)
Room 404, No. 10 Quan Fu Li
Fangcun District
Guangzhou City, Guangdong
P.R. China
|
1,802,294
|
13.53
|
%
|
|||||||
|
Golden Genesis Limited (6)(7)
Suite 2108, Nan Fung Tower
173 Des Voex Road
Hong Kong
|
9,011,469
|
67.63
|
%
|
|||||||
|
(1)
|
Under Rule 13d-3 of the Exchange Act, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights.
Golden Genesis has shared power to dispose, or direct the disposition, but no power to vote, or direct the vote of such shares, while the Tanke Shareholders have shared power to dispose, or direct the disposition, but sole power to vote, or direct the vote, of the shares held by Golden Genesis on behalf of Tanke Shareholders pursuant to the Call Option Agreement and Securities Escrow Agreement.
|
|
(2)
|
Based on 13,324,083 shares of common stock issued and outstanding as of March 30, 2013.
|
|
(3)
|
Includes (i) 3,605,161 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to Mr. Qiu pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Mr. Qiu without the prior written consent of Mr. Qiu, before all of such shares are transferred to Mr. Qiu; and (ii) 900,000 shares of common stock over which Mr. Qiu has voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that 450,000 shares will be disbursed in each of 2012 and 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Mr. Qiu), based on whether the Company achieves certain financial benchmarks for the fiscal years ending December 31, 2011 and 2012. Mr. Qiu is the CEO, President and sole director of the Company.
|
|
(4)
|
Includes (i) 2,563,670 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to Mr. Gao pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Mr. Gao without the prior written consent of Mr. Gao, before all of such shares are transferred to Mr. Gao; and (ii) 640,000 shares of common stock over which Mr. Gao has voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that 320,000 shares will be disbursed in each of 2012 and 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Mr. Gao), based on whether the Company achieves certain financial benchmarks for the fiscal years ending December 31, 2011 and 2012.
|
|
(5)
|
Includes (i) 1,602,294 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to Ms. Liang pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Ms. Liang without the prior written consent of Ms. Liang, before all of such shares are transferred to Ms. Liang; and (ii) 400,000 shares of common stock over which Ms. Liang has voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that 200,000 shares will be disbursed in each of 2012 and 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Ms. Liang), based on whether the Company achieves certain financial benchmarks for the fiscal years ending December 31, 2011 and 2012.
|
|
(6)
|
Includes an aggregate of 8,011,469 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to the Tanke Shareholders in accordance with the Call Option Agreement. Pursuant to the Call Option Agreement, Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of the Tanke Shareholders without the prior written consent of the Tanke Shareholders.
|
|
(7)
|
Includes an aggregate of 1,000,000 shares of common stock over which the Tanke Shareholders have voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that an aggregate of 1,000,000 shares will be disbursed in 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, to the Tanke Shareholders, on a pro rata basis), based on whether the Company achieves certain financial benchmarks for the fiscal year ending December 31, 2012.
We did not achieve the Adjusted Income of $7,571,111 for the year ended December 31, 2012, therefore 1,000,000 shares of common stock placed in escrow should be distributed to the Investors on a pro rata basis, pursuant to the terms of the Securities Escrow Agreement. We are in discussion with Euro Pacific and the investors on a possible waiver of the distribution or at a reduced quantity.
|
|
December 31,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Notes receivable from directors
|
$
|
-
|
$
|
239,149
|
||||
|
Others
|
-
|
327
|
||||||
|
$
|
-
|
$
|
239,476
|
|||||
|
|
Fiscal 2012
|
Fiscal 2011
|
||||||
|
Audit Fees
|
$
|
132,000
|
$
|
189,360
|
||||
|
Audit Related Fees
|
42,606
|
|||||||
|
Tax Fees
|
8,000
|
|||||||
|
All Other Fees
|
0
|
|||||||
|
Total
|
$
|
182,606
|
$
|
189,360
|
||||
|
Exhibit No.
