|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michigan
|
|
94-3096597
|
|
(State or other jurisdiction of
|
|
(I.R.S. Employer
|
|
incorporation or organization)
|
|
Identification No.)
|
|
Title of Class
|
|
Name of Each Exchange on Which Registered
|
||
|
Common Stock (No par value)
|
|
The NASDAQ Stock Market, Inc.
|
||
|
Large accelerated filer -
o
|
|
Accelerated filer -
x
|
|
Non-accelerated filer -
o
|
|
Smaller reporting company -
o
|
|
(Do not check if a smaller reporting company)
|
|
|
|
Document
|
|
Form 10-K Reference
|
|
Proxy Statement for the Annual Meeting of Shareholders scheduled for May 4, 2016
|
|
Items 10, 11, 12, 13 and 14 of Part III
|
|
|
|
Page
|
|
|
PART I
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
|
PART II
|
|
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
|
PART III
|
|
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
|
PART IV
|
|
|
Item 15.
|
||
|
|
|
|
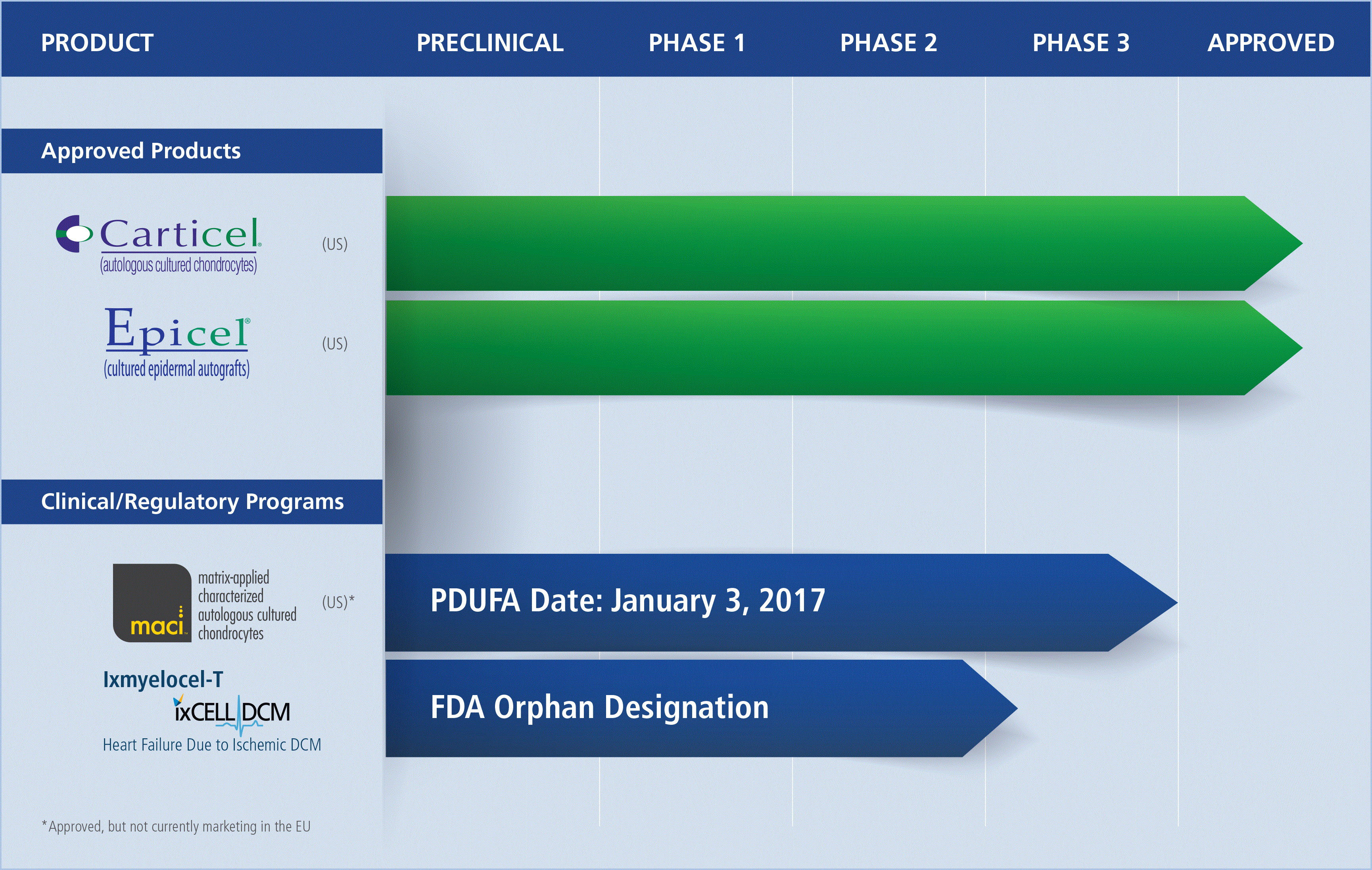
|
•
|
Fully integrate the acquired commercial stage CTRM business and improve efficiencies to reduce redundancies and related costs, as well as take advantage of complementary technology platforms;
|
|
•
|
Increase the operating income from the U.S. Carticel and Epicel business and become profitable without raising additional equity capital unless required for additional strategic transactions or other events;
|
|
•
|
Lower the manufacturing costs for Carticel through an improved ratio of Carticel unit sales to biopsies as well as other efficiencies;
|
|
•
|
Assess and capitalize on opportunities to increase revenue from Carticel and Epicel in the U.S.;
|
|
•
|
Develop and execute on a regulatory strategy for the approval of MACI in the U.S.;
|
|
•
|
Expand Epicel usage in the severely burned patient segment by increasing sales and marketing resources;
|
|
•
|
Capitalize on our recent U.S. Food and Drug Administration (FDA) approval to label Epicel for use in pediatric patients and the related determination from the FDA that Epicel meets the criteria to be sold for profit; and
|
|
•
|
Initiate and complete our pivotal phase 3 clinical trials for the treatment of the orphan indication, advanced heart failure due to ischemic DCM, and evaluate potential strategic collaborations.
|
|
•
|
The device is intended for the treatment or diagnosis of a disease or condition that occurs in pediatric patients or in a pediatric subpopulation, and such device is labeled for use in pediatric patients or in a pediatric subpopulation in which the disease or condition occurs; or
|
|
•
|
The device is intended for the treatment or diagnosis of a disease or condition that does not occur in pediatric patients or that occurs in pediatric patients in such numbers that the development of the device for such patients is impossible, highly impracticable or unsafe.
|
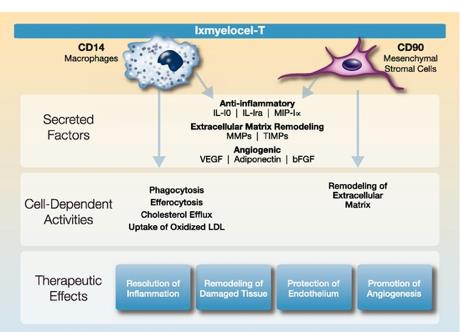
|
•
|
Patient-specific (autologous)
— We start with the patient’s own cells, which are accepted by the patient’s immune system, allowing the cells to integrate into existing functional tissues. We believe that this characteristic of our therapy eliminates both the risk of rejection and the need to use immunosuppressive therapy pre- or post-therapy. Our data also suggests that ixmyelocel-T may provide the potential for long-term engraftment and tissue repair.
|
|
•
|
Expanded
— We begin with a small amount of bone marrow from the patient (up to 60 ml) and significantly expand the number of certain cell types, primarily MSCs and M2-like anti-inflammatory macrophages, to a substantially greater number than are present in the patient’s own bone marrow (up to 200 times the number of certain cell types compared with the starting bone marrow).
|
|
•
|
Multicellular
— We believe the multiple cell types in ixmyelocel-T, which are normally found in bone marrow but in smaller quantities, possess the key functions required for reducing chronic inflammation and promoting angiogenesis and tissue repair. By reducing inflammation, we believe that ixmyelocel-T provides the ideal conditions to allow for the growth of new tissue and blood vessels.
|
|
•
|
Minimally invasive
— Our procedure for collecting bone marrow can be performed in an out-patient setting and takes approximately 15 minutes. Administration of ixmyelocel-T for the treatment of advanced heart failure due to ischemic dilated cardiomyopathy is performed in the cardiac catheterization laboratory using a cell injection catheter system in a one-time procedure. Bone marrow and bone marrow-derived therapies have been used safely and efficaciously in medicine for over three decades. Ixmyelocel-T leverages this body of scientific study and medical experience, and appears well tolerated in over 200 patients treated to date.
|
|
•
|
Phase 1—The biological product is initially tested for safety and tolerability. In the case of biological products and those for severe or life-threatening diseases, the initial human testing is generally conducted in patients. These trials may also provide early evidence on effectiveness.
|
|
•
|
Phase 2—These trials are conducted in a limited number of subjects in the target population to determine a safe and effective dosage to evaluate in Phase 3 and to identify possibly related adverse effects and safety risks. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
|
|
•
|
Phase 3—Phase 3 trials are undertaken to provide evidence of clinical efficacy and to further evaluate dosage, potency, and safety in an expanded patient population at multiple clinical trial sites. Phase 3 studies are performed after preliminary evidence suggesting effectiveness of the product has been obtained, and are intended to establish the overall benefit-risk relationship of the investigational product, and to provide an adequate basis for product approval and labeling.
|
|
•
|
Obtaining regulatory approval to commence a trial;
|
|
•
|
Reaching agreement with third-party clinical trial sites and their subsequent performance in conducting accurate and reliable trials on a timely basis;
|
|
•
|
Obtaining IRB approval to conduct a trial at a prospective site;
|
|
•
|
Recruiting patients to participate in a trial; and
|
|
•
|
Obtaining supply of the biological product.
