|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michigan
|
|
94-3096597
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
Title of Class
|
|
Name of Each Exchange on Which Registered
|
||
|
Common Stock (No par value)
|
|
The NASDAQ Stock Market, Inc.
|
||
|
Large accelerated filer -
o
|
|
Accelerated filer -
x
|
|
Non-accelerated filer -
o
|
|
Smaller reporting company -
o
|
|
(Do not check if a smaller reporting company)
|
|
Emerging growth company -
o
|
|
Document
|
|
Form 10-K Reference
|
|
Proxy Statement for the Annual Meeting of Shareholders scheduled for May 2, 2018
|
|
Items 10, 11, 12, 13 and 14 of Part III
|
|
|
|
Page
|
|
|
PART I
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
|
PART II
|
|
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
|
PART III
|
|
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
|
PART IV
|
|
|
Item 15.
|
||
|
Item 16.
|
||
|
•
|
Increase MACI revenue by increasing the number of surgeons implanting MACI and the average number of implants per surgeon;
|
|
•
|
Increase Epicel revenue by expanding the number of burn centers consistently using Epicel;
|
|
•
|
Lower the marginal manufacturing costs for MACI and Epicel through increased volume;
|
|
•
|
Generate positive operating income by keeping the growth in commercial expense lower than the growth in revenue
|
|
•
|
Phase 1—The biological product is initially tested for safety and tolerability. In the case of biological products and those for severe or life-threatening diseases, the initial human testing is generally conducted in patients. These trials may also provide early evidence on effectiveness.
|
|
•
|
Phase 2—These trials are conducted in a limited number of subjects in the target population to determine a safe and effective dosage to evaluate in Phase 3 and to identify possibly related adverse effects and safety risks. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
|
|
•
|
Phase 3—Phase 3 trials are undertaken to provide evidence of clinical efficacy and to further evaluate dosage, potency, and safety in an expanded patient population at multiple clinical trial sites. Phase 3 studies are performed after preliminary evidence suggesting effectiveness of the product has been obtained, and are intended to establish the overall benefit-risk relationship of the investigational product, and to provide an adequate basis for product approval and labeling.
|
|
•
|
A product comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced as a single entity;
|
|
•
|
Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products;
|
|
•
|
A drug, or device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, or device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or
|
|
•
|
Any investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect.
|
|
•
|
Clinical evidence, clinical studies, patient registries, or other sources of real world evidence, such as electronic health records;
|
|
•
|
The collection of larger confirmatory data sets; or
|
|
•
|
Post-approval monitoring of all patients treated with such therapy prior to approval of the therapy.
|
|
Name
|
Position
|
Age
|
Executive
Officer Since
|
|||
|
Dominick C. Colangelo
|
President and Chief Executive Officer
|
53
|
2013
|
|||
|
Daniel R. Orlando
|
Chief Operating Officer
|
52
|
2012
|
|||
|
Gerard Michel
|
Chief Financial Officer & Vice President of Corporate Development
|
54
|
2014
|
|||
|
•
|
the timing of new orders and revenue recognition for new and prior year orders;
|
|
•
|
seasonal buying patterns of our customers;
|
|
•
|
volatility in the sales of our products;
|
|
•
|
volume of revenues;
|
|
•
|
our ability to increase sales to our existing customers, particularly larger customers;
|
|
•
|
our ability to attract new customers;
|
|
•
|
our ability to develop and achieve market adoption of our products;
|
|
•
|
the impact of a recession or any other adverse global economic conditions on our business;
|
|
•
|
erosion in margins or significant fluctuations in revenues caused by changing customer demand;
|
|
•
|
the timing and cost of our sales force expansion and hiring personnel and of large expenses such as third-party professional services;
|
|
•
|
stock-based compensation expenses, which vary along with changes to our stock price;
|
|
•
|
fluctuations in foreign currency exchange rates; and
|
|
•
|
future accounting pronouncements or changes in accounting rules or our accounting policies.
|
|
•
|
The ability to maintain our manufacturing facility's compliance with FDA requirements including establishment and product fees;
|
|
•
|
The requirements to maintain marketing authorization and licenses from regulatory bodies in the United States and other countries in good standing;
|
|
•
|
The liquidity and market volatility of our equity securities;
|
|
•
|
Regulatory and manufacturing requirements and uncertainties;
|
|
•
|
Staying ahead of technological developments by competitors;
|
|
•
|
The rate and degree of progress of our product development; and
|
|
•
|
The rate of regulatory approval to proceed with clinical development programs.
|
|
•
|
Our ability to further commercialize our products;
|
|
•
|
The rate and degree of progress of our product development;
|
|
•
|
The rate of regulatory approval to proceed with clinical developmental programs;
|
|
•
|
The level of success achieved in clinical trials;
|
|
•
|
The requirements for marketing authorization from regulatory bodies in the United States and other countries;
|
|
•
|
The liquidity and market volatility of our equity securities; and
|
|
•
|
Regulatory and manufacturing requirements and uncertainties, and technological developments by competitors.
|
|
•
|
Delays in securing clinical investigators or trial sites for our clinical trials and their subsequent performance in conducting accurate and reliable trials on a timely basis;
|
|
•
|
Delays in obtaining IRB and other regulatory approvals to commence a clinical trial;
|
|
•
|
Slower than anticipated rates of patient recruitment and enrollment in our clinical trials, or failing to reach the targeted number of patients due to competition for patients from other trials;
|
|
•
|
Limited or no availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third party payers for the use of biological products supplied for use in our clinical trials;
|
|
•
|
Negative or inconclusive results from clinical trials;
|
|
•
|
Unforeseen adverse effects interrupting, delaying, or halting clinical trials of any future therapeutic product candidates, and possibly resulting in the FDA or other regulatory authorities denying approval of any future therapeutic product candidates;
|
|
•
|
Unforeseen safety issues;
|
|
•
|
Approval and introduction of new therapies or changes in standards of practice or regulatory requirements or guidance that render our clinical trial endpoints or the targeting of our proposed indications obsolete;
|
|
•
|
Inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols;
|
|
•
|
Inability to replicate in large controlled trials safety and efficacy data obtained from a limited number of patients in uncontrolled trials;
|
|
•
|
Inability or unwillingness of medical investigators to follow our clinical protocols; and
|
|
•
|
Unavailability of clinical trial supplies.
