|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
(State or Other Jurisdiction of
Incorporation or Organization)
|
20-5455398
(I.R.S. Employer
Identification Number)
|
|||
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
||
|
Common Stock, par value $0.001 per share
|
|
The NASDAQ Stock Market LLC
|
||
|
Large accelerated filer
|
o
|
Accelerated filer
|
ý
|
Non-accelerated filer
|
o
|
Smaller reporting company
|
o
|
|
(Do not check if a
smaller reporting company)
|
|||||||
|
Item No.
|
Page No.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
•
|
Revenue
for the three- and twelve-month periods ended December 31, 2016 was $18.3 million and $65.1 million, respectively, an increase of 30% and 31% over the prior year.
|
|
•
|
Afirma Gene Expression Classifier (GEC) Reported Volume
for the three- and twelve-month periods ended December 31, 2016 was 6,313 and 23,237, respectively, an increase of 13% and 20% over the prior year.
|
|
•
|
Operating Expenses
for the three- and twelve-month periods ended December 31, 2016, were $21.9 million and $93.9 million, respectively, an increase of 0% and 13% over the prior year.
|
|
•
|
Net Loss and Comprehensive Loss
for the three- and twelve-month periods ended December 31, 2016 was ($4.4) million and ($31.4) million, respectively, a 45% and 7% reduction from the prior year.
|
|
•
|
Cash and cash equivalents
was $59.2 million at December 31, 2016. During the twelve-month period ended December 31, 2016, the company raised $51.1 million in capital, including $19.2 million in net proceeds from its March 2016 debt financing and $31.9 million in net proceeds from a public offering of common stock.
|
|
•
|
Cash Burn
for the three- and twelve-month periods ended December 31, 2016 (which is defined as net cash used in operating activities and purchases of property and equipment), was $4.7 million and $32.2 million, respectively, a 33% and 3% improvement compared to the prior year.
|
|
•
|
Executed a Blues group-purchasing agreement in April 2016, accelerating Blues plan in-network contracting and overall reimbursement for the Afirma GEC thyroid cancer test. As of February 28, 2017, the company has more than 70 million Blues plan members under coverage and nearly 25 million under contract.
|
|
•
|
Expanded overall covered lives for the Afirma GEC by 50 million to nearly 225 million and overall contracted lives by 25 million to over 155 million as of February 28, 2017.
|
|
•
|
Achieved draft Medicare coverage policies for the Percepta Bronchial Genomic Classifier for use in lung cancer screening and diagnosis, leading to two final policies scheduled to become effective in March 2017.
|
|
•
|
Clinical utility and cost-effectiveness data for the Percepta classifier were presented at the American Thoracic Society and the CHEST annual meetings, further suggesting that use of the Percepta classifier changes patient care and reduces healthcare costs as intended.
|
|
•
|
Launched the Envisia Genomic Classifier at the CHEST annual meeting in October 2016, in conjunction with the presentation of new data suggesting the test’s ability to significantly improve the diagnosis of IPF without the need for risky, expensive surgery.
|
|
•
|
Presented data at the American Thyroid Association meeting in September 2016, demonstrating the potential for a next-generation Afirma GEC, planned for 2017 introduction, to substantially increase the percentage of patients with benign thyroid nodules who may be able to avoid unnecessary surgery.
|
|
•
|
Data were published in the
Journal of the National Cancer Institute
suggesting the potential for the “field of injury” technology behind Veracyte’s Percepta classifier to enable lung cancer detection using a simple, non-invasive nasal swab test.
|
|
•
|
Enhanced Afirma GEC
- We are developing a product enhancement to our current Afirma test, which we believe will maintain the Afirma GEC's high sensitivity and negative predictive value, while potentially further increasing the test's specificity and thus the number of benign surgeries that can potentially be avoided.
|
|
•
|
Risk of Recurrence
- We are in the discovery phase for a risk of recurrence classifier.
|
|
•
|
Expanded Indications for the Percepta
Classifier -
We are evaluating enhancements to our product, which we envision would allow us to expand the intended use population for our test.
|
|
•
|
Nasal Classifier
- We are in the discovery phase for a nasal test, based on our proprietary “field of injury” technology, that would potentially allow us to test patients at a different point in the clinical pathway of care.
|
|
•
|
Rx Response
- We are in the discovery phase for a test that could help guide treatment decisions for IPF patients based upon their genomic profile.
|
|
•
|
Compile a Growing Library of Peer-reviewed Studies that Demonstrate the Test Is Effective
- To date, several peer-reviewed articles and review papers have been published and have helped support our efforts aimed at widespread adoption and reimbursement of our genomic tests. In each disease area we pursue, we intend to conduct studies in order to develop robust library of evidence.
|
|
•
|
Meet the Evidence Standards Necessary to Be Consistent with Leading Clinical Guidelines
- We believe inclusion in leading clinical practice guidelines plays an important role in payers' coverage decisions. For example, the data published on the Afirma GEC to date is consistent with the recommendations of the widely-recognized American Thyroid Association and National Comprehensive Cancer Network clinical practice guidelines.
|
|
•
|
Execute an Internal Managed Care and Claims Adjudication Function as Part of Our Core Business Operations
- We believe that obtaining adequate and widespread reimbursement is a critical factor in our long-term success. We employ a team of in-house claims processing and reimbursement specialists who work with payers, physician practices and patients to obtain maximum reimbursement.
|
|
•
|
Cultivate a Network of Key Opinion Leaders -
Key opinion leaders are able to influence clinical practice by publishing research and determining whether new tests should be integrated into practice guidelines. We collaborate with key opinion leaders early in the development process to ensure our clinical studies are designed and executed in a way that clearly demonstrates the benefits of our tests to patients, physicians and payers. Ongoing studies to support real world experience with our tests are also a key component of our efforts to collaborate with physician thought leaders.
|
|
•
|
Established Payer Relationships and In-network Contracts -
We believe that positive engagement with payers leads to coverage decisions and facilitates our efforts on coverage and contract decisions for subsequent tests.
|
|
•
|
Medicare accounted for 27%, 26% and 26% of our revenue;
|
|
•
|
UnitedHealthcare accounted for 12%, 14% and 18% of our revenue; and
|
|
•
|
Aetna accounted for 8%, 9% and 11% of our revenue.
|
|
•
|
the ability of the test to answer the appropriate clinical question at the right point in the clinical pathway;
|
|
•
|
the quality and strength of clinical validation and utility data;
|
|
•
|
confidence in diagnostic results backed by analytical verification data;
|
|
•
|
the extent of reimbursement and in-network payer contracts;
|
|
•
|
inclusion in practice guidelines;
|
|
•
|
cost-effectiveness; and
|
|
•
|
ease of use.
|
|
•
|
not experimental or investigational;
|
|
•
|
pre-authorized and appropriate for the specific patient;
|
|
•
|
cost-effective;
|
|
•
|
supported by peer-reviewed publications; and
|
|
•
|
included in clinical practice guidelines.
|
|
•
|
differences between the list price for our tests and the reimbursement rates of payers;
|
|
•
|
compliance with complex federal and state regulations related to billing Medicare;
|
|
•
|
risk of government audits related to billing Medicare;
|
|
•
|
disputes among payers as to which party is responsible for payment;
|
|
•
|
differences in coverage and in information and billing requirements among payers, including the need for prior authorization and/or advanced notification;
|
|
•
|
the effect of patient co-payments or co-insurance;
|
|
•
|
changes to billing codes used for our tests;
|
|
•
|
incorrect or missing billing information; and
|
|
•
|
the resources required to manage the billing and claims appeals process.
|
|
•
|
expend significant funds to conduct substantial research and development;
|
|
•
|
conduct successful analytical and clinical studies;
|
|
•
|
scale our laboratory processes to accommodate new tests; and
|
|
•
|
build the commercial infrastructure to market and sell new products.