|
|
•
|
A product comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced as a single entity;
|
|
•
|
Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products;
|
|
•
|
A drug, or device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, or device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or
|
|
•
|
Any investigational drug, or device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect.
|
|
Name
|
Position
|
Age
|
Executive
Officer Since
|
|||
|
Dominick C. Colangelo (1)
|
President and Chief Executive Officer
|
53
|
2013
|
|||
|
Daniel R. Orlando (1)
|
Chief Operating Officer
|
50
|
2012
|
|||
|
David Recker, MD
|
Chief Medical Officer
|
58
|
2013
|
|||
|
Gerard Michel (1)
|
Chief Financial Officer & Vice President of Corporate Development
|
52
|
2014
|
|||
|
Ross Tubo, PhD
|
Chief Scientific Officer
|
56
|
2014
|
|||
|
•
|
The rate and degree of progress of our product development;
|
|
•
|
t
The ability to maintain our manufacturing facility's compliance with U.S. Food and Drug Administration (FDA) requirements including establishment and license fees;
|
|
•
|
The rate of regulatory approval to proceed with clinical development programs;
|
|
•
|
The level of success achieved in clinical trials;
|
|
•
|
The requirements to maintain marketing authorization and licenses from regulatory bodies in the United States and other countries in good standing;
|
|
•
|
The liquidity and market volatility of our equity securities;
|
|
•
|
Regulatory and manufacturing requirements and uncertainties; and
|
|
•
|
Staying ahead of technological developments by competitors.
|
|
•
|
Our ability to further commercialize our products;
|
|
•
|
The rate and degree of progress of our product development;
|
|
•
|
The rate of regulatory approval to proceed with clinical developmental programs;
|
|
•
|
The level of success achieved in clinical trials;
|
|
•
|
The requirements for marketing authorization from regulatory bodies in the United States and other countries;
|
|
•
|
The liquidity and market volatility of our equity securities; and
|
|
•
|
Regulatory and manufacturing requirements and uncertainties, and technological developments by competitors.
|
|
•
|
Does not demonstrate acceptable safety and efficacy in clinical trials, or otherwise does not meet applicable regulatory requirements for regulatory approval;
|
|
•
|
Does not offer sufficient, clinically meaningful therapeutic benefit over the standard of care/ existing therapies;
|
|
•
|
Cannot be produced in commercial quantities at an acceptable costs;
|
|
•
|
Is not accepted as a safe, efficacious, and cost-effective treatment over the standard of care and/or current therapies by the medical community and third-party payers.
|
|
•
|
The clinical safety and effectiveness of our products and their demonstrated advantage over alternative treatment methods;
|
|
•
|
Our ability to demonstrate to healthcare providers that our products provide a therapeutic advancement over standard of care or other competitive products / methods;
|
|
•
|
Our ability to educate healthcare providers on the autologous use of patient-specific human tissue, to avoid potential confusion with and differentiate ourselves from the ethical controversies associated with human fetal tissue and engineered human tissue;
|
|
•
|
Our ability to educate healthcare providers, patients and payers on the safety and adverse reactions involving our products;
|
|
•
|
Our ability to meet supply and demand and develop a core group of medical professionals familiar with and committed to the use of our products; and
|
|
•
|
Delays in securing clinical investigators or trial sites for our clinical trials;
|
|
•
|
Delays in obtaining Institutional Review Board (IRB) and other regulatory approvals to commence a clinical trial;
|
|
•
|
Slower than anticipated rates of patient recruitment and enrollment in our clinical trials, or failing to reach the targeted number of patients due to competition for patients from other trials;
|
|
•
|
Limited or no availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third party payers for the use of biological products supplied for use in our clinical trials;
|
|
•
|
Negative or inconclusive results from clinical trials;
|
|
•
|
Unforeseen adverse effects interrupting, delaying, or halting clinical trials of any future therapeutic product candidates, and possibly resulting in the FDA or other regulatory authorities denying approval of any future therapeutic product candidates;
|
|
•
|
Unforeseen safety issues;
|
|
•
|
Approval and introduction of new therapies or changes in standards of practice or regulatory requirements or guidance that render our clinical trial endpoints or the targeting of our proposed indications obsolete;
|
|
•
|
Inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols;
|
|
•
|
Inability to replicate in large controlled trials safety and efficacy data obtained from a limited number of patients in uncontrolled trials;
|
|
•
|
Inability or unwillingness of medical investigators to follow our clinical protocols; and
|
|
•
|
Unavailability of clinical trial supplies.
|
|
•
|
The recall or seizure of products;
|
|
•
|
The suspension or revocation of the authority necessary for the production or sale of a product;
|
|
•
|
The suspension of shipments from particular manufacturing facilities;
|
|
•
|
The imposition of fines and penalties;
|
|
•
|
The delay of our ability to introduce new products into the market;
|
|
•
|
Our exclusion or the exclusion of our products from being reimbursed by federal and state healthcare programs (such as Medicare, Medicaid, Veterans Administration, or VA, health programs and Civilian Health and Medical Program Uniformed Service, or CHAMPUS); and
|
|
•
|
Other civil or criminal prosecution or sanctions against us or our employees, such as fines, penalties or imprisonment.
|
|
•
|
Untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
Unanticipated expenditures to address or defend such actions;
|
|
•
|
Client notifications for repair, replacement, or refunds of a device;
|
|
•
|
Recall, detention or seizure of our products;
|
|
•
|
Operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
Denying, refusing or delaying our requests for approval of new products or proposed changes to existing products;
|
|
•
|
Operating restrictions;
|
|
•
|
Withdrawing product approvals that have already been granted;
|
|
•
|
Refusal to approve a pending marketing application, such as a BLA or supplements to a BLA submitted by us;
|
|
•
|
Refusal to grant export approval for our products; or
|
|
•
|
Criminal prosecution.
|
|
•
|
An annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic products, apportioned among these entities according to their market share in certain government healthcare programs;
|
|
•
|
Expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level, thereby potentially increasing both the volume of sales and manufacturers’ Medicaid rebate liability;
|
|
•
|
Expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
|
•
|
New requirements to report certain financial arrangements with physicians and teaching hospitals, as defined in the Affordable Care Act and its implementing regulations, including reporting any “transfer of value” made or distributed to physicians and teaching hospitals and reporting any ownership and investment interests held by physicians and their immediate family members and applicable group purchasing organizations during the preceding calendar year, with data collection required and reporting to the Centers for Medicare & Medicaid Services (CMS) required by the 90th day of each calendar year;
|
|
•
|
|
|
•
|
Expansion of health care fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;
|
|
•
|
A licensure framework for follow-on biologic products;
|
|
•
|
A new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research;
|
|
•
|
Creation of the Independent Payment Advisory Board which, beginning in 2014, has authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription products and those recommendations could have the effect of law even if Congress does not act on the recommendations; and
|
|
•
|
Establishment of a Center for Medicare Innovation at the CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending.
|
|
•
|
Others may be able to make products that are the same as or similar to our products or product candidates, but that are not covered by the claims of the patents that we own or have exclusively licensed;
|
|
•
|
We or any strategic partners might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or have exclusively licensed;
|
|
•
|
We might not have been the first to file patent applications covering certain of our inventions;
|
|
•
|
Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
|
|
•
|
It is possible that our pending patent applications will not lead to issued patents;
|
|
•
|
Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges;
|
|
•
|
Our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
|
|
•
|
We may not develop additional proprietary technologies that are patentable; and
|
|
•
|
The patents of others may have an adverse effect on our business.
|
|
•
|
Significant awards against us;
|
|
•
|
Substantial litigation costs;
|
|
•
|
Recall of the product;
|
|
•
|
Injury to our reputation;
|
|
•
|
Withdrawal of clinical trial participants; or
|
|
•
|
Adverse regulatory action.
|
|
•
|
Clinical trial results;
|
|
•
|
The amount of our cash resources and our ability to obtain additional funding;
|
|
•
|
Announcements of research activities, business developments, technological innovations or new products by us or our competitors;
|
|
•
|
Entering into or terminating strategic relationships;
|
|
•
|
Regulatory developments in both the United States and abroad;
|
|
•
|
Disputes concerning patents or proprietary rights;
|
|
•
|
Changes in our revenues or expense levels;
|
|
•
|
Seasonal or other variations in patient demand for Carticel and Epicel;
|
|
•
|
Public concern regarding the safety, efficacy or other aspects of the products or methodologies we are developing;
|
|
•
|
News or reports from other stem cell, cell therapy or regenerative medicine companies;
|
|
•
|
Reports by securities analysts;
|
|
•
|
Status of the investment markets;
|
|
•
|
Concerns related to management transitions; and
|
|
•
|
Delisting from The NASDAQ Capital Market.
|
|
•
|
convey, sell, lease or otherwise dispose of certain parts of our business or property;
|
|
•
|
change the nature of our business;
|
|
•
|
liquidate or dissolve;
|
|
•
|
enter into certain change in control or acquisition transactions;
|
|
•
|
incur or assume certain debt;
|
|
•
|
grant certain types of liens on our assets;
|
|
•
|
maintain certain collateral accounts;
|
|
•
|
pay dividends or make certain distributions to our stockholders;
|
|
•
|
make certain investments;
|
|
•
|
enter into material transactions with affiliates;
|
|
•
|
make or permit certain payments on subordinate debt; and
|
|
•
|
become an “investment company” as defined under the Investment Company Act of 1940, as amended.
|
|
•
|
That a broker or dealer approve a person’s account for transactions in penny stocks; and
|
|
•
|
The broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
|
|
•
|
Obtain financial information and investment experience objectives of the person; and
|
|
•
|
Make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
|
|
•
|
Sets forth the basis on which the broker or dealer made the suitability determination; and
|
|
•
|
That the broker or dealer received a signed, written agreement from the investor prior to the transaction.