|
|
•
|
The recall or seizure of products;
|
|
•
|
The suspension or revocation of the authority necessary for the production or sale of a product;
|
|
•
|
The suspension of shipments from particular manufacturing facilities;
|
|
•
|
The imposition of fines and penalties;
|
|
•
|
The delay of our ability to introduce new products into the market;
|
|
•
|
Our exclusion or the exclusion of our products from being reimbursed by federal and state healthcare programs (such as Medicare, Medicaid, Veterans Administration, or VA, health programs and Civilian Health and Medical Program Uniformed Service, or CHAMPUS); and
|
|
•
|
Other civil or criminal prosecution or sanctions against us or our employees, such as fines, penalties or imprisonment.
|
|
•
|
Untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
Unanticipated expenditures to address or defend such actions;
|
|
•
|
Client notifications for repair, replacement, or refunds of a device;
|
|
•
|
Recall, detention or seizure of our products;
|
|
•
|
Operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
Denying, refusing or delaying our requests for approval of new products or proposed changes to existing products;
|
|
•
|
Operating restrictions;
|
|
•
|
Withdrawing product approvals that have already been granted;
|
|
•
|
Refusal to approve a pending marketing application, such as a BLA or supplements to a BLA submitted by us;
|
|
•
|
Refusal to grant export approval for our products; or
|
|
•
|
Criminal prosecution.
|
|
•
|
Significant awards against us;
|
|
•
|
Substantial litigation costs;
|
|
•
|
Recall of the product;
|
|
•
|
Injury to our reputation;
|
|
•
|
Withdrawal of clinical trial participants; or
|
|
•
|
Adverse regulatory action.
|
|
•
|
Others may be able to make products that are the same as or similar to our products or product candidates, but that are not covered by the claims of the patents that we own or have exclusively licensed;
|
|
•
|
We or any strategic partners might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or have exclusively licensed;
|
|
•
|
We might not have been the first to file patent applications covering certain of our inventions;
|
|
•
|
Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
|
|
•
|
It is possible that our pending patent applications will not lead to issued patents;
|
|
•
|
Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges;
|
|
•
|
Our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
|
|
•
|
We may not develop additional proprietary technologies that are patentable; and
|
|
•
|
The patents of others may have an adverse effect on our business.
|
|
•
|
Clinical trial results;
|
|
•
|
The amount of our cash resources and our ability to obtain additional funding;
|
|
•
|
Announcements of research activities, business developments, technological innovations or new products by us or our competitors;
|
|
•
|
Entering into or terminating strategic relationships;
|
|
•
|
Regulatory developments in both the United States and abroad;
|
|
•
|
Disputes concerning patents or proprietary rights;
|
|
•
|
Changes in our revenues or expense levels;
|
|
•
|
Changes in our pricing policies or the pricing policies of our competitors;
|
|
•
|
Seasonal or other variations in patient demand for MACI and Epicel;
|
|
•
|
Demand for and clinical acceptance of products;
|
|
•
|
The timing of sales of products and of the introduction of new products;
|
|
•
|
Public concern regarding the safety, efficacy or other aspects of the products or methodologies we are developing;
|
|
•
|
News or reports from other stem cell, cell therapy or regenerative medicine companies;
|
|
•
|
Reports by securities analysts;
|
|
•
|
Status of the investment markets;
|
|
•
|
Loss of key personnel;
|
|
•
|
Concerns related to management transitions; and
|
|
•
|
Delisting from the NASDAQ Capital Market.
|
|
|
High
|
Low
|
||||||
|
Year ended December 31, 2016
|
|
|
|
|
||||
|
First Quarter
|
$
|
6.08
|
|
$
|
1.79
|
|
||
|
Second Quarter
|
6.03
|
|
2.07
|
|
||||
|
Third Quarter
|
2.95
|
|
2.09
|
|
||||
|
Fourth Quarter
|
4.10
|
|
2.05
|
|
||||
|
Year ended December 31, 2017
|
|
|
|
|
||||
|
First Quarter
|
$
|
3.10
|
|
$
|
2.50
|
|
||
|
Second Quarter
|
3.45
|
|
2.55
|
|
||||
|
Third Quarter
|
6.00
|
|
3.00
|
|
||||
|
Fourth Quarter
|
5.98
|
|
3.65
|
|
||||
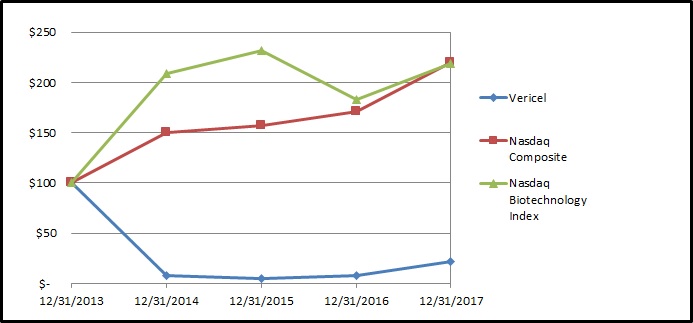
|
|
Number of Securities
to be Issued upon Exercise
of Outstanding Options,
Warrants and Rights
|
Weighted Average
Exercise Price of
Outstanding
Options, Warrants
and Rights
|
Number of Securities
Remaining Available
for Future Issuance
Under Equity
Compensation Plans
(2)
|
|||||||
|
Equity compensation plans approved by security holders (employees and directors)
(1)
|
4,528,426
|
|
$
|
3.77
|
|
4,427,722
|
|
|||
|
Employee stock purchase plan
(1)
|
27,506
|
|
$
|
4.63
|
|
570,489
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
(In thousands, except per share amounts)
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Product sales, net
|
$
|
62,760
|
|
$
|
54,383
|
|
$
|
51,168
|
|
$
|
28,796
|
|
$
|
19
|
|
|||||
|
Other
|
1,164
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Total revenue
|
63,924
|
|
54,383
|
|
51,168
|
|
28,796
|
|
19
|
|
||||||||||
|
Cost of product sales
(a)
|
30,354
|
|
28,307
|
|
26,470
|
|
17,293
|
|
4
|
|
||||||||||
|
Gross profit
|
33,570
|
|
26,076
|
|
24,698
|
|
11,503
|
|
15
|
|
||||||||||
|
Research and development
|
12,944
|
|
15,295
|
|
18,890
|
|
21,263
|
|
15,104
|
|
||||||||||
|
Selling, general and administrative
|
35,610
|
|
27,388
|
|
22,479
|
|
13,774
|
|
5,875
|
|
||||||||||
|
Loss on impairment of intangible asset
(b)
|
—
|
|
2,638
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Total operating expenses
|
48,554
|
|
45,321
|
|
41,369
|
|
35,037
|
|
20,979
|
|
||||||||||
|
Loss from operations
|
(14,984
|
)
|
(19,245
|
)
|
(16,671
|
)
|
(23,534
|
)
|
(20,964
|
)
|
||||||||||
|
Other income (expense):
|
|
|
|
|
|
|
|
|
||||||||||||
|
(Increase) decrease in fair value of warrants
(c)
|
(257
|
)
|
—
|
|
324
|
|
(27
|
)
|
5,337
|
|
||||||||||
|
Bargain purchase gain
(d)
|
—
|
|
—
|
|
—
|
|
3,473
|
|
—
|
|
||||||||||
|
Loss on extinguishment of debt
(e)
|
(860
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Interest income
|
14
|
|
8
|
|
36
|
|
24
|
|
16
|
|
||||||||||
|
Other (expense) income
|
(92
|
)
|
(15
|
)
|
(20
|
)
|
150
|
|
—
|
|
||||||||||
|
Interest expense
|
(1,107
|
)
|
(314
|
)
|
(9
|
)
|
(6
|
)
|
(11
|
)
|
||||||||||
|
Total other (expense) income
|
(2,302
|
)
|
(321
|
)
|
331
|
|
3,614
|
|
5,342
|
|
||||||||||
|
Net loss
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
$
|
(19,920
|
)
|
$
|
(15,622
|
)
|
|||||
|
Net loss per share attributable to common shareholders (Basic and Diluted)
|
$
|
(0.52
|
)
|
$
|
(1.18
|
)
|
$
|
(0.97
|
)
|
$
|
(2.23
|
)
|
$
|
(6.95
|
)
|
|||||
|
(a) Revenue from commercial operations began in June 2014 following the acquisition of the CTRM business. Prior to June 2014, we were a development stage entity which is an entity focused on early stage business activity including research and development and market research.