|
|
•
|
failure to identify a genomic signature in biomarker discovery;
|
|
•
|
inability to secure sufficient numbers of samples at an acceptable cost and on an acceptable timeframe to conduct analytical and clinical studies; or
|
|
•
|
failure of clinical validation studies to support the effectiveness of the test.
|
|
•
|
the Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which established comprehensive federal standards with respect to the privacy and security of protected health information and requirements for the use of certain standardized electronic transactions, and amendments made in 2013 to HIPAA under the Health Information Technology for Economic and Clinical Health Act, or HITECH, which strengthen and expand HIPAA privacy and security compliance requirements, increase penalties for violators, extend enforcement authority to state attorneys general, and impose requirements for breach notification;
|
|
•
|
Medicare billing and payment regulations applicable to clinical laboratories;
|
|
•
|
the Federal Anti-Kickback Statute, which prohibits knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for, or recommending of an item or service that is reimbursable, in whole or in part, by a federal healthcare program;
|
|
•
|
the Federal Stark physician self-referral law (and state equivalents), which prohibits a physician from making a referral for certain designated health services covered by the Medicare program, including laboratory and pathology services, if the physician or an immediate family member has a financial relationship with the entity providing the designated health services, unless the financial relationship falls within an applicable exception to the prohibition;
|
|
•
|
the Federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or state health care program beneficiary if the person knows or should know it is likely to influence the beneficiary's selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies;
|
|
•
|
the Federal False Claims Act, which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government;
|
|
•
|
other federal and state fraud and abuse laws, such as anti-kickback laws, prohibitions on self-referral, fee-splitting restrictions, prohibitions on the provision of products at no or discounted cost to induce physician or patient adoption, and false claims acts, which may extend to services reimbursable by any third-party payer, including private insurers;
|
|
•
|
the prohibition on reassignment of Medicare claims, which, subject to certain exceptions, precludes the reassignment of Medicare claims to any other party;
|
|
•
|
the rules regarding billing for diagnostic tests reimbursable by the Medicare program, which prohibit a physician or other supplier from marking up the price of the technical component or professional component of a diagnostic test ordered by the physician or other supplier and supervised or performed by a physician who does not "share a practice" with the billing physician or supplier;
|
|
•
|
state laws that prohibit other specified practices related to billing such as billing physicians for testing that they order, waiving co-insurance, co-payments, deductibles, and other amounts owed by patients, and billing a state Medicaid program at a price that is higher than what is charged to other payers; and
|
|
•
|
the Foreign Corrupt Practices Act of 1977, and other similar laws, which apply to our international activities.
|
|
•
|
multiple, conflicting and changing laws and regulations such as tax laws, privacy laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
|
|
•
|
failure by us to obtain regulatory approvals where required for the use of our solutions in various countries;
|
|
•
|
complexities associated with managing multiple payer reimbursement regimes, government payers or patient self-pay systems;
|
|
•
|
logistics and regulations associated with shipping tissue samples, including infrastructure conditions and transportation delays;
|
|
•
|
challenges associated with establishing laboratory partners, including proper sample collection techniques, inventory management, sample logistics, billing and promotional activities;
|
|
•
|
limits on our ability to penetrate international markets if we are not able to process tests locally;
|
|
•
|
financial risks, such as longer payment cycles, difficulty in collecting from payers, the effect of local and regional financial crises, and exposure to foreign currency exchange rate fluctuations;
|
|
•
|
natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; and
|
|
•
|
regulatory and compliance risks that relate to maintaining accurate information and control over activities that may fall within the purview of the Foreign Corrupt Practices Act of 1977, including both its books and records provisions and its anti-bribery provisions.
|
|
•
|
actual or anticipated variations in our and our competitors' results of operations;
|
|
•
|
announcements by us or our competitors of new products, commercial relationships or capital commitments;
|
|
•
|
changes in reimbursement by current or potential payers, including governmental payers;
|
|
•
|
issuance of new securities analysts' reports or changed recommendations for our stock;
|
|
•
|
fluctuations in our revenue, due in part to the way in which we recognize revenue;
|
|
•
|
actual or anticipated changes in regulatory oversight of our products;
|
|
•
|
developments or disputes concerning our intellectual property or other proprietary rights;
|
|
•
|
commencement of, or our involvement in, litigation;
|
|
•
|
announced or completed acquisitions of businesses or technologies by us or our competitors;
|
|
•
|
any major change in our management; and
|
|
•
|
general economic conditions and slow or negative growth of our markets.
|
|
•
|
authorize our board of directors to issue, without further action by the stockholders, up to 5.0 million shares of undesignated preferred stock;
|
|
•
|
require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by written consent;
|
|
•
|
specify that special meetings of our stockholders can be called only by our board of directors, our chairman of the board, or our chief executive officer;
|
|
•
|
establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors;
|
|
•
|
establish that our board of directors is divided into three classes, Class I, Class II and Class III, with each class serving staggered terms;
|
|
•
|
provide that our directors may be removed only for cause;
|
|
•
|
provide that vacancies on our board of directors may, except as otherwise required by law, be filled only by a majority of directors then in office, even if less than a quorum;
|
|
•
|
specify that no stockholder is permitted to cumulate votes at any election of directors; and
|
|
•
|
require a super-majority of votes to amend certain of the above-mentioned provisions.
|
|
Name
|
Age
|
Position
|
|||
|
Bonnie H. Anderson
|
58
|
|
Chairman, President and Chief Executive Officer
|
||
|
Julie A. Brooks
|
71
|
|
General Counsel and Secretary
|
||
|
Keith S. Kennedy
|
47
|
|
Chief Financial Officer
|
||
|
Christopher M. Hall
|
48
|
|
Chief Operating Officer
|
||
|
High
|
Low
|
||||||
|
2016
|
|
|
|
|
|||
|
Fourth Quarter
|
$
|
8.45
|
|
$
|
5.82
|
|
|
|
Third Quarter
|
$
|
7.96
|
|
$
|
4.83
|
|
|
|
Second Quarter
|
$
|
5.98
|
|
$
|
4.81
|
|
|
|
First Quarter
|
$
|
7.31
|
|
$
|
4.21
|
|
|
|
2015
|
|
|
|
|
|||
|
Fourth Quarter
|
$
|
8.15
|
|
$
|
4.69
|
|
|
|
Third Quarter
|
$
|
12.47
|
|
$
|
4.59
|
|
|
|
Second Quarter
|
$
|
12.20
|
|
$
|
7.24
|
|
|
|
First Quarter
|
$
|
9.74
|
|
$
|
6.50
|
|
|
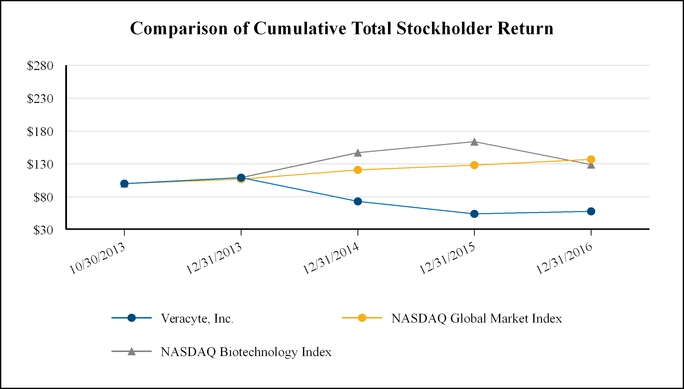
|
October 30,
2013 |
December 31,
2013 |
December 31,
2014 |
December 31,
2015 |
December 31,
2016 |
|||||||||||||||
|
Veracyte, Inc.