|
|
|
High
|
Low
|
||||||
|
Year ended December 31, 2014
|
|
|
|
|
||||
|
First Quarter
|
$
|
6.49
|
|
$
|
3.31
|
|
||
|
Second Quarter
|
5.05
|
|
3.51
|
|
||||
|
Third Quarter
|
4.08
|
|
2.61
|
|
||||
|
Fourth Quarter
|
3.04
|
|
2.68
|
|
||||
|
Year ended December 31, 2015
|
|
|
|
|
||||
|
First Quarter
|
$
|
3.95
|
|
$
|
2.75
|
|
||
|
Second Quarter
|
3.76
|
|
3.02
|
|
||||
|
Third Quarter
|
3.61
|
|
2.40
|
|
||||
|
Fourth Quarter
|
2.71
|
|
1.71
|
|
||||
|
|
Number of Securities
to be Issued upon Exercise
of Outstanding Options,
Warrants and Rights
|
Weighted Average
Exercise Price of
Outstanding
Options, Warrants
and Rights
|
Number of Securities
Remaining Available
for Future Issuance
Under Equity
Compensation Plans
(2)
|
|||||||
|
Equity compensation plans approved by security holders (employees and directors)
(1)
|
2,523,400
|
|
$
|
6.36
|
|
1,962,168
|
|
|||
|
Employee stock purchase plan
(1)
|
63,193
|
|
$
|
2.19
|
|
936,807
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
(In thousands, except per share amounts)
|
2015
|
2014
|
2013
|
2012
|
2011
|
|||||||||||||||
|
Revenues:
|
|
|
|
|
|
|
||||||||||||||
|
Product sales
(a)
|
$
|
51,168
|
|
$
|
28,796
|
|
$
|
19
|
|
$
|
21
|
|
$
|
18
|
|
|||||
|
Total revenues
|
51,168
|
|
28,796
|
|
19
|
|
21
|
|
18
|
|
||||||||||
|
Costs and expenses:
|
|
|
|
|
|
|
||||||||||||||
|
Cost of product sales
(a)
|
26,470
|
|
17,293
|
|
4
|
|
6
|
|
4
|
|
||||||||||
|
Gross profit
|
24,698
|
|
11,503
|
|
15
|
|
15
|
|
14
|
|
||||||||||
|
Research and development
|
18,890
|
|
21,263
|
|
15,104
|
|
26,025
|
|
21,330
|
|
||||||||||
|
Selling, general and administrative
|
22,479
|
|
13,774
|
|
5,875
|
|
7,750
|
|
7,724
|
|
||||||||||
|
Total operating expenses
|
41,369
|
|
35,037
|
|
20,979
|
|
33,775
|
|
29,054
|
|
||||||||||
|
Loss from operations
|
(16,671
|
)
|
(23,534
|
)
|
(20,964
|
)
|
(33,760
|
)
|
(29,040
|
)
|
||||||||||
|
Other income (expense):
|
|
|
|
|
|
|
||||||||||||||
|
(Increase) decrease in fair value of warrants
(b)
|
324
|
|
(27
|
)
|
5,337
|
|
4,248
|
|
9,329
|
|
||||||||||
|
Bargain purchase gain
(c)
|
—
|
|
3,473
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Foreign currency translation gain (loss)
|
(67
|
)
|
152
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Interest income
|
36
|
|
24
|
|
16
|
|
50
|
|
53
|
|
||||||||||
|
Other income (expense)
|
47
|
|
(2
|
)
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Interest expense
|
(9
|
)
|
(6
|
)
|
(11
|
)
|
(12
|
)
|
(10
|
)
|
||||||||||
|
Total other income (expense)
|
331
|
|
3,614
|
|
5,342
|
|
4,286
|
|
9,372
|
|
||||||||||
|
Net loss
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
$
|
(29,474
|
)
|
$
|
(19,668
|
)
|
|||||
|
Net loss per share attributable to common shareholders (Basic and Diluted)
|
$
|
(0.97
|
)
|
$
|
(2.23
|
)
|
$
|
(6.95
|
)
|
$
|
(16.25
|
)
|
$
|
(10.18
|
)
|
|||||
|
(a) Revenue from commercial operations began in June 2014 following the acquisition of the CTRM business. Prior to June 2014, we were a development stage entity.
|
||||||||||||||||||||
|
(b) Fluctuations in the fair value of the warrants are due to the reduction in the time to maturity and changes in our stock price.
|
||||||||||||||||||||
|
(c) The bargain purchase gain is a result of the CTRM business acquisition.
|
||||||||||||||||||||
|
|
December 31,
|
|||||||||||||||||||
|
(In thousands, except per share amounts)
|
2015
|
2014
|
2013
|
2012
|
2011
|
|||||||||||||||
|
Cash
|
$
|
14,581
|
|
$
|
30,343
|
|
$
|
8,059
|
|
$
|
13,638
|
|
$
|
5,530
|
|
|||||
|
Working capital (deficit)
|
15,235
|
|
29,661
|
|
3,155
|
|
8,331
|
|
(14,495
|
)
|
||||||||||
|
Property and equipment, net
|
4,049
|
|
2,892
|
|
739
|
|
1,188
|
|
1,564
|
|
||||||||||
|
Total assets
|
34,309
|
|
47,579
|
|
9,215
|
|
15,178
|
|
7,739
|
|
||||||||||
|
Total liabilities
|
12,179
|
|
11,938
|
|
5,321
|
|
5,665
|
|
20,710
|
|
||||||||||
|
Total shareholders' equity (deficit)
|
22,130
|
|
35,641
|
|
3,894
|
|
(32,100
|
)
|
(12,971
|
)
|
||||||||||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Net revenues
|
$
|
51,168
|
|
$
|
28,796
|
|
$
|
19
|
|
|||
|
Cost of product sales
|
26,470
|
|
17,293
|
|
4
|
|
||||||
|
Gross profit
|
24,698
|
|
11,503
|
|
15
|
|
||||||
|
Total operating expenses
|
41,369
|
|
35,037
|
|
20,979
|
|
||||||
|
Loss from operations
|
(16,671
|
)
|
(23,534
|
)
|
(20,964
|
)
|
||||||
|
Other income (expense)
|
331
|
|
141
|
|
5,342
|
|
||||||
|
Bargain purchase gain
|
—
|
|
3,473
|
|
—
|
|
||||||
|
Total other income
|
331
|
|
3,614
|
|
5,342
|
|
||||||
|
Net loss
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Revenue by product (In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Carticel
|
$
|
35,203
|
|
$
|
22,267
|
|
$
|
—
|
|
|||
|
Epicel
|
15,242
|
|
5,989
|
|
—
|
|
||||||
|
Bone Marrow
|
714
|
|
354
|
|
—
|
|
||||||
|
MACI
|
9
|
|
186
|
|
—
|
|
||||||
|
Other
|
—
|
|
—
|
|
19
|
|
||||||
|
|
$
|
51,168
|
|
$
|
28,796
|
|
$
|
19
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
(In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Gross profit
|
$
|
24,698
|
|
$
|
11,503
|
|
$
|
15
|
|
|||
|
Gross profit %
|
48.3
|
%
|
39.9
|
%
|
78.9
|
%
|
||||||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Research and development costs
|
$
|
18,890
|
|
$
|
21,263
|
|
$
|
15,104
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Dilated Cardiomyopathy
|
$
|
8,937
|
|
$
|
15,099
|
|
$
|
7,881
|
|
|||
|
Critical Limb Ischemia
|
—
|
|
801
|
|
7,223
|
|
||||||
|
MACI
|
5,497
|
|
3,752
|
|
—
|
|
||||||
|
Carticel
|
2,798
|
|
1,008
|
|
—
|
|
||||||
|
Epicel
|
1,658
|
|
603
|
|
—
|
|
||||||
|
Total research and development expenses
|
$
|
18,890
|
|
$
|
21,263
|
|
$
|
15,104
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Selling, general and administrative costs
|
$
|
22,479
|
|
$
|
13,774
|
|
$
|
5,875
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
(Increase) decrease in fair value of warrants
|
$
|
324
|
|
$
|
(27
|
)
|
$
|
5,337
|
|
|||
|
Bargain purchase gain
|
—
|
|
3,473
|
|
—
|
|
||||||
|
Foreign currency translation gain (loss)
|
(67
|
)
|
152
|
|
—
|
|
||||||
|
Interest income
|
36
|
|
24
|
|
16
|
|
||||||
|
Other income (expense)
|
47
|
|
(2
|
)
|
—
|
|
||||||
|
Interest expense
|
(9
|
)
|
(6
|
)
|
(11
|
)
|
||||||
|
Total other income (expense)
|
$
|
331
|
|
$
|
3,614
|
|
$
|
5,342
|
|
|||
|
|
Years Ended December 31,
|
|||||||||||
|
(in thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Cost of goods sold
|
$
|
308
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Research and development
|
555
|
|
197
|
|
75
|
|
||||||
|
General, selling and administrative
|
1,884
|
|
642
|
|
851
|
|
||||||
|
Total non-cash stock-based compensation expense
|
$
|
2,747
|
|
$
|
839
|
|
$
|
926
|
|
|||
|
|
|
Payments Due by Period
|
||||||||||||||||||||||||||
|
Contractual Obligations
|
Total
|
2016
|
2017
|
2018
|
2019
|
2020
|
More than
5 Years |
|||||||||||||||||||||
|
Operating leases
|
$
|
27,693
|
|
$
|
4,310
|
|
$
|
4,890
|
|
$
|
4,572
|
|
$
|
4,260
|
|
$
|
4,386
|
|
$
|
5,275
|
|
|||||||
|
Purchase commitments
|
300
|
|
300
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Capital leases
|
118
|
|
43
|
|
43
|
|
32
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Total
|
$
|
28,111
|
|
$
|
4,653
|
|
$
|
4,933
|
|
$
|
4,604
|
|
$
|
4,260
|
|
$
|
4,386
|
|
$
|
5,275
|
|
|||||||
|
|
Page
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
Consolidated Balance Sheets as of December 31, 2015 and December 31, 2014
|
|
|
Consolidated Statements of Operations for the years ended December 31, 2015, 2014 and 2013
|
|
|
Consolidated Statements of Shareholders’ Equity from December 31, 2013 to December 31, 2015
|
|
|
Consolidated Statements of Comprehensive Loss
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2015, 2014 and 2013
|
|
|
Notes to Consolidated Financial Statements
|
|
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
||||||
|
ASSETS
|
|
|
|
|
||||
|
Current assets:
|
|
|
|
|
||||
|
Cash
|
$
|
14,581
|
|
$
|
30,343
|
|
||
|
Accounts receivable (net of allowance for doubtful accounts of $68 and $40, respectively)