|
||||||||||||||||||||
|
(b) The loss on impairment of intangible asset in 2016 is related to write-off of the commercial use rights for certain products (primarily Carticel). Upon the approval of MACI in December 2016 and the replacement of Carticel with MACI, it was determined the Carticel related intangible asset was fully impaired as of December 31, 2016.
|
||||||||||||||||||||
|
(c) Fluctuations in the fair value of the warrants are due to the reduction in the time to maturity and changes in our stock price.
|
||||||||||||||||||||
|
(d) The bargain purchase gain is a result of the CTRM business acquisition.
|
||||||||||||||||||||
|
(e) In December 2017 we modified our debt arrangement which resulted in a loss incurred for fees expensed upon the extinguishment of the old debt and fees related to the new debt discussed in note 6.
|
||||||||||||||||||||
|
|
December 31,
|
|||||||||||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Cash
|
$
|
26,862
|
|
$
|
22,978
|
|
$
|
14,581
|
|
$
|
30,343
|
|
$
|
8,059
|
|
|||||
|
Working capital
(a)
|
37,416
|
|
31,870
|
|
15,235
|
|
29,661
|
|
3,155
|
|
||||||||||
|
Property and equipment, net
|
4,071
|
|
3,875
|
|
4,049
|
|
2,892
|
|
739
|
|
||||||||||
|
Total assets
|
54,577
|
|
48,598
|
|
34,309
|
|
47,579
|
|
9,215
|
|
||||||||||
|
Total liabilities
|
32,037
|
|
23,890
|
|
12,179
|
|
11,938
|
|
5,321
|
|
||||||||||
|
Total shareholders' equity (deficit)
|
22,540
|
|
24,708
|
|
22,130
|
|
35,641
|
|
3,894
|
|
||||||||||
|
(a) We define working capital as current assets less current liabilities.
|
||||||||||||||||||||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Net revenues
|
$
|
63,924
|
|
$
|
54,383
|
|
$
|
51,168
|
|
|||
|
Cost of product sales
|
30,354
|
|
28,307
|
|
26,470
|
|
||||||
|
Gross profit
|
33,570
|
|
26,076
|
|
24,698
|
|
||||||
|
Total operating expenses
|
48,554
|
|
45,321
|
|
41,369
|
|
||||||
|
Loss from operations
|
(14,984
|
)
|
(19,245
|
)
|
(16,671
|
)
|
||||||
|
Other (expense) income
|
(2,302
|
)
|
(321
|
)
|
331
|
|
||||||
|
Net loss
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Net revenue by product (In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Carticel and MACI
|
$
|
43,902
|
|
$
|
38,871
|
|
$
|
35,212
|
|
|||
|
Epicel
|
18,858
|
|
15,512
|
|
15,242
|
|
||||||
|
Bone Marrow
|
—
|
|
—
|
|
714
|
|
||||||
|
License Revenue
|
1,164
|
|
—
|
|
$
|
—
|
|
|||||
|
|
$
|
63,924
|
|
$
|
54,383
|
|
$
|
51,168
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Gross profit
|
$
|
33,570
|
|
$
|
26,076
|
|
$
|
24,698
|
|
|||
|
Gross profit %
|
52.5
|
%
|
47.9
|
%
|
48.3
|
%
|
||||||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Research and development costs
|
$
|
12,944
|
|
$
|
15,295
|
|
$
|
18,890
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Dilated Cardiomyopathy
|
$
|
4,909
|
|
$
|
8,195
|
|
$
|
8,937
|
|
|||
|
MACI
|
5,390
|
|
2,811
|
|
5,497
|
|
||||||
|
Carticel
|
424
|
|
2,153
|
|
2,798
|
|
||||||
|
Epicel
|
2,221
|
|
2,136
|
|
1,658
|
|
||||||
|
Total research and development costs
|
$
|
12,944
|
|
$
|
15,295
|
|
$
|
18,890
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Selling, general and administrative costs
|
$
|
35,610
|
|
$
|
27,388
|
|
$
|
22,479
|
|
|||
|
Loss on impairment of intangible asset
|
—
|
|
2,638
|
|
—
|
|
||||||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
(Increase) decrease in fair value of warrants
|
$
|
(257
|
)
|
$
|
—
|
|
$
|
324
|
|
|||
|
Loss on extinguishment of debt
|
(860
|
)
|
—
|
|
—
|
|
||||||
|
Foreign currency translation (loss)
|
—
|
|
—
|
|
—
|
|
||||||
|
Interest income
|
14
|
|
8
|
|
36
|
|
||||||
|
Other (expense) income
|
(92
|
)
|
(15
|
)
|
(20
|
)
|
||||||
|
Interest expense
|
(1,107
|
)
|
(314
|
)
|
(9
|
)
|
||||||
|
Total other (expense) income
|
$
|
(2,302
|
)
|
$
|
(321
|
)
|
$
|
331
|
|
|||
|
|
Years Ended December 31,
|
|||||||||||
|
(in thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Cost of goods sold
|
$
|
428
|
|
$
|
427
|
|
$
|
308
|
|
|||
|
Research and development
|
506
|
|
497
|
|
555
|
|
||||||
|
General, selling and administrative
|
1,746
|
|
1,575
|
|
1,884
|
|
||||||
|
Total non-cash stock-based compensation expense
|
$
|
2,680
|
|
$
|
2,499
|
|
$
|
2,747
|
|
|||
|
|
|
Payments Due by Period
|
||||||||||||||||||||||||||
|
Contractual Obligations
|
Total
|
2018
|
2019
|
2020
|
2021
|
2022
|
More than
5 Years |
|||||||||||||||||||||
|
Operating leases
|
$
|
19,336
|
|
$
|
4,878