|
$
|
100.00
|
|
$
|
109.00
|
|
$
|
73.00
|
|
$
|
54.00
|
|
$
|
58.00
|
|
||||
|
NASDAQ Global Market Index
|
$
|
100.00
|
|
$
|
107.00
|
|
$
|
121.00
|
|
$
|
128.00
|
|
$
|
137.00
|
|
||||
|
NASDAQ Biotechnology Index
|
$
|
100.00
|
|
$
|
109.00
|
|
$
|
147.00
|
|
$
|
164.00
|
|
$
|
129.00
|
|
||||
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
Statements of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Revenue
|
$
|
65,085
|
|
$
|
49,503
|
|
$
|
38,190
|
|
$
|
21,884
|
|
$
|
11,628
|
|
||||
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Cost of revenue(1)
|
25,462
|
|
21,497
|
|
16,606
|
|
12,607
|
|
7,584
|
|
|||||||||
|
Research and development(1)
|
15,324
|
|
12,796
|
|
9,804
|
|
7,810
|
|
6,608
|
|
|||||||||
|
Selling and marketing(1)
|
28,248
|
|
25,293
|
|
21,932
|
|
12,540
|
|
8,447
|
|
|||||||||
|
General and administrative(1)
|
23,787
|
|
22,583
|
|
18,854
|
|
12,100
|
|
7,918
|
|
|||||||||
|
Intangible asset amortization
|
1,067
|
|
800
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total operating expenses(1)
|
93,888
|
|
82,969
|
|
67,196
|
|
45,057
|
|
30,557
|
|
|||||||||
|
Loss from operations
|
(28,803
|
)
|
(33,466
|
)
|
(29,006
|
)
|
(23,173
|
)
|
(18,929
|
)
|
|||||||||
|
Interest expense
|
(2,757
|
)
|
(378
|
)
|
(439
|
)
|
(233
|
)
|
—
|
|
|||||||||
|
Other income (expense), net
|
202
|
|
140
|
|
72
|
|
(2,174
|
)
|
280
|
|
|||||||||
|
Net loss
|
$
|
(31,358
|
)
|
$
|
(33,704
|
)
|
$
|
(29,373
|
)
|
$
|
(25,580
|
)
|
$
|
(18,649
|
)
|
||||
|
Net loss per common share, basic and diluted
|
$
|
(1.09
|
)
|
$
|
(1.30
|
)
|
$
|
(1.36
|
)
|
$
|
(6.15
|
)
|
$
|
(28.68
|
)
|
||||
|
Shares used in computing net loss per common share, basic and diluted
|
28,830,472
|
|
25,994,193
|
|
21,639,374
|
|
4,158,664
|
|
650,333
|
|
|||||||||
|
Other Operating Data:
|
|
|
|
|
|
|
|
|
|||||||||||
|
GECs reported
|
23,237
|
|
19,421
|
|
14,061
|
|
9,716
|
|
4,993
|
|
|||||||||
|
(1)
|
Includes employee stock-based compensation as follows:
|
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
Cost of revenue
|
$
|
126
|
|
$
|
100
|
|
$
|
51
|
|
$
|
34
|
|
$
|
26
|
|
||||
|
Research and development
|
1,322
|
|
1,178
|
|
790
|
|
250
|
|
131
|
|
|||||||||
|
Selling and marketing
|
1,594
|
|
1,326
|
|
707
|
|
169
|
|
111
|
|
|||||||||
|
General and administrative
|
3,336
|
|
2,998
|
|
2,000
|
|
794
|
|
407
|
|
|||||||||
|
Total stock-based compensation
|
$
|
6,378
|
|
$
|
5,602
|
|
$
|
3,548
|
|
$
|
1,247
|
|
$
|
675
|
|
||||
|
|
As of December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
Cash and cash equivalents
|
$
|
59,219
|
|
$
|
39,084
|
|
$
|
35,014
|
|
$
|
71,220
|
|
$
|
14,002
|
|
||||
|
Working capital
|
62,093
|
|
33,192
|
|
26,203
|
|
61,019
|
|
7,390
|
|
|||||||||
|
Total assets
|
101,034
|
|
75,247
|
|
64,839
|
|
79,630
|
|
19,067
|
|
|||||||||
|
Convertible preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
63,372
|
|
|||||||||
|
Accumulated deficit
|
(180,084
|
)
|
(148,726
|
)
|
(115,022
|
)
|
(85,649
|
)
|
(60,069
|
)
|
|||||||||
|
Total stockholders' equity (deficit)
|
59,581
|
|
51,252
|
|
41,374
|
|
56,443
|
|
(58,471
|
)
|
|||||||||
|
•
|
Revenue
for the three- and twelve-month periods ended December 31, 2016 was $18.3 million and $65.1 million, respectively, an increase of 30% and 31% over the prior year.
|
|
•
|
Afirma Gene Expression Classifier (GEC) Reported Volume
for the three- and twelve-month periods ended December 31, 2016 was 6,313 and 23,237, respectively, an increase of 13% and 20% over the prior year.
|
|
•
|
Operating Expenses
for the three- and twelve-month periods ended December 31, 2016, were $21.9 million and $93.9 million, respectively, an increase of 0% and 13% over the prior year.
|
|
•
|
Net Loss and Comprehensive Loss
for the three- and twelve-month periods ended December 31, 2016 was ($4.4) million and ($31.4) million, respectively, a 45% and 7% reduction from the prior year.
|
|
•
|
Cash and cash equivalents
was $59.2 million at December 31, 2016. During the twelve-month period ended December 31, 2016, the company raised $51.1 million in capital, including $19.2 million in net proceeds from its March 2016 debt financing and $31.9 million in net proceeds from a public offering of common stock.
|
|
•
|
Cash Burn
for the three- and twelve-month periods ended December 31, 2016 (which is defined as net cash used in operating activities and purchases of property and equipment), was $4.7 million and $32.2 million, respectively, a 33% and 3% improvement compared to the prior year.
|
|
•
|
Executed a Blues group-purchasing agreement in April 2016, accelerating Blues plan in-network contracting and overall reimbursement for the Afirma GEC thyroid cancer test. As of February 28, 2017, the company has more than 70 million Blues plan members under coverage and nearly 25 million under contract.
|
|
•
|
Expanded overall covered lives for the Afirma GEC by 50 million to nearly 225 million and overall contracted lives by 25 million to over 155 million as of February 28, 2017.
|
|
•
|
Achieved draft Medicare coverage policies for the Percepta Bronchial Genomic Classifier for use in lung cancer screening and diagnosis, leading to two final policies scheduled to become effective in March 2017.
|
|
•
|
Clinical utility and cost-effectiveness data for the Percepta classifier were presented at the American Thoracic Society and the CHEST annual meetings, further suggesting that use of the Percepta classifier changes patient care and reduces healthcare costs as intended.
|
|
•
|
Launched the Envisia Genomic Classifier at the CHEST annual meeting in October 2016, in conjunction with the presentation of new data suggesting the test’s ability to significantly improve the diagnosis of IPF without the need for risky, expensive surgery.
|
|
•
|
Presented data at the American Thyroid Association meeting in September 2016, demonstrating the potential for a next-generation Afirma GEC, planned for 2017 introduction, to substantially increase the percentage of patients with benign thyroid nodules who may be able to avoid unnecessary surgery.
|
|
•
|
Data were published in the
Journal of the National Cancer Institute
suggesting the potential for the “field of injury” technology behind Veracyte’s Percepta classifier to enable lung cancer detection using a simple, non-invasive nasal swab test.