|
10,919
|
|
8,191
|
|
||||
|
Inventory
|
1,379
|
|
1,920
|
|
||||
|
Other current assets
|
464
|
|
1,036
|
|
||||
|
Total current assets
|
27,343
|
|
41,490
|
|
||||
|
Property and equipment, net
|
4,049
|
|
2,892
|
|
||||
|
Intangible assets
|
2,917
|
|
3,197
|
|
||||
|
Total assets
|
$
|
34,309
|
|
$
|
47,579
|
|
||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|
|
|
|
||||
|
Current liabilities:
|
|
|
|
|
||||
|
Accounts payable
|
$
|
7,588
|
|
$
|
5,824
|
|
||
|
Accrued expenses
|
3,603
|
|
4,714
|
|
||||
|
Warrant liabilities
|
757
|
|
1,081
|
|
||||
|
Other
|
160
|
|
210
|
|
||||
|
Total current liabilities
|
12,108
|
|
11,829
|
|
||||
|
Long term debt
|
71
|
|
109
|
|
||||
|
Total liabilities
|
12,179
|
|
11,938
|
|
||||
|
COMMITMENTS AND CONTINGENCIES (Note 16)
|
|
|
|
|
||||
|
Shareholders’ equity:
|
|
|
|
|
||||
|
Series A non-voting convertible preferred stock, no par value: shares authorized and reserved — 1; shares issued and outstanding — 1
|
3,150
|
|
—
|
|
||||
|
Series B-2 voting convertible preferred stock, no par value: shares authorized and reserved — 39, shares issued and outstanding — 12
|
38,389
|
|
38,389
|
|
||||
|
Common stock, no par value; shares authorized — 75,000; shares issued and outstanding — 23,789 and 23,786, respectively
|
307,766
|
|
305,008
|
|
||||
|
Treasury stock — 1,250 shares
|
(3,150
|
)
|
—
|
|
||||
|
Other comprehensive loss
|
—
|
|
(71
|
)
|
||||
|
Accumulated deficit
|
(324,025
|
)
|
(307,685
|
)
|
||||
|
Total shareholders’ equity
|
22,130
|
|
35,641
|
|
||||
|
Total liabilities and shareholders’ equity
|
$
|
34,309
|
|
$
|
47,579
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Revenues:
|
|
|
|
|
|
|
||||||
|
Product sales
|
$
|
51,168
|
|
$
|
28,796
|
|
$
|
19
|
|
|||
|
Total revenues
|
51,168
|
|
28,796
|
|
19
|
|
||||||
|
Costs and expenses:
|
|
|
|
|
|
|
||||||
|
Cost of product sales
|
26,470
|
|
17,293
|
|
4
|
|
||||||
|
Gross profit
|
24,698
|
|
11,503
|
|
15
|
|
||||||
|
Research and development
|
18,890
|
|
21,263
|
|
15,104
|
|
||||||
|
Selling, general and administrative
|
22,479
|
|
13,774
|
|
5,875
|
|
||||||
|
Total operating expenses
|
41,369
|
|
35,037
|
|
20,979
|
|
||||||
|
Loss from operations
|
(16,671
|
)
|
(23,534
|
)
|
(20,964
|
)
|
||||||
|
Other income (expense):
|
|
|
|
|
|
|
||||||
|
(Increase) decrease in fair value of warrants
|
324
|
|
(27
|
)
|
5,337
|
|
||||||
|
Bargain purchase gain
|
—
|
|
3,473
|
|
—
|
|
||||||
|
Foreign currency translation gain (loss)
|
(67
|
)
|
152
|
|
—
|
|
||||||
|
Interest income
|
36
|
|
24
|
|
16
|
|
||||||
|
Other income (expense)
|
47
|
|
(2
|
)
|
—
|
|
||||||
|
Interest expense
|
(9
|
)
|
(6
|
)
|
(11
|
)
|
||||||
|
Total other income (expense)
|
331
|
|
3,614
|
|
5,342
|
|
||||||
|
Net loss
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||
|
Net loss per share attributable to common shareholders (Basic and Diluted) (see note 10)
|
$
|
(0.97
|
)
|
$
|
(2.23
|
)
|
$
|
(6.95
|
)
|
|||
|
Weighted average number of common shares outstanding (Basic and Diluted)
|
23,760
|
|
11,642
|
|
3,016
|
|
||||||
|
|
Preferred Stock
|
Common Stock
|
Treasury Stock
|
Accumulated
Other Comprehensive |
Accumulated
|
Total
Shareholders’ |
|||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Loss
|
Deficit
|
Equity
|
||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2013
|
12
|
|
$
|
38,389
|
|
4,723
|
|
$
|
253,270
|
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(287,765
|
)
|
$
|
3,894
|
|
|||||||||
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
(19,920
|
)
|
(19,920
|
)
|
|||||||||||||||||
|
Compensation expense related to stock options granted
|
|
|
|
|
|
|
839
|
|
|
|
|
|
839
|
|
|||||||||||||||||||
|
Exercise of stock purchase warrants
|
|
|
|
|
408
|
|
2,490
|
|
|
|
|
|
2,490
|
|
|||||||||||||||||||
|
Issuance of common stock, net of issuance costs of $3,167
|
|
|
|
|
18,655
|
|
48,409
|
|
|
|
|
|
48,409
|
|
|||||||||||||||||||
|
Foreign currency translation adjustment
|
|
|
|
|
|
|
(71
|
)
|
|
(71
|
)
|
||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2014
|
12
|
|
$
|
38,389
|
|
23,786
|
|
$
|
305,008
|
|
—
|
|
$
|
—
|
|
(71
|
)
|
$
|
(307,685
|
)
|
$
|
35,641
|
|
||||||||||
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
(16,340
|
)
|
(16,340
|
)
|
|||||||||||||||||||
|
Common stock exchanged for preferred stock and held in treasury shares
|
1
|
|
3,150
|
|
|
|
|
|
(1,250
|
)
|
(3,150
|
)
|
—
|
|
|||||||||||||||||||
|
Compensation expense related to stock options granted
|
2,747
|
|
|
|
|
|
|
|
2,747
|
|
|||||||||||||||||||||||
|
Stock option exercises
|
3
|
|
11
|
|
|
|
|
|
|
|
|
|
11
|
|
|||||||||||||||||||
|
Foreign currency translation adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
71
|
|
|
|
71
|
|
|||||||||||||||
|
BALANCE, DECEMBER 31, 2015
|
13
|
|
$
|
41,539
|
|
23,789
|
|
$
|
307,766
|
|
(1,250
|
)
|
$
|
(3,150
|
)
|
$
|
—
|
|
$
|
(324,025
|
)
|
$
|
22,130
|
|
|||||||||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Net loss
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||
|
Other comprehensive loss
|
|
|
|
|
|
|
||||||
|
Foreign currency translation
|
71
|
|
(71
|
)
|
—
|
|
||||||
|
Comprehensive loss
|
$
|
(16,269
|
)
|
$
|
(19,991
|
)
|
$
|
(15,622
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Operating activities:
|
|
|
|
|
|
|
||||||
|
Net loss
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used for operating activities:
|
|
|
|
|
|
|
||||||
|
Depreciation and amortization
|
1,592
|
|
752
|
|
489
|
|
||||||
|
Stock compensation expense
|
2,747
|
|
839
|
|
926
|
|
||||||
|
Inventory provision
|
627
|
|
—
|
|
—
|
|
||||||
|
Change in fair value of warrants
|
(324
|
)
|
27
|
|
(5,337
|
)
|
||||||
|
Bargain purchase gain
|
—
|
|
(3,473
|
)
|
—
|
|
||||||
|
Foreign currency translation loss (gain)
|
67
|
|
(152
|
)
|
—
|
|
||||||
|
(Gain) loss on sale of fixed assets
|
(35
|
)
|
139
|
|
—
|
|
||||||
|
Write down of asset retirement obligation
|
(268
|
)
|
(1,102
|
)
|
—
|
|
||||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
||||||
|
Inventory
|
(86
|
)
|
119
|
|
—
|
|
||||||
|
Accounts receivable
|
(2,728
|
)
|
(8,139
|
)
|
—
|
|
||||||
|
Other current assets
|
572
|
|
(455
|
)
|
(65
|
)
|
||||||
|
Accounts payable
|
1,726
|
|
2,773
|
|
(571
|
)
|
||||||
|
Accrued expenses
|
(764
|
)
|
3,007
|
|
237
|
|
||||||
|
Asset retirement obligation
|
(80
|
)
|
—
|
|
—
|
|
||||||
|
Other non-current assets and liabilities, net
|
(52
|
)
|
175
|
|
—
|
|
||||||
|
Net cash used for operating activities
|
(13,346
|
)
|
(25,410
|
)
|
(19,943
|
)
|
||||||
|
Investing activities:
|
|
|
|
|
|
|
||||||
|
Acquisition of CTRM business, net of cash acquired
|
—
|
|
(1,450
|
)
|
—
|
|
||||||
|
Expenditures for property, plant and equipment
|
(2,427
|
)
|
(829
|
)
|
(40
|
)
|
||||||
|
Other
|
35
|
|
101
|
|
—
|
|
||||||
|
Net cash used for investing activities
|
(2,392
|
)
|
(2,178
|
)
|
(40
|
)
|
||||||
|
Financing activities:
|
|
|
|
|
|
|
||||||
|
Net proceeds from issuance of common stock and warrants
|
11
|
|
49,934
|
|
14,438
|
|
||||||
|
Payments on long-term debt
|
(35
|
)
|
(8
|
)
|
(34
|
)
|
||||||
|
Net cash provided by financing activities
|
(24
|
)
|
49,926
|
|
14,404
|
|
||||||
|
Effect of exchange rate changes on cash
|
—
|
|
(54
|
)
|
—
|
|
||||||
|
Net increase (decrease) in cash
|
(15,762
|
)
|
22,284
|
|
(5,579
|
)
|
||||||
|
Cash at beginning of period
|
30,343
|
|
8,059
|
|
13,638
|
|
||||||
|
Cash at end of period
|
$
|
14,581
|
|
$
|
30,343
|
|
$
|
8,059
|
|
|||
|
Supplemental cash flow information (non-cash):
|
|
|
|
|
|
|
||||||
|
Acquisition of business through promissory note
|
$
|
—
|
|
$
|
2,500
|
|
$
|
—
|
|
|||
|
Accretion of convertible preferred stock
|
$
|
—
|
|
$
|
—
|
|
$
|
1,263
|
|
|||
|
Common shares exchanged for preferred stock
|
$
|
3,150
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Warrants exchanged for common stock
|
$
|
—
|
|
$
|
965
|
|
$
|
—
|
|
|||
|
Additions to equipment in process included in accounts payable
|
$
|
42
|
|
$
|
199
|
|
$
|
—
|
|
|||
|
Equipment acquired under capital lease
|
$
|
—
|
|
$
|
153
|
|
$
|
—
|
|
|||
|
4.