|
|
$
|
4,564
|
|
$
|
4,575
|
|
$
|
4,558
|
|
$
|
761
|
|
$
|
—
|
|
|||||||
|
Purchase commitments
|
3,055
|
|
713
|
|
608
|
|
578
|
|
578
|
|
578
|
|
—
|
|
||||||||||||||
|
Capital leases
|
92
|
|
80
|
|
6
|
|
2
|
|
2
|
|
2
|
|
—
|
|
||||||||||||||
|
Debt and Interest
|
$
|
18,656
|
|
$
|
1,726
|
|
$
|
6,038
|
|
$
|
5,603
|
|
$
|
5,289
|
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Total
|
$
|
41,139
|
|
$
|
7,397
|
|
$
|
11,216
|
|
$
|
10,758
|
|
$
|
10,427
|
|
$
|
1,341
|
|
$
|
—
|
|
|||||||
|
|
Page
|
|
|
December 31,
|
|||||||
|
|
2017
|
2016
|
||||||
|
ASSETS
|
|
|
|
|
||||
|
Current assets:
|
|
|
|
|
||||
|
Cash
|
$
|
26,862
|
|
$
|
22,978
|
|
||
|
Accounts receivable (net of allowance for doubtful accounts of $249 and $225, respectively)
|
18,270
|
|
17,093
|
|
||||
|
Inventory
|
3,793
|
|
3,488
|
|
||||
|
Other current assets
|
1,581
|
|
1,164
|
|
||||
|
Total current assets
|
50,506
|
|
44,723
|
|
||||
|
Property and equipment, net
|
4,071
|
|
3,875
|
|
||||
|
Total assets
|
$
|
54,577
|
|
$
|
48,598
|
|
||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|
|
|
|
||||
|
Current liabilities:
|
|
|
|
|
||||
|
Accounts payable
|
$
|
5,552
|
|
$
|
6,535
|
|
||
|
Accrued expenses
|
5,573
|
|
4,523
|
|
||||
|
Short term deferred rent
|
420
|
|
3
|
|
||||
|
Warrant liabilities
|
1,014
|
|
757
|
|
||||
|
Current portion of term loan credit agreement (net of deferred costs of $67 and $110, respectively)
|
350
|
|
779
|
|
||||
|
Other
|
181
|
|
256
|
|
||||
|
Total current liabilities
|
13,090
|
|
12,853
|
|
||||
|
Revolving and term loan credit agreement (net of deferred costs of $196 and $293, respectively)
|
16,888
|
|
9,318
|
|
||||
|
Long term deferred rent
|
2,059
|
|
1,687
|
|
||||
|
Other long term debt
|
—
|
|
32
|
|
||||
|
Total liabilities
|
32,037
|
|
23,890
|
|
||||
|
COMMITMENTS AND CONTINGENCIES (Note 15)
|
|
|
|
|
||||
|
Shareholders’ equity:
|
|
|
|
|
||||
|
Series B-2 non-voting convertible preferred stock, no par value: shares authorized and reserved — 39; shares issued and outstanding — 0 and 12, respectively
|
—
|
|
38,389
|
|
||||
|
Common stock, no par value; shares authorized — 75,000; shares issued and outstanding — 35,861 and 31,595, respectively
|
383,020
|
|
329,720
|
|
||||
|
Warrants
|
397
|
|
190
|
|
||||
|
Accumulated deficit
|
(360,877
|
)
|
(343,591
|
)
|
||||
|
Total shareholders’ equity
|
22,540
|
|
24,708
|
|
||||
|
Total liabilities and shareholders’ equity
|
$
|
54,577
|
|
$
|
48,598
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Product sales, net
|
$
|
62,760
|
|
$
|
54,383
|
|
$
|
51,168
|
|
|||
|
Other
|
1,164
|
|
—
|
|
—
|
|
||||||
|
Total revenue
|
$
|
63,924
|
|
$
|
54,383
|
|
$
|
51,168
|
|
|||
|
Cost of product sales
|
30,354
|
|
28,307
|
|
26,470
|
|
||||||
|
Gross profit
|
33,570
|
|
26,076
|
|
24,698
|
|
||||||
|
Research and development
|
12,944
|
|
15,295
|
|
18,890
|
|
||||||
|
Selling, general and administrative
|
35,610
|
|
27,388
|
|
22,479
|
|
||||||
|
Loss on impairment of intangible asset
|
—
|
|
2,638
|
|
—
|
|
||||||
|
Total operating expenses
|
48,554
|
|
45,321
|
|
41,369
|
|
||||||
|
Loss from operations
|
(14,984
|
)
|
(19,245
|
)
|
(16,671
|
)
|
||||||
|
Other income (expense):
|
|
|
|
|
|
|
||||||
|
(Increase) decrease in fair value of warrants
|
(257
|
)
|
—
|
|
324
|
|
||||||
|
Loss on extinguishment of debt
|
(860
|
)
|
—
|
|
—
|
|
||||||
|
Interest income
|
14
|
|
8
|
|
36
|
|
||||||
|
Other (expense) income
|
(92
|
)
|
(15
|
)
|
(20
|
)
|
||||||
|
Interest expense
|
(1,107
|
)
|
(314
|
)
|
(9
|
)
|
||||||
|
Total other (expense) income
|
(2,302
|
)
|
(321
|
)
|
331
|
|
||||||
|
Net loss
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
|||
|
Net loss per share attributable to common shareholders (Basic and Diluted)
|
$
|
(0.52
|
)
|
$
|
(1.18
|
)
|
$
|
(0.97
|
)
|
|||
|
Weighted average number of common shares outstanding (Basic and Diluted)
|
33,355
|
|
23,093
|
|
23,760
|
|
||||||
|
|
Preferred Stock
|
Common Stock
|
Treasury Stock
|
Warrants
|
Accumulated
Other Comprehensive |
Accumulated
|
Total
Shareholders’ |
||||||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Amount
|
Loss
|
Deficit
|
Equity
|
|||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2014
|
12
|
|
38,389
|
|
23,786
|
|
305,008
|
|
—
|
|
—
|
|
—
|
|
(71
|
)
|
(307,685
|
)
|
35,641
|
|
|||||||||||||||||
|
Net loss
|
|
|
|
|
|
|
|
|
|
|
|
|
(16,340
|
)
|
(16,340
|
)
|
|||||||||||||||||||||
|