|
|
|
Year Ended
December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Medicare
|
27
|
%
|
26
|
%
|
26
|
%
|
||
|
UnitedHealthcare
|
12
|
%
|
14
|
%
|
18
|
%
|
||
|
Aetna
|
8
|
%
|
9
|
%
|
11
|
%
|
||
|
47
|
%
|
49
|
%
|
55
|
%
|
|||
|
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
|
2016
|
Change
|
%
|
2015
|
Change
|
%
|
2014
|
||||||||||||||||||
|
Revenue
|
$
|
65,085
|
|
$
|
15,582
|
|
31
|
%
|
$
|
49,503
|
|
$
|
11,313
|
|
30
|
%
|
$
|
38,190
|
|
||||||
|
Operating expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Cost of revenue
|
25,462
|
|
3,965
|
|
18
|
%
|
21,497
|
|
4,891
|
|
29
|
%
|
16,606
|
|
|||||||||||
|
Research and development
|
15,324
|
|
2,528
|
|
20
|
%
|
12,796
|
|
2,992
|
|
31
|
%
|
9,804
|
|
|||||||||||
|
Selling and marketing
|
28,248
|
|
2,955
|
|
12
|
%
|
25,293
|
|
3,361
|
|
15
|
%
|
21,932
|
|
|||||||||||
|
General and administrative
|
23,787
|
|
1,204
|
|
5
|
%
|
22,583
|
|
3,729
|
|
20
|
%
|
18,854
|
|
|||||||||||
|
Intangible asset amortization
|
1,067
|
|
267
|
|
33
|
%
|
800
|
|
800
|
|
—
|
|
—
|
|
|||||||||||
|
Total operating expenses
|
93,888
|
|
10,919
|
|
13
|
%
|
82,969
|
|
15,773
|
|
23
|
%
|
67,196
|
|
|||||||||||
|
Loss from operations
|
(28,803
|
)
|
4,663
|
|
14
|
%
|
(33,466
|
)
|
(4,460
|
)
|
(15
|
)%
|
(29,006
|
)
|
|||||||||||
|
Interest expense
|
(2,757
|
)
|
(2,379
|
)
|
629
|
%
|
(378
|
)
|
61
|
|
14
|
%
|
(439
|
)
|
|||||||||||
|
Other income (expense), net
|
202
|
|
62
|
|
44
|
%
|
140
|
|
68
|
|
94
|
%
|
72
|
|
|||||||||||
|
Net loss and comprehensive loss
|
$
|
(31,358
|
)
|
$
|
2,346
|
|
7
|
%
|
$
|
(33,704
|
)
|
$
|
(4,331
|
)
|
(15
|
)%
|
$
|
(29,373
|
)
|
||||||
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
|
2016
|
%
|
2015
|
%
|
2014
|
%
|
||||||||||||||
|
Revenue recognized on an accrual basis
|
$
|
47,099
|
|
72
|
%
|
$
|
27,043
|
|
55
|
%
|
$
|
12,545
|
|
33
|
%
|
|||||
|
Revenue recognized when cash is received
|
17,986
|
|
28
|
%
|
22,460
|
|
45
|
%
|
25,645
|
|
67
|
%
|
||||||||
|
Total
|
$
|
65,085
|
|
100
|
%
|
$
|
49,503
|
|
100
|
%
|
$
|
38,190
|
|
100
|
%
|
|||||
|
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
|
2016
|
Change
|
%
|
2015
|
Change
|
%
|
2014
|
||||||||||||||||||
|
Cost of revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Reagents, chips, consumables and related
|
$
|
9,639
|
|
$
|
2,131
|
|
28
|
%
|
$
|
7,508
|
|
$
|
2,238
|
|
42
|
%
|
$
|
5,271
|
|
||||||
|
Cytopathology fees and related costs
|
5,963
|
|
427
|
|
8
|
%
|
5,536
|
|
975
|
|
21
|
%
|
4,561
|
|
|||||||||||
|
Sample collection
|
3,458
|
|
334
|
|
11
|
%
|
3,124
|
|
593
|
|
23
|
%
|
2,531
|
|
|||||||||||
|
Direct labor
|
3,195
|
|
667
|
|
26
|
%
|
2,528
|
|
719
|
|
40
|
%
|
1,809
|
|
|||||||||||
|
Other
|
3,207
|
|
406
|
|
14
|
%
|
2,801
|
|
366
|
|
15
|
%
|
2,434
|
|
|||||||||||
|
Total
|
$
|
25,462
|
|
$
|
3,965
|
|
18
|
%
|
$
|
21,497
|
|
$
|
4,891
|
|
29
|
%
|
$
|
16,606
|
|
||||||
|
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
|
2016
|
Change
|
%
|
2015
|
Change
|
%
|
2014
|
||||||||||||||||||
|
Research and development expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Personnel-related expense
|
$
|
6,846
|
|
$
|
932
|
|
16
|
%
|
$
|
5,914
|
|
$
|
1,380
|
|
30
|
%
|
$
|
4,534
|
|
||||||
|
Stock-based compensation expense
|
1,322
|
|
144
|
|
12
|
%
|
1,178
|
|
388
|
|
49
|
%
|
790
|
|
|||||||||||
|
Direct R&D expense
|
4,202
|
|
796
|
|
23
|
%
|
3,406
|
|
672
|
|
25
|
%
|
2,734
|
|
|||||||||||
|
Other expense
|
2,954
|
|
656
|
|
29
|
%
|
2,298
|
|
552
|
|
32
|
%
|
1,746
|
|
|||||||||||
|
Total
|
$
|
15,324
|
|
$
|
2,528
|
|
20
|
%
|
$
|
12,796
|
|
$
|
2,992
|
|
31
|
%
|
$
|
9,804
|
|
||||||
|
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
|
2016
|
Change
|
%
|
2015
|
Change
|
%
|
2014
|
||||||||||||||||||
|
Selling and marketing expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Genzyme co-promotion expense, net
|
$
|
5,103
|
|
$
|
(264
|
)
|
|
(5
|
)%
|
$
|
5,367
|
|
$
|
(4,366
|
)
|
|
(45
|
)%
|
$
|
9,733
|
|
||||
|
Personnel-related expense
|
15,473
|
|
$
|
3,406
|
|
|
28
|
%
|
12,067
|
|
3,946
|
|
|
49
|
%
|
8,121
|
|
||||||||
|
Stock-based compensation expense
|
1,594
|
|
$
|
268
|
|
|
20
|
%
|
1,326
|
|
619
|
|
|
88
|
%
|
707
|
|
||||||||
|
Direct marketing expense
|
2,957
|
|
$
|
89
|
|
|
3
|
%
|
2,868
|
|
1,324
|
|
|
86
|
%
|
1,544
|
|
||||||||
|
Other expense
|
3,121
|
|
$
|
(544
|
)
|
|
(15
|
)%
|
3,665
|
|
1,838
|
|
|
101
|
%
|
1,827
|
|
||||||||
|
Total
|
$
|
28,248
|
|
$
|
2,955
|
|
|
12
|
%
|
$
|
25,293
|
|
$
|
3,361
|
|
|
15
|
%
|
$
|
21,932
|
|
||||
|
|
Year Ended December 31,
|
||||||||||||||||||||||||
|
|
2016
|
Change
|
%
|
2015
|
Change
|
%
|
2014
|
||||||||||||||||||
|
General and administrative expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Personnel-related expense
|
$
|
12,630
|
|
$
|
2,235
|
|
22
|
%
|
$
|
10,395
|
|
$
|
832
|
|
9
|
%
|
$
|
9,563
|
|
||||||
|
Stock-based compensation expense
|
3,336
|
|
$
|
338
|
|
11
|
%
|
2,998
|
|
998
|
|
50
|
%
|
2,000
|
|
||||||||||
|
Professional fees expense
|
4,455
|
|
$
|
(623
|
)
|
(12
|
)%
|
5,078
|
|
553
|
|
12
|
%
|
4,525
|
|
||||||||||
|
Rent and other facilities expense
|
2,470
|
|
$
|
(156
|
)
|
(6
|
)%
|
2,626
|
|
1,122
|
|
75
|
%
|
1,504
|
|
||||||||||
|
Other expense
|
896
|
|
$
|
(590
|
)
|
(40
|
)%
|
1,486
|
|
224
|
|
18
|
%
|
1,262
|
|
||||||||||
|
Total
|
$
|
23,787
|
|
$
|
1,204
|
|
5
|
%
|
$
|
22,583
|
|
$
|
3,729
|
|
20
|
%
|
$
|
18,854
|
|
||||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Cash used in operating activities
|
$
|
(27,982
|
)
|
$
|
(26,965
|
)
|
$
|
(27,632
|
)
|
||
|
Cash used in investing activities
|
(4,212
|
)
|
(6,698
|
)
|
(9,010
|
)
|
|||||
|
Cash provided by financing activities
|
52,329
|
|
37,733
|
|
436
|
|
|||||
|
|
Payments Due by Period
|
||||||||||||||||||
|
|
Fiscal Year 2017
|
Fiscal Year 2018 to 2019
|
Fiscal Year 2020 to 2021
|
Fiscal Year 2022 and Beyond
|
Total
|
||||||||||||||
|
Operating lease obligations
|
$
|
2,143
|
|
$
|
4,128
|
|
$
|
4,226
|
|
$
|
9,812
|
|
$
|
20,309
|
|
||||
|
Long-term debt obligations
|
—
|
|
—
|
|
22,212
|
|
3,173
|
|
25,385
|
|
|||||||||
|
Supplies purchase commitments
|
1,727
|
|
—
|
|
—
|
|
—
|
|
1,727
|
|
|||||||||
|
Capital lease obligation
|
317
|
|
634
|
|
—
|
|
—
|
|
951
|
|
|||||||||
|
Total
|
$
|
4,187
|
|
$
|
4,762
|
|
$
|
26,438
|
|
$
|
12,985
|
|
$
|
48,372
|
|
||||
|
|
Page No.