|
Acquisitions
|
|
Acquisition consideration (In thousands):
|
Fair Value
|
||
|
Cash payment
|
$
|
4,000
|
|
|
Promissory note
|
2,500
|
|
|
|
Total acquisition consideration
|
$
|
6,500
|
|
|
Purchase price allocation (In thousands):
|
Fair Value
|
||
|
Cash
|
$
|
5,050
|
|
|
Accounts receivable
|
53
|
|
|
|
Inventory
|
2,039
|
|
|
|
Other current assets
|
192
|
|
|
|
Accounts payable and accrued expenses
|
(939
|
)
|
|
|
Asset retirement obligation
|
(1,600
|
)
|
|
|
Property and equipment
|
1,818
|
|
|
|
Intangible assets
|
3,360
|
|
|
|
Bargain purchase gain
|
(3,473
|
)
|
|
|
Total consideration
|
$
|
6,500
|
|
|
Year Ended December 31,
|
||||||||
|
(in thousands)
|
2014
|
2013
|
||||||
|
Pro forma revenue
|
$
|
44,906
|
|
$
|
43,863
|
|
||
|
Pro forma net loss
|
(30,115
|
)
|
(49,124
|
)
|
||||
|
Pro forma net loss per share - basic and diluted
|
(3.10
|
)
|
(18.06
|
)
|
||||
|
(In thousands)
|
2015
|
2014
|
||||||
|
Raw materials
|
$
|
1,228
|
|
$
|
1,078
|
|
||
|
Work-in-process
|
131
|
|
458
|
|
||||
|
Finished goods
|
20
|
|
384
|
|
||||
|
Inventory
|
$
|
1,379
|
|
$
|
1,920
|
|
||
|
(In thousands)
|
2015
|
2014
|
||||||
|
Machinery and equipment
|
$
|
3,280
|
|
$
|
3,135
|
|
||
|
Furniture, fixtures and office equipment
|
931
|
|
777
|
|
||||
|
Computer equipment and software
|
2,662
|
|
667
|
|
||||
|
Leasehold improvements
|
2,393
|
|
1,691
|
|
||||
|
Construction in process
|
421
|
|
1,019
|
|
||||
|
|
9,687
|
|
7,289
|
|
||||
|
Less accumulated depreciation
|
(5,638
|
)
|
(4,397
|
)
|
||||
|
Property and Equipment
|
$
|
4,049
|
|
$
|
2,892
|
|
||
|
(In thousands)
|
2015
|
2014
|
||||||
|
Commercial rights
|
$
|
3,360
|
|
$
|
3,360
|
|
||
|
Less accumulated amortization
|
(443
|
)
|
(163
|
)
|
||||
|
Intangible assets
|
$
|
2,917
|
|
$
|
3,197
|
|
||
|
Calendar Years Ending December 31, (In thousands)
|
|
|||
|
2016
|
$
|
280
|
|
|
|
2017
|
280
|
|
||
|
2018
|
280
|
|
||
|
2019
|
280
|
|
||
|
2020
|
280
|
|
||
|
Thereafter
|
1,517
|
|
||
|
Total
|
$
|
2,917
|
|
|
|
(In thousands)
|
2015
|
2014
|
|||||||
|
Bonus
|
$
|
1,956
|
|
$
|
2,044
|
|
|||
|
Employee related accruals
|
1,341
|
|
1,281
|
|
|||||
|
Accrued expenses
|
75
|
|
605
|
|
|||||
|
Asset retirement obligation
(a)
|
—
|
|
348
|
|
|||||
|
Other
|
231
|
|
436
|
|
|||||
|
Accrued expenses
|
$
|
3,603
|
|
$
|
4,714
|
|
|||
|
(a) The reduction in the asset retirement obligation is based on a change to the estimate of the obligation to restore the Denmark facility to its original state and final payment of the obligation.
|
|||||||||
|
|
Year Ended December 31,
|
|||||
|
Service-Based Stock Options
|
2015
|
2014
|
2013
|
|||
|
Expected dividend rate
|
—%
|
—%
|
—%
|
|||
|
Expected stock price volatility
|
77.4 – 88.1%
|
82.4 – 88.2%
|
74.0 – 87.9%
|
|||
|
Risk-free interest rate
|
1.5 – 2.0%
|
1.7 – 2.2%
|
0.1 – 2.1%
|
|||
|
Expected life (years)
|
5.5 – 6.3
|
5.5 – 6.3
|
5.0 – 6.3
|
|||
|
Service-Based Stock Options
|
Options
|
Weighted Average
Exercise Price
|
Weighted Average
Remaining
Contractual Term
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Outstanding at December 31, 2012
|
499,374
|
|
$
|
47.60
|
|
7.5
|
$
|
—
|
|
||||
|
Granted
|
75,751
|
|
$
|
21.32
|
|
|
|
|
|||||
|
Exercised
|
—
|
|
$
|
—
|
|
|
$
|
—
|
|
||||
|
Expired
|
(164,189
|
)
|
$
|
51.76
|
|
|
|
|
|||||
|
Forfeited
|
(113,076
|
)
|
$
|
45.38
|
|
|
|
|
|||||
|
Outstanding at December 31, 2013
|
297,860
|
|
$
|
39.53
|
|
7.9
|
$
|
—
|
|
||||
|
Granted
|
242,029
|
|
$
|
3.91
|
|
|
|
|
|||||
|
Exercised
|
—
|
|
$
|
—
|
|
|
$
|
—
|
|
||||
|
Expired
|
(32,012
|
)
|
$
|
42.63
|
|
|
|
|
|||||
|
Forfeited
|
(30,347
|
)
|
$
|
32.13
|
|
|
|
|
|||||
|
Outstanding at December 31, 2014
|
477,530
|
|
$
|
36.43
|
|
8.0
|
$
|
—
|
|
||||
|
Granted
|
2,216,600
|
|
$
|
3.11
|
|
||||||||
|
Exercised
|
(3,566
|
)
|
$
|
3.02
|
|
$
|
1,000
|
|
|||||
|
Expired
|
(17,791
|
)
|
$
|
40.02
|
|
||||||||
|
Forfeited
|
(149,373
|
)
|
$
|
3.35
|
|
||||||||
|
Outstanding at December 31, 2015
|
2,523,400
|
|
$
|
6.36
|
|
8.7
|
$
|
5,000
|
|
||||
|
Exercisable at December 31, 2015
|
661,229
|
|
$
|
14.27
|
|
7.9
|
$
|
—
|
|
||||
|
|
Year Ended December 31,
|
|||||||||||
|
(Amounts in thousands, except per share amounts)
|
2015
|
2014
|
2013
|
|||||||||
|
Numerator:
|
|
|
|
|
||||||||
|
Net loss
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||
|
Less: earnings attributable to convertible preferred stock
|
6,736
|
|
6,005
|
|
5,352
|
|
||||||
|
Numerator of basic and diluted EPS
|
$
|
(23,076
|
)
|
$
|
(25,925
|
)
|
$
|
(20,974
|
)
|
|||
|
Denominator:
|
|
|
|
|
|
|
||||||
|
Denominator for basic and diluted EPS: weighted-average common shares outstanding
|
23,760
|
|
11,642
|
|
3,016
|
|
||||||
|
Net loss per share attributable to common shareholders (basic and diluted)
|
$
|
(0.97
|
)
|
$
|
(2.23
|
)
|
$
|
(6.95
|
)
|
|||
|
|
August 2013
Warrants
|
|
|
Exercise price
|
$4.80
|
|
|
Expiration date
|
August 16, 2018
|
|
|
Total shares issuable on exercise
|
724,950
|
|
|
August 2013 Warrants
|
December 31, 2015
|
December 31, 2014
|
||||||
|
Closing stock price
|
$
|
2.58
|
|
$
|
3.04
|
|
||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
||||
|
Expected stock price volatility
|
91.4
|
%
|
83.2
|
%
|
||||
|
Risk-free interest rate
|
1.31
|
%
|
1.20
|
%
|
||||
|
Expected life (years)
|
2.63
|
|
3.63
|
|
||||
|
•
|
Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
|
|
•
|
Level 2: Quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability;
|
|
•
|
Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
|
|
|
December 31, 2015
|
December 31, 2014
|
||||||||||||||||||||||||||||||
|
|
Fair value measurement category
|
Fair value measurement category
|
||||||||||||||||||||||||||||||
|
(In thousands)
|
Total
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||||||||||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Warrant liabilities
|
$
|
757
|
|
$
|
—
|
|
$
|
757
|
|
$
|
—
|
|
$
|
1,081
|
|
$
|
—
|
|
$
|
1,061
|
|
$
|
20
|
|
||||||||
|
Warrant Liabilities (In thousands)
|
|
|||
|
Balance at December 31, 2013
|
$
|
2,019
|
|
|
|
Warrant exercises
|
(965
|
)
|
||
|
Increase in fair value
|
27
|
|
||
|
Balance at December 31, 2014
|
1,081
|
|
||
|
Decrease in fair value
|
(324
|
)
|
||
|