Common stock exchanged for preferred stock and held in treasury shares
|
1
|
|
3,150
|
|
|
|
|
|
(1,250
|
)
|
(3,150
|
)
|
|
|
|
|
—
|
|
|||||||||||||||||||
|
Compensation expense related to stock options granted
|
|
|
|
|
|
|
2,747
|
|
|
|
|
|
2,747
|
|
|||||||||||||||||||||||
|
Stock option exercises
|
|
|
|
|
3
|
|
11
|
|
|
|
|
|
11
|
|
|||||||||||||||||||||||
|
Foreign currency translation adjustment
|
|
|
|
|
71
|
|
|
71
|
|
||||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2015
|
13
|
|
$
|
41,539
|
|
23,789
|
|
$
|
307,766
|
|
(1,250
|
)
|
$
|
(3,150
|
)
|
—
|
|
—
|
|
$
|
(324,025
|
)
|
$
|
22,130
|
|
||||||||||||
|
Net loss
|
(19,566
|
)
|
(19,566
|
)
|
|||||||||||||||||||||||||||||||||
|
Conversion of Series A preferred stock for common stock
|
(1
|
)
|
(3,150
|
)
|
|
|
|
|
1,250
|
|
3,150
|
|
—
|
|
|||||||||||||||||||||||
|
Compensation expense related to stock options granted, net of forfeitures
|
2,499
|
|
2,499
|
|
|||||||||||||||||||||||||||||||||
|
Issuance of common stock, net of issuance costs of $1,653
|
7,538
|
|
18,868
|
|
18,868
|
|
|||||||||||||||||||||||||||||||
|
Stock option exercises
|
39
|
|
120
|
|
120
|
|
|||||||||||||||||||||||||||||||
|
Shares issued under the Employee Stock Purchase Plan
|
229
|
|
467
|
|
467
|
|
|||||||||||||||||||||||||||||||
|
Issuance of warrants
|
|
|
190
|
|
190
|
|
|||||||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2016
|
12
|
|
$
|
38,389
|
|
31,595
|
|
$
|
329,720
|
|
—
|
|
$
|
—
|
|
$
|
190
|
|
$
|
—
|
|
$
|
(343,591
|
)
|
$
|
24,708
|
|
||||||||||
|
Net loss
|
(17,286
|
)
|
(17,286
|
)
|
|||||||||||||||||||||||||||||||||
|
Conversion of Series B-1 or B-2 preferred stock for common stock (Note 9)
|
(12
|
)
|
(38,389
|
)
|
1,094
|
|
38,389
|
|
|
|
|
|
—
|
|
|||||||||||||||||||||||
|
Compensation expense related to stock options granted, net of forfeitures
|
2,680
|
|
2,680
|
|
|||||||||||||||||||||||||||||||||
|
Issuance of common stock, net of issuance costs of $311
|
1,983
|
|
7,188
|
|
7,188
|
|
|||||||||||||||||||||||||||||||
|
Stock option exercises
|
199
|
|
608
|
|
608
|
|
|||||||||||||||||||||||||||||||
|
Shares issued under the Employee Stock Purchase Plan
|
173
|
|
425
|
|
425
|
|
|||||||||||||||||||||||||||||||
|
Issuance of warrants (Note 11)
|
|
|
207
|
|
207
|
|
|||||||||||||||||||||||||||||||
|
Exercise of warrants resulting in the issuance of common stock (Note 11)
|
817
|
|
4,010
|
|
4,010
|
|
|||||||||||||||||||||||||||||||
|
BALANCE, DECEMBER 31, 2017
|
—
|
|
|
$
|
—
|
|
|
35,861
|
|
|
$
|
383,020
|
|
|
—
|
|
|
$
|
—
|
|
|
$
|
397
|
|
$
|
—
|
|
|
$
|
(360,877
|
)
|
|
$
|
22,540
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Net loss
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
|||
|
Foreign currency translation
|
—
|
|
—
|
|
71
|
|
||||||
|
Comprehensive loss
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,269
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Operating activities:
|
|
|
|
|
|
|
||||||
|
Net loss
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used for operating activities:
|
|
|
|
|
|
|
||||||
|
Depreciation and amortization
|
1,612
|
|
1,886
|
|
1,592
|
|
||||||
|
Impairment of intangible asset
|
—
|
|
2,638
|
|
—
|
|
||||||
|
Stock compensation expense
|
2,680
|
|
2,499
|
|
2,747
|
|
||||||
|
Inventory provision
|
352
|
|
137
|
|
627
|
|
||||||
|
Change in fair value of warrants
|
257
|
|
—
|
|
(324
|
)
|
||||||
|
Loss on extinguishment of debt
|
860
|
|
—
|
|
—
|
|
||||||
|
Foreign currency translation loss
|
37
|
|
5
|
|
67
|
|
||||||
|
Gain on sale of fixed assets
|
(115
|
)
|
—
|
|
(35
|
)
|
||||||
|
Deferred rent expense
|
(13
|
)
|
670
|
|
—
|
|
||||||
|
Write down of asset retirement obligation
|
—
|
|
—
|
|
(268
|
)
|
||||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
||||||
|
Inventory
|
(657
|
)
|
(2,245
|
)
|
(86
|
)
|
||||||
|
Deferred Rent
|
798
|
|
898
|
|
—
|
|
||||||
|
Accounts receivable
|
(1,177
|
)
|
(6,174
|
)
|
(2,728
|
)
|
||||||
|
Other current assets
|
(261
|
)
|
(701
|
)
|
572
|
|
||||||
|
Accounts payable
|
(1,361
|
)
|
(1,076
|
)
|
1,726
|
|
||||||
|
Accrued expenses
|
1,050
|
|
920
|
|
(764
|
)
|
||||||
|
Other non-current assets and liabilities, net
|
41
|
|
217
|
|
(132
|
)
|
||||||
|
Net cash used for operating activities
|
(13,183
|
)
|
(19,892
|
)
|
(13,346
|
)
|
||||||
|
Investing activities:
|
|
|
|
|
|
|
||||||
|
Expenditures for property, plant and equipment
|
(1,510
|
)
|
(1,415
|
)
|
(2,427
|
)
|