|
|
As of December 31,
|
|||||||
|
2016
|
2015
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
59,219
|
|
$
|
39,084
|
|
|
|
Accounts receivable
|
8,756
|
|
3,503
|
|
|||
|
Supplies inventory
|
3,475
|
|
3,767
|
|
|||
|
Prepaid expenses and other current assets
|
2,057
|
|
1,442
|
|
|||
|
Restricted cash
|
120
|
|
118
|
|
|||
|
Total current assets
|
73,627
|
|
47,914
|
|
|||
|
Property and equipment, net
|
11,480
|
|
10,314
|
|
|||
|
Finite-lived intangible assets, net
|
14,133
|
|
15,200
|
|
|||
|
Goodwill
|
1,057
|
|
1,057
|
|
|||
|
Restricted cash
|
603
|
|
603
|
|
|||
|
Other assets
|
134
|
|
159
|
|
|||
|
Total assets
|
$
|
101,034
|
|
$
|
75,247
|
|
|
|
Liabilities and Stockholders' Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
2,424
|
|
$
|
5,085
|
|
|
|
Accrued liabilities
|
9,110
|
|
8,689
|
|
|||
|
Deferred Genzyme co-promotion fee
|
—
|
|
948
|
|
|||
|
Total current liabilities
|
11,534
|
|
14,722
|
|
|||
|
Long-term debt
|
24,918
|
|
4,990
|
|
|||
|
Capital lease liability, net of current portion
|
599
|
|
—
|
|
|||
|
Deferred rent, net of current portion
|
4,402
|
|
4,283
|
|
|||
|
Total liabilities
|
41,453
|
|
23,995
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Stockholders' equity:
|
|||||||
|
Preferred stock, $0.001 par value; 5,000,000 shares authorized, no shares issued and outstanding as of December 31, 2016 and 2015
|
—
|
|
—
|
|
|||
|
Common stock, $0.001 par value; 125,000,000 shares authorized, 33,762,278 and 27,685,291 shares issued and outstanding as of December 31, 2016 and 2015, respectively
|
34
|
|
28
|
|
|||
|
Additional paid-in capital
|
239,631
|
|
199,950
|
|
|||
|
Accumulated deficit
|
(180,084
|
)
|
(148,726
|
)
|
|||
|
Total stockholders' equity
|
59,581
|
|
51,252
|
|
|||
|
Total liabilities and stockholders' equity
|
$
|
101,034
|
|
$
|
75,247
|
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Revenue
|
$
|
65,085
|
|
$
|
49,503
|
|
$
|
38,190
|
|
||
|
Operating Expenses:
|
|||||||||||
|
Cost of revenue
|
25,462
|
|
21,497
|
|
16,606
|
|
|||||
|
Research and development
|
15,324
|
|
12,796
|
|
9,804
|
|
|||||
|
Selling and marketing
|
28,248
|
|
25,293
|
|
21,932
|
|
|||||
|
General and administrative
|
23,787
|
|
22,583
|
|
18,854
|
|
|||||
|
Intangible asset amortization
|
1,067
|
|
800
|
|
—
|
|
|||||
|
Total operating expenses
|
93,888
|
|
82,969
|
|
67,196
|
|
|||||
|
Loss from operations
|
(28,803
|
)
|
(33,466
|
)
|
(29,006
|
)
|
|||||
|
Interest expense
|
(2,757
|
)
|
(378
|
)
|
(439
|
)
|
|||||
|
Other income, net
|
202
|
|
140
|
|
72
|
|
|||||
|
Net loss and comprehensive loss
|
$
|
(31,358
|
)
|
$
|
(33,704
|
)
|
$
|
(29,373
|
)
|
||
|
Net loss per common share, basic and diluted
|
$
|
(1.09
|
)
|
$
|
(1.30
|
)
|
$
|
(1.36
|
)
|
||
|
Shares used to compute net loss per common share, basic and diluted
|
28,830,472
|
|
25,994,193
|
|
21,639,374
|
|
|||||
|
|
Common Stock
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Total
Stockholders'
Equity
|
||||||||||||||
|
|
||||||||||||||||||
|
|
||||||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||
|
Balance at December 31, 2013
|
21,143,313
|
|
$
|
21
|
|
$
|
142,071
|
|
$
|
(85,649
|
)
|
$
|
56,443
|
|
||||
|
Issuance of common stock on exercise of stock options
|
402,100
|
|
1
|
|
674
|
|
—
|
|
675
|
|
||||||||
|
Issuance of common stock on cashless exercise of stock warrant
|
13,739
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Common stock subject to repurchase
|
|
|
—
|
|
3
|
|
—
|
|
3
|
|
||||||||
|
Issuance of common stock for acquisition
|
964,377
|
|
1
|
|
10,077
|
|
|
|
10,078
|
|
||||||||
|
Stock-based compensation expense (employee)
|
—
|
|
—
|
|
3,388
|
|
—
|
|
3,388
|
|
||||||||
|
Stock-based compensation expense (non-employee)
|
—
|
|
—
|
|
160
|
|
—
|
|
160
|
|
||||||||
|
Net loss and comprehensive loss
|
—
|
|
—
|
|
—
|
|
(29,373
|
)
|
(29,373
|
)
|
||||||||
|
Balance at December 31, 2014
|
22,523,529
|
|
23
|
|
156,373
|
|
(115,022
|
)
|
41,374
|
|
||||||||
|
Issuance of common stock on exercise of stock options
|
253,787
|
|
—
|
|
722
|
|
—
|
|
722
|
|
||||||||
|
Sale of common stock in a private placement, net of issuance costs of $2,742
|
4,907,975
|
|
5
|
|
37,253
|
|
—
|
|
37,258
|
|
||||||||
|
Stock-based compensation expense (employee)
|
—
|
|
—
|
|
5,302
|
|
—
|
|
5,302
|
|
||||||||
|
Stock-based compensation expense (non-employee)
|
—
|
|
—
|
|
110
|
|
—
|
|
110
|
|
||||||||
|
Stock-based compensation expense (ESPP)
|
—
|
|
—
|
|
190
|
|
—
|
|
190
|
|
||||||||
|
Net loss and comprehensive loss
|
—
|
|
—
|
|
—
|
|
(33,704
|
)
|
(33,704
|
)
|
||||||||
|
Balance at December 31, 2015
|
27,685,291
|
|
28
|
|
199,950
|
|
(148,726
|
)
|
51,252
|
|
||||||||
|
Issuance of common stock on exercise of stock options
|
212,740
|
|
—
|
|
538
|
|
—
|
|
538
|
|
||||||||
|
Issuance of common stock under employee stock purchase plan (ESPP)
|
140,947
|
|
—
|
|
678
|
|
—
|
|
678
|
|
||||||||
|
Sale of common stock in a public offering, net of issuance costs of $2,247
|
5,723,300
|
|
6
|
|
32,087
|
|
32,093
|
|
||||||||||
|
Stock-based compensation expense (employee)
|
—
|
|
—
|
|
6,046
|
|
—
|
|
6,046
|
|
||||||||
|
Stock-based compensation expense (non-employee)
|
—
|
|
—
|
|
15
|
|
—
|
|
15
|
|
||||||||
|
Stock-based compensation expense (ESPP)
|
—
|
|
—
|
|
317
|
|
—
|
|
317
|
|
||||||||
|
Net loss and comprehensive loss
|
—
|
|
—
|
|
—
|
|
(31,358
|
)
|
(31,358
|
)
|
||||||||
|
Balance at December 31, 2016
|
33,762,278
|
|
$
|
34
|
|
$
|
239,631
|
|
$
|
(180,084
|
)
|
$
|
59,581
|
|
||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Operating activities
|
|
|
|
|
|
|
|||||
|
Net loss
|
$
|