Balance at December 31, 2015
|
$
|
757
|
|
|
|
|
Year Ended December 31,
|
|||||||
|
(In thousands)
|
2015
|
2014
|
||||||
|
Beginning balance
|
$
|
20
|
|
$
|
85
|
|
||
|
Decrease in fair value
|
(20
|
)
|
(65
|
)
|
||||
|
Ending balance
|
$
|
—
|
|
$
|
20
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
U.S. loss
|
$
|
(16,235
|
)
|
$
|
(18,078
|
)
|
$
|
(15,622
|
)
|
|||
|
Non U.S. loss
|
(105
|
)
|
(1,842
|
)
|
—
|
|
||||||
|
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2015
|
2014
|
2013
|
|||||||||
|
Loss before income taxes
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||
|
Federal statutory rate
|
34
|
%
|
34
|
%
|
34
|
%
|
||||||
|
Taxes computed at federal statutory rate
|
(5,556
|
)
|
(6,773
|
)
|
(5,311
|
)
|
||||||
|
State taxes (net of federal benefit)
|
(392
|
)
|
(463
|
)
|
—
|
|
||||||
|
Warrants
|
(118
|
)
|
(10
|
)
|
(1,815
|
)
|
||||||
|
Nondeductible stock compensation
|
543
|
|
48
|
|
81
|
|
||||||
|
Michigan NOL benefit
|
—
|
|
—
|
|
(791
|
)
|
||||||
|
Net operating loss expirations
|
—
|
|
655
|
|
612
|
|
||||||
|
Write-off of Section 382 limited NOL’s
|
—
|
|
67,781
|
|
—
|
|
||||||
|
Write-off of Section 383 limited R&D credits
|
—
|
|
1,600
|
|
—
|
|
||||||
|
Other
|
57
|
|
352
|
|
(27
|
)
|
||||||
|
Adjustment to prior year filed returns
|
(5,203
|
)
|
—
|
|
—
|
|
||||||
|
Change in valuation allowance
|
10,669
|
|
(63,190
|
)
|
7,251
|
|
||||||
|
Reported income taxes
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
|
Year Ended December 31,
|
|||||||
|
(In thousands)
|
2015
|
2014
|
||||||
|
Net operating loss carryforwards
|
$
|
13,998
|
|
$
|
7,092
|
|
||
|
Employee benefits and stock compensation
|
2,485
|
|
1,897
|
|
||||
|
Research and development costs
|
4,903
|
|
2,184
|
|
||||
|
Fixed assets
|
453
|
|
254
|
|
||||
|
Intangible assets
|
(477
|
)
|
(510
|
)
|
||||
|
Asset retirement obligation
|
—
|
|
127
|
|
||||
|
Inventory reserve
|
510
|
|
186
|
|
||||
|
Other, net
|
143
|
|
115
|
|
||||
|
Total deferred tax assets
|
22,015
|
|
11,345
|
|
||||
|
Valuation allowance
|
(22,015
|
)
|
(11,345
|
)
|
||||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Unrecognized
Income Tax
|
|||
|
(In thousands)
|
Benefits
|
|||
|
Balance at December 31, 2013
|
$
|
900
|
|
|
|
Decrease in prior year tax positions
|
(900
|
)
|
||
|
Balance at December 31, 2014 and 2015
|
$
|
—
|
|
|
|
|
|
Payments Due by Period
|
||||||||||||||||||||||||||
|
Contractual Obligations
|
Total
|
2016
|
2017
|
2018
|
2019
|
2020
|
More than
5 Years |
|||||||||||||||||||||
|
Operating leases
|
$
|
27,693
|
|
$
|
4,310
|
|
$
|
4,890
|
|
$
|
4,572
|
|
$
|
4,260
|
|
$
|
4,386
|
|
$
|
5,275
|
|
|||||||
|
Purchase commitments
|
300
|
|
300
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Capital leases
|
118
|
|
43
|
|
43
|
|
32
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Total
|
$
|
28,111
|
|
$
|
4,653
|
|
$
|
4,933
|
|
$
|
4,604
|
|
$
|
4,260
|
|
$
|
4,386
|
|
$
|
5,275
|
|
|||||||
|
In thousands, except per share data)
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Year
|
|||||||||||||||
|
2015
|
||||||||||||||||||||
|
Revenues
|
$
|
10,849
|
|
$
|
13,590
|
|
$
|
11,309
|
|
$
|
15,420
|
|
$
|
51,168
|
|
|||||
|
Gross profit
|
5,281
|
|
6,689
|
|
4,537
|
|
8,191
|
|
24,698
|
|
||||||||||
|
Loss from operations
|
(4,572
|
)
|
(2,265
|
)
|
(4,877
|
)
|
(4,957
|
)
|
(16,671
|
)
|
||||||||||
|
Net loss
|
(4,862
|
)
|
(2,152
|
)
|
(4,416
|
)
|
(4,910
|
)
|
(16,340
|
)
|
||||||||||
|
Net loss per share (Basic and Diluted)
|
(0.27
|
)
|
(0.16
|
)
|
(0.26
|
)
|
(0.28
|
)
|
(0.97
|
)
|
||||||||||
|
2014
|
||||||||||||||||||||
|
Revenues
(a)
|
$
|
—
|
|
$
|
4,432
|
|
$
|
9,658
|
|
$
|
14,706
|
|
$
|
28,796
|
|
|||||
|
Gross profit (loss)
|
—
|
|
(577
|
)
|
4,126
|
|
7,954
|
|
11,503
|
|
||||||||||
|
Loss from operations
|
(4,645
|
)
|
(8,522
|
)
|
(8,022
|
)
|
(2,345
|
)
|
(23,534
|
)
|
||||||||||
|
Net loss
(b)
|
(5,995
|
)
|
(4,638
|
)
|
(6,917
|
)
|
(2,370
|
)
|
(19,920
|
)
|
||||||||||
|
Net loss per share (Basic and Diluted)
|
(1.26
|
)
|
(0.94
|
)
|
(0.82
|
)
|
(0.17
|
)
|
(2.23
|
)
|
||||||||||
|
(a) Revenue from commercial operations began in June 2014 following the acquisition of the CTRM business. Prior to June 2014, Vericel was a development stage entity.
|
||||||||||||||||||||
|
(b) The net loss in the second quarter of 2014 includes a $3.5 million bargain purchase gain as a result of the CTRM business acquisition.
|
||||||||||||||||||||
|
1)
|
Remove inappropriate permissions. When the permissions error was located in January 2016, inappropriate access was immediately removed. IT staff responsible for the maintenance of Active Directory Group assignments made changes to the Controller’s permissions and by January 25, 2016, the Controller’s permissions were corrected to remove the incompatible access.
|
|
2)
|
Enable and/or design reporting functionality that provides an audit trail for journal entries, module access and other relevant user actions. In addition to the corrections to the Controller’s permissions, the FastPath software vendor was contacted to customize an additional report that is needed to document the audit trail / life cycle of each journal entry in the ERP. The new report captures journal entries that originate in the general ledger together with the user that initiated each journal entry, the user the changed each journal entry, and the user that posted each journal entry in the ERP.
|
|
3)
|
Review remaining conflicts and confirm whether Controller’s review would effectively mitigate the risk associated with the permissions.
|
|
Date: March 14, 2016
|
|
|
|
Vericel Corporation
|
|
|
|
|
|
/s/ DOMINICK C. COLANGELO
|
|
|
Dominick C. Colangelo
|
|
|
President and Chief Executive Officer
|
|
|
(Principal Executive Officer)
|
|
Signature
|
|
Title
|
|
|
|
|
|
/s/ DOMINICK C. COLANGELO
|
|
President and Chief Executive Officer, Director
|
|
Dominick C. Colangelo
|
|
(Principal Executive Officer)
|
|
|
|
|
|
/s/ GERARD J. MICHEL
|
|
Chief Financial Officer and Vice President
|
|
Gerard J. Michel
|
|
of Corporate Development
|
|
|
|
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
/s/ ROBERT L. ZERBE, M.D.
|
|
Chairman of the Board of Directors
|
|
Robert L. Zerbe, M.D.