||||||
|
Other
|
—
|
|
—
|
|
35
|
|
||||||
|
Net cash used for investing activities
|
(1,510
|
)
|
(1,415
|
)
|
(2,392
|
)
|
||||||
|
Financing activities:
|
|
|
|
|
|
|
||||||
|
Net proceeds from issuance of common stock
|
8,220
|
|
19,455
|
|
11
|
|
||||||
|
Deferred financing costs
|
(30
|
)
|
(213
|
)
|
—
|
|
||||||
|
Proceeds from exercise of warrants (Note 11)
|
4,010
|
|
—
|
|
—
|
|
||||||
|
Borrowings under revolving and term loan credit agreements
|
14,793
|
|
12,710
|
|
—
|
|
||||||
|
Warrants issued in connection with debt arrangement
|
207
|
|
190
|
|
—
|
|
||||||
|
Payments on term loan credit agreement
|
(889
|
)
|
(2,400
|
)
|
—
|
|
||||||
|
Payments on long-term debt
|
(7,151
|
)
|
(38
|
)
|
(35
|
)
|
||||||
|
Fee on long-term debt
|
(583
|
)
|
—
|
|
—
|
|
||||||
|
Net cash provided by (used in) financing activities
|
18,577
|
|
29,704
|
|
(24
|
)
|
||||||
|
Net increase (decrease) in cash
|
3,884
|
|
8,397
|
|
(15,762
|
)
|
||||||
|
Cash at beginning of period
|
22,978
|
|
14,581
|
|
30,343
|
|
||||||
|
Cash at end of period
|
$
|
26,862
|
|
$
|
22,978
|
|
$
|
14,581
|
|
|||
|
Supplemental cash flow information (non-cash):
|
|
|
|
|
|
|
||||||
|
Shares exchanged between common and preferred stock
|
(38,389
|
)
|
(3,150
|
)
|
3,150
|
|
||||||
|
Additions to equipment in process included in accounts payable
|
341
|
|
18
|
|
42
|
|
||||||
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
||||||||
|
Interest paid (net of interest capitalized)
|
$
|
931
|
|
$
|
226
|
|
$
|
—
|
|
||
|
Income tax withholding paid
|
100
|
|
—
|
|
—
|
|
|||||
|
Revenue Concentration
|
Accounts Receivable Concentration
|
|||||||||||||
|
Year Ended December 31,
|
December 31,
|
|||||||||||||
|
2017
|
2016
|
2015
|
2017
|
2016
|
||||||||||
|
Carticel and MACI
|
35
|
%
|
31
|
%
|
66
|
%
|
46
|
%
|
1
|
%
|
||||
|
Epicel
|
10
|
%
|
11
|
%
|
12
|
%
|
3
|
%
|
5
|
%
|
||||
|
(In thousands)
|
2017
|
2016
|
||||||
|
Raw materials
|
$
|
3,532
|
|
$
|
3,214
|
|
||
|
Work-in-process
|
226
|
|
257
|
|
||||
|
Finished goods
|
35
|
|
17
|
|
||||
|
Inventory
|
$
|
3,793
|
|
$
|
3,488
|
|
||
|
(In thousands)
|
2017
|
2016
|
||||||
|
Machinery and equipment
|
$
|
1,249
|
|
$
|
3,150
|
|
||
|
Furniture, fixtures and office equipment
|
872
|
|
931
|
|
||||
|
Computer equipment and software
|
3,536
|
|
3,147
|
|
||||
|
Leasehold improvements
|
4,213
|
|
3,332
|
|
||||
|
Construction in process
|
822
|
|
408
|
|
||||
|
|
10,692
|
|
10,968
|
|
||||
|
Less accumulated depreciation
|
(6,621
|
)
|
(7,093
|
)
|
||||
|
Property and Equipment
|
$
|
4,071
|
|
$
|
3,875
|
|
||
|
(In thousands)
|
2017
|
2016
|
|||||||
|
Bonus
|
$
|
2,693
|
|
$
|
2,433
|
|
|||
|
Employee related accruals
|
2,389
|
|
1,668
|
|
|||||
|
Other accrued expenses
|
491
|
|
422
|
|
|||||
|
Accrued expenses
|
$
|
5,573
|
|
$
|
4,523
|
|
|||
|
6.
|
|
|
(in thousands)
|
|||
|
Years Ending December 31,
|
Amount
|
|
|
|
2018
|
$
|
417
|
|
|
2019
|
5,000
|
|
|
|
2020
|
5,000
|
|
|
|
2021
|
7,083
|
|
|
|
2022
|
—
|
|
|
|
Thereafter
|
—
|
|
|
|
|
$
|
17,500
|
|
|
|
Years Ended December 31,
|
|||||||||||
|
(in thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Cost of goods sold
|
$
|
428
|
|
$
|
427
|
|
$
|
308
|
|
|||
|
Research and development
|
506
|
|
497
|
|
555
|
|
||||||
|
General, selling and administrative
|
1,746
|
|
1,575
|
|
1,884
|
|
||||||
|
Total non-cash stock-based compensation expense
|
$
|
2,680
|
|
$
|
2,499
|
|
$
|
2,747
|
|
|||
|
|
Year Ended December 31,
|
|||||
|
Service-Based Stock Options
|
2017
|
2016
|
2015
|
|||
|
Expected dividend rate
|
—%
|
—%
|
—%
|
|||
|
Expected stock price volatility
|
79.7 – 88.2%
|
78.7 – 92.2%
|
77.4 – 88.1%
|
|||
|
Risk-free interest rate
|
1.39 – 2.3%
|
1.1 – 2.1%
|
1.5 – 2.0%
|
|||
|
Expected life (years)
|
5.5 - 6.3
|
5.5 – 6.3
|
5.5 – 6.3
|
|||
|
Service-Based Stock Options
|
Options
|
Weighted Average
Exercise Price
|
Weighted Average
Remaining
Contractual Term
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Outstanding at December 31, 2016
|
3,355,692
|
|
$
|
4.66
|
|
8.2
|
$
|
610
|
|
||||
|
Granted
|
1,686,210
|
|
$
|
2.82
|
|
||||||||
|
Exercised
|
(198,564
|
)
|
$
|
3.06
|
|
|
|
||||||
|
Expired
|
(114,318
|
)
|
$
|
17.98
|
|
||||||||
|
Forfeited
|
(200,594
|
)
|
$
|
3.32
|
|
||||||||
|
Outstanding at December 31, 2017
|
4,528,426
|
|
$
|
3.77
|
|
8.0
|
$
|
10,776
|
|
||||
|
Exercisable at December 31, 2017
|
2,099,655
|
|
$
|
4.76
|
|
|
$
|
4,618
|
|
||||
|
|