(31,358
|
)
|
$
|
(33,704
|
)
|
$
|
(29,373
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation and amortization
|
3,511
|
|
2,254
|
|
1,175
|
|
|||||
|
Bad debt expense
|
68
|
|
105
|
|
54
|
|
|||||
|
Loss on disposal of property and equipment
|
12
|
|
—
|
|
—
|
|
|||||
|
Genzyme co-promotion fee amortization
|
(948
|
)
|
(1,897
|
)
|
(2,269
|
)
|
|||||
|
Stock-based compensation
|
6,378
|
|
5,602
|
|
3,548
|
|
|||||
|
Conversion of accrued interest to long-term debt
|
385
|
|
—
|
|
—
|
|
|||||
|
Amortization of debt discount and issuance costs
|
173
|
|
46
|
|
97
|
|
|||||
|
Interest on debt balloon payment and prepayment penalty
|
206
|
|
79
|
|
81
|
|
|||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|||||
|
Accounts receivable
|
(5,321
|
)
|
(558
|
)
|
(1,961
|
)
|
|||||
|
Supplies inventory
|
292
|
|
(71
|
)
|
(1,129
|
)
|
|||||
|
Prepaid expenses and current other assets
|
(415
|
)
|
304
|
|
(38
|
)
|
|||||
|
Other assets
|
25
|
|
(42
|
)
|
(46
|
)
|
|||||
|
Accounts payable
|
(1,441
|
)
|
(3,546
|
)
|
1,874
|
|
|||||
|
Accrued liabilities and deferred rent
|
451
|
|
4,463
|
|
355
|
|
|||||
|
Net cash used in operating activities
|
(27,982
|
)
|
(26,965
|
)
|
(27,632
|
)
|
|||||
|
Investing activities
|
|
|
|
|
|
|
|||||
|
Purchases of property and equipment
|
(4,210
|
)
|
(6,165
|
)
|
(2,024
|
)
|
|||||
|
Cash remitted for acquisition, net of cash received
|
—
|
|
—
|
|
(6,916
|
)
|
|||||
|
Change in restricted cash
|
(2
|
)
|
(533
|
)
|
(70
|
)
|
|||||
|
Net cash used in investing activities
|
(4,212
|
)
|
(6,698
|
)
|
(9,010
|
)
|
|||||
|
Financing activities
|
|
|
|
|
|
|
|||||
|
Proceeds from the issuance of long-term debt, net of debt issuance costs
|
24,452
|
|
—
|
|
—
|
|
|||||
|
Proceeds from issuance of common stock in a private placement, net of issuance costs
|
—
|
|
37,258
|
|
—
|
|
|||||
|
Proceeds from issuance of common stock in a public offering, net of issuance costs
|
31,949
|
|
|||||||||
|
Commissions and issuance costs relating to initial public offering
|
—
|
|
—
|
|
(129
|
)
|
|||||
|
Payment of long-term debt
|
(5,000
|
)
|
—
|
|
—
|
|
|||||
|
Payment of end-of-term debt obligation and prepayment penalty
|
(288
|
)
|
—
|
|
(110
|
)
|
|||||
|
Payment of deferred stock offering costs
|
—
|
|
(247
|
)
|
—
|
|
|||||
|
Proceeds from the exercise of common stock options and employee stock purchases
|
1,216
|
|
722
|
|
675
|
|
|||||
|
Net cash provided by financing activities
|
52,329
|
|
37,733
|
|
436
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
20,135
|
|
4,070
|
|
(36,206
|
)
|
|||||
|
Cash and cash equivalents at beginning of period
|
39,084
|
|
35,014
|
|
71,220
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
59,219
|
|
$
|
39,084
|
|
$
|
35,014
|
|
||
|
Supplementary cash flow information of non-cash investing and financing activities:
|
|
|
|
|
|
|
|||||
|
Fair value of common stock issued for acquisition
|
—
|
|
—
|
|
$
|
10,078
|
|
||||
|
Non-cash issuance of long-term debt
|
—
|
|
—
|
|
5,000
|
|
|||||
|
Non-cash repayment of long-term debt
|
—
|
|
—
|
|
(5,000
|
)
|
|||||
|
Net receivable for reimbursement of public offering issuance costs
|
$
|
144
|
|
—
|
|
—
|
|
||||
|
Purchases of property and equipment included in accounts payable and accrued liabilities
|
363
|
|
$
|
1,825
|
|
383
|
|
||||
|
Issuance of common stock from the non-cash exercise of common stock warrants
|
—
|
|
—
|
|
187
|
|
|||||
|
Supplementary cash flow information:
|
|||||||||||
|
Cash paid for interest on debt
|
2,149
|
|
278
|
|
307
|
|
|||||
|
Cash paid for tax
|
7
|
|
22
|
|
—
|
|
|||||
|
|
Year Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Medicare
|
27
|
%
|
|
26
|
%
|
|
26
|
%
|
|
UnitedHealthcare
|
12
|
%
|
|
14
|
%
|
|
18
|
%
|
|
Aetna
|
8
|
%
|
|
9
|
%
|
|
11
|
%
|
|
47
|
%
|
|
49
|
%
|
|
55
|
%
|
|
|
|
December 31,
|
||||
|
|
2016
|
2015
|
|||
|
Medicare
|
18
|
%
|
31
|
%
|
|
|
UnitedHealthcare
|
8
|
%
|
25
|
%
|
|
|
Aetna
|
4
|
%
|
23
|
%
|
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
|
2016
|
2015
|
2014
|
|||||||||||||||||
|
Revenue recognized on the accrual basis
|
$
|
47,099
|
|
72
|
%
|
$
|
27,043
|
|
55
|
%
|
$
|
12,545
|
|
33
|
%
|
|||||
|
Revenue recognized on the cash basis
|
17,986
|
|
28
|
%
|
22,460
|
|
45
|
%
|
25,645
|
|
67
|
%
|
||||||||
|
Total
|
$
|
65,085
|
|
100
|
%
|
$
|
49,503
|
|
100
|
%
|
$
|
38,190
|
|
100
|
%
|
|||||
|
|
Year Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Shares of common stock subject to outstanding options
|
5,093,454
|
|
4,086,640
|
|
3,035,614
|
|
||
|
Employee stock purchase plan
|
36,651
|
|
15,561
|
|
—
|
|
||
|
Restricted stock units
|
25,000
|
|
—
|
|
—
|
|
||
|
Total common stock equivalents
|
5,155,105
|
|
4,102,201
|
|
3,035,614
|
|
||
|
Veracyte common stock
|
$
|
10,078
|
|
|
Cash
|
2,725
|
|
|
|
Payment of outstanding indebtedness
|
4,290
|
|
|
|
Total acquisition consideration
|
$
|
17,093
|
|
|
Cash and cash equivalents
|
$
|
29
|
|
|
Other assets, net
|
7
|
|
|
|
In-process research and development (IPR&D)
|
16,000
|
|
|
|
Goodwill
|
1,057
|
|
|
|
Total net assets acquired
|
$
|
17,093
|
|
|
|
Year Ended
December 31, |
||||||
|
|
2014
|
2013
|
|||||
|
Revenue
|
$
|
38,190
|
|
$
|
21,884
|
|
|
|
Net loss
|
$
|
(29,090
|
)
|
$
|
(28,605
|
)
|
|
|
•
|
The reversal of costs related to transaction bonuses and other payments to employees and acquisition-related expenses directly related to the Merger of
$2.2 million
for the year ended
December 31, 2014
; and
|
|
•
|
the elimination of interest expense related to Allegro indebtedness of
$2.3 million
and
$4.5 million
for the years ended
December 31, 2014
and
2013
, respectively.