|
Description
|
|
|
2.1
|
Share Exchange Agreement, dated January 3, 2011, by and among Greyhound Commissary, Inc. (as now known as Tanke Biosciences Corporation, the “Company”) Golden Genesis Limited (“Golden Genesis”) and China Flying Development Limited (“China Flying”) (1)
|
|
|
3.1
|
Articles of Incorporation of the Company (2)
|
|
|
3.2
|
Certificate of Amendment to Articles of Incorporation for reverse stock split (3)
|
|
|
3.3
|
Certificate of Amendment to Articles of Incorporation for change of corporate name (3)
|
|
|
3.4
|
Bylaws of the Company (2)
|
|
|
4.1
|
Form of Note issued to the investors (the “Investors”) in the private placement of 6,669,627 units (the “Private Placement”), dated February 9, 2011 (3)
|
|
|
4.2
|
Form of Warrant issued to the Investors in the Private Placement, dated February 9, 2011 (3)
|
|
|
4.3
|
Form of Agent Warrant issued to Euro Pacific Capital, Inc. and to Newbridge Securities Corporation, dated February 9, 2011 (3)
|
|
|
10.1
|
Securities Purchase Agreement, dated February 9, 2011, by and among the Company, the Investors and, with respect to certain sections thereof, Euro Pacific Capital, Inc. and Newbridge Securities Corporation (8)
|
|
|
10.2
|
Registration Rights Agreement, dated February 9, 2011, by and among the Company and the Investors (3)
|
|
|
10.3
|
Securities Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, Golden Genesis and Escrow, LLC, as escrow agent (3)
|
|
|
10.4
|
Interest Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, and Escrow, LLC, as escrow agent (3)
|
|
|
10.5
|
Consulting Services Agreement, dated January 3, 2011, between Guangzhou Tanke Industry Co., Ltd. (“Guangzhou Tanke”) and Guangzhou Kanghui Agricultural Technology Co., Ltd. (the “WFOE”) (3)
|
|
|
10.6
|
Operating Agreement, dated January 3, 2011, by and among Guixiong Qiu, Bi Gao, Xiuzhen Liang and Bing Teng (the “Tanke Shareholders”), Guangzhou Tanke and the WFOE (3)
|
|
|
10.7
|
Voting Rights Proxy Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (3)
|
|
|
10.8
|
Equity Pledge Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (3)
|
|
|
10.9
|
Option Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (3)
|
|
|
10.10
|
Call Option Agreement, dated January 3, 2011, between Golden Genesis, Wong Kwai Ho and the Tanke Shareholders (3)
|
|
|
10.11
|
Employment Agreement, dated July 26, 2011, by and between the Company and Gilbert Lee (4)
|
|
|
99.1
|
Unofficial English translation of land lease agreement dated April 15, 2006, between Guangzhou Baoyuhua Industry Co., Ltd. and Guangzhou Tanke (7)
|
|
|
99.2
|
Unofficial English translation of form of employment agreement (8)
|
|
|
99.3
|
Unofficial English translation of promissory note dated December 24, 2010 signed by Guixiong Qiu, Bi Gao and Xiuzhen Liang (6)
|
|
|
99.4
|
Unofficial English translation of Loan Agreement dated May 2009 (8)
|
|
|
21.1
|
List of the Company’s Subsidiaries (5)
|
|
| 23.1 |
Consent of EFP Rotenberg LLP *
|
|
|
31.1
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 *
|
|
|
31.2
|
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 *
|
|
|
32.1
|
Certification of Principal Executive Officer of the Company, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 **
|
|
|
32.2
|
Certification of Principal Financial Officer of the Company, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 **
|
|
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
*
|
Filed herewith
|
|
**
|
Furnished herewith
|
|
(1)
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on January 6, 2011.
|
|
(2)
|
Incorporated by reference to the Company’s Registration Statement on Form 10 filed with the SEC on December 16, 2008.
|
|
(3)
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(4)
|
Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on August 1, 2011.
|
|
(5)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1 filed with the SEC on February14, 2011.
|
|
(6)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A filed with the SEC on May 16, 2011.
|
|
(7)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A filed with the SEC on August 24, 2011.
|
|
(8)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1/A filed with the SEC on February 10, 2012.
|
|
TANKE BIOSCIENCES CORPORATION
|
|||
|
Date:
April 15, 2013
|
By:
|
/s/ Guixiong Qiu
|
|
|
Guixiong Qiu
|
|||
|
Chief Executive Officer (Duly Authorized Officer and Principal Executive Officer
)
|
|||
|
Date:
April 15, 2013
|
By:
|
/s/ Gilbert Lee
|
|
|
Gilbert Lee
|
|||
|
Chief Financial Officer (Principal Financial Officer)
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/ Guixiong Qiu
|
||||
|
Guixiong Qiu
|
|
Chief Executive Officer and
Chairman of the Board of Directors
(Principal Executive Officer)
|
April 15, 2013
|
|
|
/s/ Gilbert Lee
|
||||
|
Gilbert Lee
|
Chief Financial Officer (Principal Financial Officer)
|
April 15, 2013
|
||