Year Ended December 31,
|
|||||||||||
|
(Amounts in thousands, except per share amounts)
|
2017
|
2016
|
2015
|
|||||||||
|
Numerator:
|
|
|
|
|
||||||||
|
Net loss
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
|||
|
Less: earnings attributable to convertible preferred stock
|
—
|
|
7,579
|
|
6,736
|
|
||||||
|
Numerator of basic and diluted EPS
|
$
|
(17,286
|
)
|
$
|
(27,145
|
)
|
$
|
(23,076
|
)
|
|||
|
Denominator:
|
|
|
|
|
|
|
||||||
|
Denominator for basic and diluted EPS: weighted-average common shares outstanding
|
33,355
|
|
23,093
|
|
23,760
|
|
||||||
|
Net loss per share attributable to common shareholders (basic and diluted)
|
$
|
(0.52
|
)
|
$
|
(1.18
|
)
|
$
|
(0.97
|
)
|
|||
|
|
August 2013 Warrants
|
September 2016 Warrants
|
December 2017 Warrants
|
|||
|
Exercise price
|
$4.80
|
$2.48
|
$4.27
|
|||
|
Expiration date
|
August 16, 2018
|
September 9, 2022
|
December 6, 2023
|
|||
|
Total shares issuable on exercise
|
724,950
|
117,074
|
53,902
|
|||
|
August 2013 Warrants
|
December 31, 2017
|
December 31, 2016
|
||||||
|
Closing stock price
|
$
|
5.45
|
|
$
|
3.00
|
|
||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
||||
|
Expected stock price volatility
|
63.7
|
%
|
97.9
|
%
|
||||
|
Risk-free interest rate
|
1.65
|
%
|
1.03
|
%
|
||||
|
Expected life (years)
|
0.62
|
|
1.62
|
|
||||
|
September 2016 Warrants
|
September 9, 2016
|
|||
|
Closing stock price
|
$
|
2.20
|
|
|
|
Expected dividend rate
|
—
|
%
|
||
|
Expected stock price volatility
|
89.8
|
%
|
||
|
Risk-free interest rate
|
1.4
|
%
|
||
|
Expected life (years)
|
6.00
|
|
||
|
December 2017 Warrants
|
December 6, 2017
|
|||
|
Closing stock price
|
$
|
5.10
|
|
|
|
Expected dividend rate
|
—
|
%
|
||
|
Expected stock price volatility
|
86.4
|
%
|
||
|
Risk-free interest rate
|
2.2
|
%
|
||
|
Expected life (years)
|
6.00
|
|
||
|
•
|
Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
|
|
•
|
Level 2: Quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability;
|
|
•
|
Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
|
|
|
December 31, 2017
|
December 31, 2016
|
||||||||||||||||||||||||||||||
|
|
Fair value measurement category
|
Fair value measurement category
|
||||||||||||||||||||||||||||||
|
(In thousands)
|
Total
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||||||||||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Warrant liabilities
|
$
|
1,014
|
|
$
|
—
|
|
$
|
1,014
|
|
$
|
—
|
|
$
|
757
|
|
$
|
—
|
|
$
|
757
|
|
$
|
—
|
|
||||||||
|
Warrant Liabilities (In thousands)
|
|
|||
|
Balance at December 31, 2015
|
$
|
757
|
|
|
|
Change in fair value
|
—
|
|
||
|
Balance at December 31, 2016
|
757
|
|
||
|
Change in fair value
|
257
|
|
||
|
Balance at December 31, 2017
|
$
|
1,014
|
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
U.S. loss
|
$
|
(17,066
|
)
|
$
|
(19,302
|
)
|
$
|
(16,235
|
)
|
|||
|
Non U.S. loss
|
(220
|
)
|
(264
|
)
|
(105
|
)
|
||||||
|
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
(In thousands)
|
2017
|
2016
|
2015
|
|||||||||
|
Loss before income taxes
|
$
|
(17,286
|
)
|
$
|
(19,566
|
)
|
$
|
(16,340
|
)
|
|||
|
Federal statutory rate
|
34
|
%
|
34
|
%
|
34
|
%
|
||||||
|
Taxes computed at federal statutory rate
|
(5,877
|
)
|
(6,652
|
)
|
(5,556
|
)
|
||||||
|
State taxes (net of federal benefit)
|
(1,106
|
)
|
(1,016
|
)
|
(392
|
)
|
||||||
|
Warrants
|
—
|
|
—
|
|
(118
|
)
|
||||||
|
Nondeductible stock compensation
|
563
|
|
549
|
|
543
|
|
||||||
|
Federal and State Rate Change
|
11,749
|
|
(614
|
)
|
—
|
|
||||||
|
Other
|
116
|
|
56
|
|
57
|
|
||||||
|
Adjustment to prior year filed returns
|
—
|
|
—
|
|
(5,203
|
)
|
||||||
|
Change in valuation allowance
|
(5,445
|
)
|
7,677
|
|
10,669
|
|
||||||
|
Reported income taxes
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
|
Year Ended December 31,
|
|||||||
|
(In thousands)
|
2017
|
2016
|
||||||
|
Net operating loss carryforwards
|
$
|
10,487
|
|
$
|
10,343
|
|
||
|
Employee benefits and stock compensation
|
2,128
|
|
2,896
|
|
||||
|
Research and development costs
|
8,275
|
|
13,659
|
|
||||
|
Fixed assets
|
508
|
|
700
|
|
||||
|
Inventory reserve
|
2,568
|
|
1,898
|
|
||||
|
Other, net
|
282
|
|
196
|
|
||||
|
Total deferred tax assets
|
24,248
|
|
29,692
|
|
||||
|
Valuation allowance
|
(24,248
|
)
|
(29,692
|
)
|
||||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
||
|
|
|
Payments Due by Period
|
||||||||||||||||||||||||||
|
Contractual Obligations
|