|
|
|
Year Ended December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Leasehold improvements
|
$
|
5,861
|
|
$
|
789
|
|
|
|
Laboratory equipment
|
6,441
|
|
5,501
|
|
|||
|
Computer equipment
|
1,177
|
|
1,046
|
|
|||
|
Software, including software developed for internal use
|
1,937
|
|
1,353
|
|
|||
|
Furniture and fixtures
|
1,131
|
|
242
|
|
|||
|
Construction-in-process
|
1,769
|
|
6,823
|
|
|||
|
Total property and equipment, at cost
|
18,316
|
|
15,754
|
|
|||
|
Accumulated depreciation and amortization
|
(6,836
|
)
|
(5,440
|
)
|
|||
|
Total property and equipment, net
|
$
|
11,480
|
|
$
|
10,314
|
|
|
|
|
Year Ended December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Accrued compensation expense
|
$
|
6,120
|
|
$
|
4,212
|
|
|
|
Accrued Genzyme co-promotion fees
|
—
|
|
2,089
|
|
|||
|
Accrued other
|
2,990
|
|
2,388
|
|
|||
|
Total accrued liabilities
|
$
|
9,110
|
|
$
|
8,689
|
|
|
|
•
|
Level I: Inputs which include quoted prices in active markets for identical assets and liabilities.
|
|
•
|
Level II: Inputs other than Level I that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
•
|
Level III: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
|
|
Year Ending December 31,
|
Amounts
|
||
|
2017
|
$
|
2,143
|
|
|
2018
|
2,102
|
|
|
|
2019
|
2,026
|
|
|
|
2020
|
2,082
|
|
|
|
2021
|
2,144
|
|
|
|
Thereafter
|
9,812
|
|
|
|
Total minimum lease payments
|
$
|
20,309
|
|
|
|
December 31, 2016
|
||
|
Debt principal
|
$
|
25,385
|
|
|
Unamortized debt issuance costs
|
(467
|
)
|
|
|
Net debt obligation
|
$
|
24,918
|
|
|
Year Ending December 31,
|
|||
|
2020
|
$
|
9,519
|
|
|
2021
|
12,693
|
|
|
|
2022
|
3,173
|
|
|
|
Total
|
$
|
25,385
|
|
|
|
|||
|
|
2015
|
||
|
Debt and unpaid accrued end-of-term payment
|
$
|
5,082
|
|
|
Unamortized note discount
|
(92
|
)
|
|
|
Net debt obligation
|
$
|
4,990
|
|
|
|
Year Ended
December 31, |
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Nominal interest
|
$
|
2,378
|
|
$
|
253
|
|
$
|
296
|
|
||
|
Amortization and write-off of debt discount and debt issuance costs
|
173
|
|
46
|
|
62
|
|
|||||
|
End-of-term payment interest and prepayment penalty
|
206
|
|
79
|
|
81
|
|
|||||
|
Total
|
$
|
2,757
|
|
$
|
378
|
|
$
|
439
|
|
||
|
|
December 31,
|
||||
|
|
2016
|
2015
|
|||
|
Stock options and restricted stock units issued and outstanding
|
5,251,832
|
|
4,179,521
|
|
|
|
Stock options and restricted stock units available for grant under stock option plans
|
887,724
|
|
1,058,359
|
|
|
|
Common stock available for the Employee Stock Purchase Plan
|
609,053
|
|
750,000
|
|
|
|
Total
|
6,748,609
|
|
5,987,880
|
|
|
|
|
Shares
Available
for Grant
|
Stock Options
Outstanding |
Weighted
Average Exercise Price |
Weighted Average
Remaining Contractual Life (Years) |
Aggregate
Intrinsic Value |
||||||||||
|
Balance—December 31, 2015
|
1,058,359
|
|
4,179,521
|
|
$
|
8.03
|
|
7.50
|
$
|
6,511
|
|
||||
|
Additional shares authorized
|
1,114,416
|
|
—
|
|
|
|
|
|
|
||||||
|
Granted - stock options
|
(1,618,250
|
)
|
1,618,250
|
|
6.30
|
|
|
|
|
||||||
|
Granted - restricted stock units
|
(25,000
|
)
|
25,000
|
|
|||||||||||
|
Canceled
|
358,199
|
|
(358,199
|
)
|
10.29
|
|
|
|
|
||||||
|
Exercised
|
—
|
|
(212,740
|
)
|
2.53
|
|
|
|
|
||||||
|
Balance—December 31, 2016
|
887,724
|
|
5,251,832
|
|
$
|
7.56
|
|
7.24
|
$
|
8,515
|
|
||||
|
Options vested and exercisable—December 31, 2016
|
|
|
2,628,328
|
|
$
|
7.42
|
|
5.89
|
$
|
6,008
|
|
||||
|
Options vested and expected to vest—December 31, 2016
|
|
|
4,962,370
|
|
$
|
7.58
|
|
7.16
|
$
|
8,254
|
|
||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Cost of revenue
|
$
|
126
|
|
$
|
100
|
|
$
|
51
|
|
||
|
Research and development
|
1,322
|
|
1,178
|
|
790
|
|
|||||
|
Selling and marketing
|
1,594
|
|
1,326
|
|
707
|
|
|||||
|
General and administrative
|
3,336
|
|
2,998
|
|
2,000
|
|
|||||
|
Total stock-based compensation expense
|
$
|
6,378
|
|
$
|
5,602
|
|
$
|
3,548
|
|
||
|
|
Year Ended December 31,
|
||||
|
|
2016
|
2015
|
2014
|
||
|
Weighted-average volatility
|
52.49 - 56.36%
|
52.56 - 68.82%
|
70.19 - 78.54%
|
||
|
Weighted-average expected term (years)
|
5.50 - 6.27
|
5.50 - 6.08
|
5.50 - 6.08
|
||
|
Risk-free interest rate
|
1.16 - 2.09%
|
1.55 - 2.03%
|
1.66 - 2.04%
|
||
|
Expected dividend yield
|
—
|
—
|
—
|
||
|
|
Year Ended December 31,
|
||||
|
|
2016
|
2015
|
2014
|
||
|
Weighted-average volatility
|
52.77 - 65.85%
|
64.72 - 74.48%
|
73.20 - 74.48%
|
||
|
Weighted-average expected term (years)
|
7.80 - 8.56
|
7.92 - 10.00
|
8.75 - 10.00
|
||
|
Risk-free interest rate
|
1.39 - 2.30%
|
1.78 - 2.29%
|
2.09 - 2.20%
|
||
|
Expected dividend yield
|
—
|
—
|
—
|
||
|
Year Ended December 31,
|
|||
|
|
2016
|
2015
|
|
|
Weighted-average volatility
|
46.38 - 75.72%
|
53.57 - 58.10%
|
|
|
Weighted-average expected term (years)
|
0.50 - 1.00
|
0.49 - 0.99
|
|
|
Risk-free interest rate
|
0.40 - 0.50%
|
0.17 - 0.33%
|
|
|
Expected dividend yield
|
—
|
—
|
|
|
|
Year Ended December, 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
U.S. federal taxes at statutory rate
|
$
|
(10,662
|
)
|
$
|
(11,459
|
)
|
$
|
(9,987
|
)
|
||
|
State tax (net of federal benefit)
|
20
|
|
(30
|
)
|
5
|
|
|||||
|
Permanent differences
|
153
|
|
96
|
|
64
|
|
|||||
|
Incentive stock options
|
1,095
|
|
789
|
|
672
|
|
|||||
|
Tax credits
|
(677
|
)
|