|
|
|
|
|
|
|
|
/s/ ALAN L. RUBINO
|
|
Director
|
|
Alan L. Rubino
|
|
|
|
|
|
|
|
/s/ HEIDI M. HAGEN
|
|
Director
|
|
Heidi M. Hagen
|
|
|
|
|
|
|
|
/s/ STEVEN C. GILMAN
|
|
Director
|
|
Steven C. Gilman
|
|
|
|
|
|
|
|
/s/ KEVIN F. MCLAUGHLIN
|
|
Director
|
|
Kevin F. McLaughlin
|
|
|
|
|
|
|
|
/s/ PAUL K. WOTTON
|
|
Director
|
|
Paul K. Wotton
|
|
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
3.1
|
|
Restated Articles of Incorporation of the Company, filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on December 17, 2009, incorporated herein by reference.
|
|
|
|
|
|
3.2
|
|
Certificate of Amendment to Restated Articles of Incorporation of the Company dated February 9, 2010, filed as Exhibit 3.2 to the Company’s Post-Effective Amendment No. 1 to Form S-1 filed on March 31, 2010, incorporated herein by reference.
|
|
|
|
|
|
3.3
|
|
Certificate of Amendment to Restated Articles of Incorporation of the Company dated March 22, 2011, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on March 25, 2011, incorporated herein by reference.
|
|
|
|
|
|
3.4
|
|
Certificate of Amendment to the Restated Articles of Incorporation of the Company, dated November 21, 2014, attached as Exhibit 3.1 to Vericel’s Current Report on Form 8-K filed on November 24, 2014, incorporated herein by reference.
|
|
|
|
|
|
3.5
|
|
Certificate of Designation, Preferences and Rights, of the Company classifying and designating the Series A Junior Participating Cumulative Preferred Stock, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-A filed on August 12, 2011, incorporated herein by reference.
|
|
|
|
|
|
3.6
|
|
Amended and Restated Certificate of Designations, Preferences and Rights, of the Company classifying and designating the Series B-1 Non-Voting Convertible Preferred Stock and the Series B-2 Voting Convertible Preferred Stock, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on August 12, 2013, incorporated herein by reference.
|
|
3.7**
|
Certificate of Designations, Preferences and Rights and Limitations of Series A Convertible Preferred Stock.
|
|
|
|
|
|
|
3.8
|
|
Bylaws, as amended, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on November 12, 2010, incorporated herein by reference.
|
|
|
|
|
|
4.1
|
|
Form of Senior Indenture for Senior Debt Securities, filed as Exhibit 4.1 to the Company’s Registration Statement on Form S-3 filed on June 29, 2015 and incorporated herein by reference.
|
|
|
|
|
|
4.2
|
|
Form of Indenture for Subordinated Debt Securities, filed as Exhibit 4.3 to the Company’s Registration Statement on Form S-3 filed on June 29, 2015 and incorporated herein by reference.
|
|
|
|
|
|
4.3
|
|
Shareholder Rights Agreement, dated as of August 11, 2011, between the Company and Continental Stock Transfer & Trust Company, as Rights Agent, attached as Exhibit 4.3 to the Company’s Current Report on Form 8-A filed on August 12, 2011, incorporated herein by reference.
|
|
|
|
|
|
4.4
|
|
Amendment to Shareholder Rights Agreement, dated as of March 9, 2012, between the Company and Continental Stock Transfer & Trust Company, as Rights Agent, attached as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 9, 2012, incorporated herein by reference.
|
|
|
|
|
|
10.1 #
|
|
Form of Indemnification Agreement, attached as Exhibit 10.1 to the Company’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference.
|
|
|
|
|
|
10.2 #
|
|
Amended and Restated 1992 Incentive and Non-Qualified Stock Option Plan and forms of agreements thereunder, attached as Exhibit 10.5 to the Company’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference.
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
10.4
|
|
License Agreement, dated March 13, 1992, between the Company and the University of Michigan and amendments thereto dated March 13, 1992, October 8, 1993 and June 21, 1995, attached as Exhibit 10.17 to the Company’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference.
|
|
10.5 #
|
|
2001 Stock Option Plan, attached as Exhibit 10.72 to the Company’s Annual Report on Form 10-K for the year ended June 30, 2002, incorporated herein by reference.
|
|
|
|
|
|
10.6 #
|
|
2004 Equity Incentive Plan, attached as Exhibit 10.82 to Amendment No. 1 to the Company’s Quarterly Report on Form 10-Q/A for the quarter ended September 30, 2004, incorporated herein by reference.
|
|
|
|
|
|
10.7 #
|
|
Form of Option and Restricted Stock Award Agreements for Grants under 2004 Equity Incentive Plan, attached as Exhibit 10.84 to the Company’s Annual Report on Form 10-K for the year ended June 30, 2005, incorporated herein by reference.
|
|
|
|
|
|
10.8
|
|
Amendment dated December 5, 2002 to License Agreement with the University of Michigan, attached as Exhibit 10.87 to the Company’s Annual Report on Form 10-K for the year ended June 30, 2005, incorporated herein by reference.
|
|
|
|
|
|
10.9 #
|
|
2004 Equity Incentive Plan, as amended, attached as Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on November 8, 2006, incorporated herein by reference.
|
|
|
|
|
|
10.10 #
|
|
Forms of Grant Notice and Stock Option Agreement for Grants under 2004 Equity Incentive Plan, as amended, attached as Exhibit 99.2 to the Company’s Current Report on Form 8-K filed on November 8, 2006, incorporated herein by reference.
|
|
|
|
|
|
10.11
|
|
Form of Purchase Agreement, attached as Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference.
|
|
|
|
|
|
10.12
|
|
Form of Warrant, attached as Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference.
|
|
|
|
|
|
10.13
|
|
Standard Lease between the Company and Domino’s Farms Office Park, L.L.C. dated January 31, 2007, as amended, (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed with the SEC on April 9, 2013).
|
|
|
|
|
|
10.15
|
|
Class A Warrant Agreement, dated as of January 21, 2010, by and between the Registrant and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 27, 2010).
|
|
|
|
|
|
10.16
|
|
Class B Warrant Agreement, dated as of January 21, 2010, by and between the Registrant and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on January 27, 2010).
|
|
|
|
|
|
10.17
|
|
Underwriting Agreement, dated as of January 15, 2010, and between the Registrant and Oppenheimer & Co. Inc. (incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 15, 2010).
|
|
|
|
|
|
10.18 #
|
|
Form of indemnification agreement entered into between the Company and each of its directors, attached as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on August 31, 2010, incorporated herein by reference.
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
10.19
|
|
Amended Code of Business Conduct and Ethics, attached as Exhibit 14.1 to the Company’s Current Report on Form 8-K filed on August 31, 2010, incorporated herein by reference.
|
|
10.20*
|
|
Contract Manufacturing and Supply Agreement, dated as of November 8, 2010, by and between Vention Medical (formerly ATEK Medical, LLC) and the Company (incorporated herein by reference to Exhibit 10.30 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010).
|
|
|
|
|
|
10.21
|
|
Warrant agreement, dated as of December 15, 2010, by and between the Registrant and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on December 16, 2010).
|
|
|
|
|
|
10.22
|
|
Underwriting Agreement, dated as of December 10, 2010, and between the Registrant and Stifel, Nicolaus & Company, Incorporated, Needham & Company, LLC and Roth Capital Partners (incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed with the SEC on December 10, 2010).
|
|
|
|
|
|
10.26#
|
|
Senior Executive Incentive Bonus Plan (incorporated herein by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K, filed on March 25, 2011).
|
|
|
|
|
|
10.27
|
|
At Market Issuance Sales Agreement, dated June 16, 2011, by and among the Company and MLV & Co. LLC (“MLV”) (formerly McNicoll, Lewis & Vlak LLC),(incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 16, 2011).
|
|
|
|
|
|
10.28
|
|
Master Services Agreement by and between the Company and PPD, made and entered into as of September 23, 2011 (the “Master Services Agreement”) (incorporated herein by reference to Exhibit 10.28 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012).
|
|
|
|
|
|
10.29
|
|
Project Addendum to the Master Services Agreement, dated as of November 16, 2011 (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC November 22, 2011).
|
|
|
|
|
|
10.3
|
|
Registration Rights Agreement, dated March 9, 2012, between the Company and Eastern Capital Limited, attached as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on March 9, 2012, incorporated herein by reference.
|
|
|
|
|
|
10.31
|
|
Securities Purchase Agreement, dated as of March 9, 2012, by and between the Company and Eastern Capital Limited (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on March 9, 2012).
|
|
|
|
|
|
10.32#
|
|
Employment Agreement, dated as of April 3, 2013, by and between the Company and Daniel R. Orlando (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 9, 2013).
|
|
10.35
|
Form of Warrant Exchange Agreement, dated June 27, 2012 (incorporated herein by reference to Exhibit 10.1 to the Company’s Report on Form 8-K, filed on June 27, 2012).
|
|
|
|
|
|
|
10.36#
|
Executive Resignation Agreement, executed on December 14, 2012, by and between the Company and Tim M. Mayleben (incorporated herein by reference to Exhibit 10.36 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012, filed on March 18, 2013).
|
|
|
|
|
|
|
10.37#
|
Executive Employment Agreement, executed March 4, 2013 and effective March 1, 2013, by and between the Company and Dominick C. Colangelo (incorporated herein by reference to Exhibit 10.1 to the Company’s Report on Form 8-K, filed on March 9, 2013).