Total
|
2018
|
2019
|
2020
|
2021
|
2022
|
More than
5 Years |
|||||||||||||||||||||
|
Operating leases
|
$
|
19,336
|
|
$
|
4,878
|
|
$
|
4,564
|
|
$
|
4,575
|
|
$
|
4,558
|
|
$
|
761
|
|
$
|
—
|
|
|||||||
|
Purchase commitments
|
3,055
|
|
713
|
|
608
|
|
578
|
|
578
|
|
578
|
|
—
|
|
||||||||||||||
|
Capital leases
|
$
|
92
|
|
$
|
80
|
|
$
|
6
|
|
$
|
2
|
|
$
|
2
|
|
$
|
2
|
|
$
|
—
|
|
|||||||
|
Total
|
22,483
|
|
5,671
|
|
5,178
|
|
5,155
|
|
5,138
|
|
1,341
|
|
—
|
|
||||||||||||||
|
In thousands, except per share data)
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Year
|
|||||||||||||||
|
2017
|
||||||||||||||||||||
|
Revenues
|
$
|
9,361
|
|
$
|
16,953
|
|
$
|
14,260
|
|
$
|
23,350
|
|
$
|
63,924
|
|
|||||
|
Gross profit
|
2,252
|
|
9,283
|
|
7,074
|
|
14,961
|
|
33,570
|
|
||||||||||
|
Loss from operations
|
(9,623
|
)
|
(2,521
|
)
|
(4,031
|
)
|
1,191
|
|
(14,984
|
)
|
||||||||||
|
Net (loss) profit
|
(9,778
|
)
|
(2,388
|
)
|
(5,407
|
)
|
287
|
|
(17,286
|
)
|
||||||||||
|
Net (loss) profit per share (Basic and Diluted)
|
(0.31
|
)
|
(0.07
|
)
|
(0.16
|
)
|
0.01
|
|
(0.52
|
)
|
||||||||||
|
2016
|
||||||||||||||||||||
|
Revenues
|
$
|
14,108
|
|
$
|
12,823
|
|
$
|
10,929
|
|
$
|
16,523
|
|
$
|
54,383
|
|
|||||
|
Gross profit (loss)
|
7,548
|
|
5,523
|
|
4,073
|
|
8,932
|
|
26,076
|
|
||||||||||
|
Loss from operations
|
(1,992
|
)
|
(4,984
|
)
|
(6,380
|
)
|
(5,889
|
)
|
(19,245
|
)
|
||||||||||
|
Net loss
|
(3,650
|
)
|
(3,044
|
)
|
(6,675
|
)
|
(6,197
|
)
|
(19,566
|
)
|
||||||||||
|
Net loss per share (Basic and Diluted)
|
(0.24
|
)
|
(0.22
|
)
|
(0.38
|
)
|
(0.34
|
)
|
(1.18
|
)
|
||||||||||
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
3.1
|
|
|
|
|
|
|
|
3.2
|
|
|
|
|
|
|
|
3.3
|
|
|
|
|
|
|
|
3.4
|
|
|
|
|
|
|
|
3.5
|
||
|
|
|
|
|
3.6
|
|
|
|
|
|
|
|
4.1
|
|
|
|
|
|
|
|
4.2
|
|
|
|
|
|
|
|
4.3
|
|
|
|
|
|
|
|
4.4
|
|
|
|
|
|
|
|
10.1 #
|
|
|
|
|
|
|
|
10.2 #
|
|
|
|
|
|
|
|
10.3 #
|
|
|
|
|
|
|
|
10.4 #
|
|
|
|
Exhibit No.
|
|
Description
|
|
10.5
|
|
|
|
|
|
|
|
10.6 #
|
|
|
|
|
|
|
|
10.7 #
|
|
|
|
|
|
|
|
10.8
|
|
|
|
|
|
|
|
10.9
|
|
|
|
|
|
|
|
10.10 #
|
||
|
10.11
|
||
|
|
|
|
|
10.12
|
||
|
|
|
|
|
10.13
|
||
|
|
|
|
|
10.14 #
|
||
|
|
|
|
|
10.16
|
.
|
|
|
10.17
|
||
|
10.18 †
|
||
|
10.19
|
||
|
Exhibit No.
|
|
Description
|
|
10.20
|
||
|
10.21 †
|
||
|
10.22
|
||
|
10.23 †
|
||
|
10.24
|
||
|
10.25 †
|
||
|
10.26 †
|
||
|
10.27
|
||
|
10.28 †
|
||
|
10.29 †
|
||
|
10.30 †
|
||
|
10.31
|
||
|
10.32
|
||
|
10.33 †
|
||
|
Exhibit No.
|
|
Description
|
|
10.34 †
|
|
|
|
10.35 †
|
||
|
10.36 †
|
||
|
10.37 †
|
||
|
10.38 †
|
||
|
10.39 #
|
||
|
10.40 #
|
||
|
10.41 #
|
||
|
10.42
|
||
|
10.43
|
||
|
10.44
|
||
|
10.45
|
||
|
10.46 †
|
||
|
10.47 †
|
||
|
10.48
|
||
|
Exhibit No.
|
|
Description
|
|
10.49
|
||
|
10.50
|
||
|
10.51
|
||
|
10.52
|
||
|
10.53
|
||
|
10.54
|
||
|
10.55
|
||
|
10.56** †
|
||
|
10.57** †
|
||
|
10.58
|
||
|
21.1**
|
||
|
|
|
|
|
23.1**
|
||
|
|
|
|
|
31.1**
|
||
|
|
|
|
|
31.2**
|
||
|
|
|
|
|
32.1**
|
||
|
|
|
|
|
101.INS**
|
XBRL Instance Document
|
|
|
|
|
|
|
101.SCH**
|
XBRL Taxonomy Extension Schema Document
|
|
|
|
|
|
|
101.CAL**
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
|
|
|
|
101.LAB**
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
101.PRE**
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
|
|
|
|
101.DEF**
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
Date:
|
March 5, 2018
|
|
|
Vericel Corporation
|
||
|
|
|
|
|
|
/s/ DOMINICK C. COLANGELO
|
|
|
|
Dominick C. Colangelo
|
|
|
|
President and Chief Executive Officer
|
|
|
|
(Principal Executive Officer)
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
/s/ DOMINICK C. COLANGELO
|
|
President and Chief Executive Officer, Director
|
|
Dominick C. Colangelo
|
|
(Principal Executive Officer)
|
|
|
|
|
|
/s/ GERARD J. MICHEL
|
|
Chief Financial Officer and Vice President
|
|
Gerard J. Michel
|
|
of Corporate Development
|
|
|
|
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
/s/ ROBERT L. ZERBE, M.D.
|
|
Chairman of the Board of Directors
|
|
Robert L. Zerbe, M.D.
|
|
|
|
|
|
|
|
/s/ ALAN L. RUBINO
|
|
Director
|
|
Alan L. Rubino
|
|
|
|
|
|
|
|
/s/ HEIDI M. HAGEN
|
|
Director
|
|
Heidi M. Hagen
|
|
|
|
|
|
|
|
/s/ STEVEN C. GILMAN
|
|
Director
|
|
Steven C. Gilman
|
|
|
|
|
|
|
|
/s/ KEVIN F. MCLAUGHLIN
|
|
Director
|
|
Kevin F. McLaughlin
|
|
|
|
|
|
|
|
/s/ PAUL K. WOTTON
|
|
Director
|
|
Paul K. Wotton
|
|
|