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
10.38
|
Form of Warrant Exercise Agreement, dated September 24, 2013 (incorporated herein by reference to Exhibit 10 to the Company’s Report on Form 8-K, filed on September 27, 2013).
|
|
|
|
|
|
|
10.39
|
Consulting Services Agreement, executed January 9, 2014 and effective January 1, 2014, by and between the Company and Ronnda L. Bartel (incorporated herein by reference to Exhibit 10.1 to the Company’s Report on Form 8-K, filed on January 14, 2014).
|
|
|
|
|
|
|
10.4
|
Underwriting Agreement, dated as of August 13, 2013, by and between the Company and Aegis Capital Corp. (incorporated herein by reference to Exhibit 1.1 to the Company’s Registration Statement on Form S-1 (File No. 333-188186) filed on August 13, 2013).
|
|
|
|
|
|
|
10.41
|
Amendment No.1 to At Market Issuance Sales Agreement, dated November 29, 2013, by and between the Company and MLV (incorporated herein by reference to Exhibit 1.1 to the Company’s Report on Form 8-K, filed on November 29, 2013).
|
|
|
|
|
|
|
10.42
|
Purchase Agreement, dated as of January 21, 2014, by and between the Company and Lincoln Park Capital Fund, LLC (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 27, 2014).
|
|
|
|
|
|
|
10.43
|
Registration Rights Agreement, dated as of January 21, 2014, by and between the Company and Lincoln Park Capital Fund, LLC (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on January 27, 2014).
|
|
|
10.44
|
Asset Purchase Agreement, dated as of April 19, 2014, by and between the Company and Sanofi (incorporated herein by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on June 2, 2014).
|
|
|
|
|
|
|
10.45#
|
Employment Agreement, dated May 13, 2014, by and between the Company and Gerard J. Michel (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 4, 2014).
|
|
|
|
|
|
|
10.46**
|
Transition Services Agreement, dated as of May 30, 2014, by and between the Company and Genzyme Corporation, as amended.
|
|
|
|
|
|
|
10.47**
|
Transition Supply Agreement, dated as of May 30, 2014, by and between the Company and Genzyme Corporation, as amended.
|
|
|
|
|
|
|
10.48
|
Form of Warrant Exercise Agreement, dated July 9, 2014 (incorporated herein by reference to Exhibit 10 to the Company’s Report on Form 8-K, filed on July 11, 2014).
|
|
|
10.51#
|
Employment Agreement, dated September 25, 2014, by and between the Company and Ross Tubo (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 25, 2014).
|
|
|
|
|
|
|
10.52#
|
Employment Agreement, dated September 25, 2014, by and between the Company and David Recker (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on September 25, 2014).
|
|
|
|
|
|
|
10.53#
|
Second Amended and Restated 2009 Omnibus Incentive Plan (previously filed as Appendix II to the Company’s definitive proxy statement on Schedule 14A, filed on October 21, 2014 and incorporated herein by reference).
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
10.54#
|
Employment Agreement, dated November 6, 2014, by and between the Company and Gerard Michael (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 12, 2014).
|
|
|
|
|
|
|
10.55#
|
Amended and Restated Non-employee Director Compensation Guidelines.
|
|
|
10.56
|
Securities Exchange Agreement, dated December 18, 2015, by and between the Company and Stonepine Capital, LP (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 18, 2015).
|
|
|
10.57**
|
Lease Agreement, dated November 30, 2005, by and between the Company and Up 64 Sidney Street, LLC, as amended.
|
|
|
10.58**
|
Lease Agreement, dated January 23, 2008, by and between the Company and Up 64 Sidney Street, LLC, as amended.
|
|
|
10.61*
|
ACI-Maix Supply Agreement, dated October 20, 2015, by and between the Company and Matricel GmbH (incorporated herein by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2015 filed on November 11, 2015).
|
|
|
10.62
|
Vericel Corporation 2015 Employee Stock Purchase Plan (incorporated herein by reference to Appendix I of the Company’s Proxy Statement on Schedule 14A for the fiscal year ended December 31, 2014, filed on March 25, 2015).
|
|
|
10.63**
|
Third Amendment to Standard Lease between the Company and Domino's Farms Office Park, L.L.C., dated March 2, 2015.
|
|
|
21.1**
|
Subsidiaries of Registrant.
|
|
|
|
|
|
|
23.1**
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
|
|
|
|
31.1**
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
|
31.2**
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
|
32.1**
|
Certification of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
|
101.INS**
|
XBRL Instance Document
|
|
|
|
|
|
|
101.SCH**
|
XBRL Taxonomy Extension Schema Document
|
|
|
|
|
|
|
101.CAL**
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
|
|
|
|
101.LAB**
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
|
|
|
|
101.PRE**
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
|
|
|
|
101.DEF**
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
TERM
|
|
DEFINITION
|
|
Adverse Event
|
|
Any adverse change in health or “side-effect” that occurs in a person participating in a clinical trial, from the time they consent to joining the trial until a pre-specified period of time after their treatment has been completed.
|
|
Autologous (Patient Specific)
|
|
Originating from the patient receiving treatment. (Vericel uses only autologous cells)
|
|
BLA — Biologics License Application
|
|
An application containing product safety, efficacy and manufacturing information required by the FDA to market biologics products in the U.S.
|
|
CLI — Critical Limb Ischemia
|
|
A vascular disease characterized by insufficient blood flow in the lower extremities that causes severe pain, tissue loss or both.
|
|
CMC — Chemistry, Manufacturing, and Control
|
|
The composition, manufacture, and control of the drug substance and the drug product. It is information on the identification, quality, purity, and strength of the investigational product.
|
|
Controlled Clinical Trial
|
|
A clinical study that compares patients receiving a specific treatment to patients receiving an alternate treatment for the condition of interest. The alternate treatment may be another active treatment, standard of care for the condition and/or a placebo (inactive) treatment.
|
|
DCM — Dilated Cardiomyopathy
|
|
A chronic cardiac disease where expansion of the patient’s heart reduces the pumping function to a point that the normal circulation of blood cannot be maintained.
|
|
Double-Blind Clinical Trial
|
|
Clinical trials in which neither the patient nor the physician know if the patient received the experimental treatment or a control/placebo.
|
|
FDA — Food & Drug Administration
|
|
The U.S. FDA ensures that medicines, medical devices, and radiation-emitting consumer products are safe and effective. Authorized by Congress to enforce the Federal Food, Drug, and Cosmetic Act and several other public health laws, the agency monitors the manufacture, import, transport, storage, and sale of $1 trillion worth of goods annually.
|
|
GMP — Good Manufacturing Practice
|
|
GMP regulations require that manufacturers, processors, and packagers of drugs, medical devices, some food, and blood take proactive steps to ensure that their products are safe, pure, and effective. GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mix-ups, and errors.
|
|
Hematopoietic Stem Cells
|
|
Stem cells that give rise to all the blood cell types including myeloid (monocytes and macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets, dendritic cells), and lymphoid lineages (T-cells, B-cells, NK-cells).
|
|
IMPACT-DCM
|
|
Vericel’s U.S. Phase 2 dilated cardiomyopathy clinical trial.
|
|
IND — Investigational New Drug
|
|
An application submitted to the FDA for a new drug or biologic that, if allowed, will be used in a clinical trial.
|
|
Ischemia
|
|
A shortage or inadequate flow of blood to a body part (commonly an organ or tissue) caused by a constriction or obstruction of the blood vessels supplying it.
|
|
LVEF — Left Ventricular Ejection Fraction
|
|
The fraction of blood pumped out of the left ventricle with each heartbeat.
|
|
Mesenchymal stromal cells
|
|
Connective tissue cells that, in the case of bone marrow derived MSC, function to support blood forming cells and secrete anti-inflammatory factors.
|
|
M2 anti-inflammatory macrophages
|
|
Specialized blood cells that remove damaged tissue and bacteria and secrete anti-inflammatory factors.
|
|
Open-label Clinical Trial
|
|
A trial in which both the treating physician and the patient know whether they are receiving the experimental treatment or control/placebo treatment.
|
|
Orphan Drug Designation
|
|
“Orphan drug” refers to a drug or biologic that is intended for use in the treatment of a rare disease or condition. Orphan drug designation from the U.S. Food and Drug Association (FDA) qualifies the sponsor to receive certain benefits from the Government in exchange for developing the drug for a rare disease or condition. The drug must then go through the FDA marketing approval process like any other drug or biologic which evaluates for safety and efficacy. Usually a sponsor receives a quicker review time and lower application fees for an orphan product.
|
|
TERM
|
|
DEFINITION
|
|
Phase 1 Clinical Trial
|
|
A Phase 1 trial represents an initial study in a small group of patients to test for safety and other relevant factors.
|
|
Phase 2 Clinical Trial
|
|
A Phase 2 trial represents a study in a moderate number of patients to assess the safety and efficacy of a product.
|
|
Phase 2b Clinical Trial
|
|
A Phase 2b trial is a moderately-sized Phase 2 trial that is more specifically designed assess the efficacy of a product than a Phase 2a trial.
|
|
Phase 3 Clinical Trial
|
|
Phase 3 studies are initiated to establish safety and efficacy in an expanded patient population at multiple clinical trial sites and are generally larger than trials in earlier phases of development.
|
|
Prospective Clinical Trial
|
|
A clinical trial in which participants are identified and then followed throughout the study going forward in time.
|
|
Randomized Clinical Trial
|
|
A clinical trial in which the participants are assigned randomly to different treatment groups.
|
|
Somatic Cell
|
|
Any of the cells responsible for forming the body of an organism such as internal organs, bones, skin, connective tissues and blood.
|
|
Stem Cell
|
|
Unspecialized (undifferentiated) cells that retain the ability to divide throughout a lifetime and give rise to more specialized (differentiated) cells which take the place of cells that die or are lost. In culture, these undifferentiated cells possess the ability to divide for indefinite periods in culture and may give rise to highly specialized cells.
|