(581
|
)
|
(461
|
)
|
|||||
|
Change in valuation allowance
|
10,071
|
|
11,185
|
|
9,707
|
|
|||||
|
Total
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Deferred tax assets:
|
|
|
|
|
|
|
|||||
|
Net operating loss carryforwards
|
$
|
61,674
|
|
$
|
52,262
|
|
$
|
41,971
|
|
||
|
Research and development credits
|
3,174
|
|
2,497
|
|
1,916
|
|
|||||
|
Stock-based compensation
|
2,847
|
|
1,825
|
|
826
|
|
|||||
|
Genzyme co-promotion agreement
|
—
|
|
330
|
|
995
|
|
|||||
|
Accruals, deferred rent and other
|
4,511
|
|
4,698
|
|
3,381
|
|
|||||
|
Gross deferred tax assets
|
72,206
|
|
61,612
|
|
49,089
|
|
|||||
|
Valuation allowance
|
(65,975
|
)
|
(55,101
|
)
|
(43,439
|
)
|
|||||
|
Net deferred tax assets
|
6,231
|
|
6,511
|
|
5,650
|
|
|||||
|
Deferred tax liabilities:
|
|
|
|
|
|
|
|||||
|
Property and equipment
|
(1,180
|
)
|
(1,215
|
)
|
(60
|
)
|
|||||
|
In-process research and development
|
(5,051
|
)
|
(5,296
|
)
|
(5,590
|
)
|
|||||
|
Gross deferred tax liabilities
|
(6,231
|
)
|
(6,511
|
)
|
(5,650
|
)
|
|||||
|
Net deferred tax liabilities
|
(6,231
|
)
|
(6,511
|
)
|
(5,650
|
)
|
|||||
|
Net deferred taxes
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Unrecognized tax benefits, beginning of period
|
$
|
1,871
|
|
$
|
1,571
|
|
$
|
727
|
|
||
|
Gross increases—tax position in prior period
|
—
|
|
—
|
|
548
|
|
|||||
|
Gross decreases—tax position in prior period
|
—
|
|
—
|
|
—
|
|
|||||
|
Gross increases—current period tax position
|
351
|
|
300
|
|
296
|
|
|||||
|
Lapse of statute of limitations
|
—
|
|
—
|
|
—
|
|
|||||
|
Unrecognized tax benefits, end of period
|
$
|
2,222
|
|
$
|
1,871
|
|
$
|
1,571
|
|
||
|
Quarter Ended
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||
|
2016:
|
|
|
|
|
|
|
|
|
|||||||
|
Revenue
|
$
|
13,550
|
|
$
|
14,675
|
|
$
|
18,603
|
|
$
|
18,257
|
|
|||
|
Net loss
|
(10,075
|
)
|
(11,243
|
)
|
(5,637
|
)
|
(4,403
|
)
|
|||||||
|
Net loss per common share, basic and diluted
|
(0.36
|
)
|
(0.40
|
)
|
(0.20
|
)
|
(0.14
|
)
|
|||||||
|
Shares used to compute net loss per common share, basic and diluted
|
27,817,993
|
|
27,859,918
|
|
27,916,819
|
|
31,705,603
|
|
|||||||
|
2015:
|
|
|
|
|
|
|
|
|
|||||||
|
Revenue
|
$
|
11,218
|
|
$
|
11,908
|
|
$
|
12,335
|
|
$
|
14,042
|
|
|||
|
Net loss
|
(7,610
|
)
|
(9,136
|
)
|
(8,945
|
)
|
(8,013
|
)
|
|||||||
|
Net loss per common share, basic and diluted
|
(0.34
|
)
|
(0.35
|
)
|
(0.32
|
)
|
(0.29
|
)
|
|||||||
|
Shares used to compute net loss per common share, basic and diluted
|
22,539,723
|
|
26,048,934
|
|
27,640,806
|
|
27,672,806
|
|
|||||||
|
(a)
|
Documents filed as part of this report
|
|
(b)
|
Exhibits
|
|
Exhibit Number
|
Description
|
||
|
3.1
|
|
Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed November 8, 2013).
|
|
|
|
|
||
|
3.2
|
|
Amended and Restated Bylaws of the Registrant (incorporated by reference to Exhibit 3.2 to the Registrant's Current Report on Form 8-K filed November 8, 2013).
|
|
|
|
|
||
|
4.1
|
|
Form of Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
||
|
4.2
|
|
Second Amended and Restated Investors Rights Agreement, dated November 6, 2012, between the Registrant and certain investors (incorporated by reference to Exhibit 4.2 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
||
|
4.3
|
|
Amendment to Second Amended and Restated Investors Rights Agreement, dated June 14, 2013, between the Registrant and certain investors (incorporated by reference to Exhibit 4.3 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
||
|
10.1
|
|
#
|
Form of Indemnification Agreement between the Registrant and its officers and directors (incorporated by reference to Exhibit 10.1 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.2
|
|
#
|
2008 Stock Plan and forms of agreements thereunder (incorporated by reference to Exhibit 10.2 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.3
|
|
#
|
2013 Stock Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
Exhibit Number
|
Description
|
||
|
10.4
|
|
# *
|
Forms of agreements under the 2013 Stock Incentive Plan.
|
|
|
|
|
|
|
10.5
|
|
#
|
Veracyte, Inc. Employee Stock Purchase Plan (incorporated by reference to Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2015, as amended).
|
|
|
|
|
|
|
10.6
|
|
|
Lease Agreement between Riata Holdings, L.P., as landlord, and the Registrant, as tenant, dated November 28, 2012 (incorporated by reference to Exhibit 10.6 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.7
|
|
|
First Amendment to Lease Agreement dated as of January 7, 2014 by and between Riata Holdings, L.P. and the Registrant (incorporated by reference to Exhibit 10.7 to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2013 filed March 20, 2014).
|
|
|
|
|
|
|
10.8
|
|
|
Office Building Lease by and between American Fund US Investments LP and the Registrant dated April 29, 2015 (incorporated by reference to Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2015, as amended).
|
|
10.9
|
|
*
|
First Amendment to Office Building Lease dated May 3, 2016 by and between American Fund US Investments LP and the Registrant.
|
|
10.10
|
|
*
|
Second Amendment to Office Building Lease dated February 8, 2017 by and between CRP 6000 Shoreline, L.L.C. and the Registrant.
|
|
|
|
|
|
|
10.11
|
|
#
|
Employment Agreement, dated as of February 15, 2008, between Bonnie Anderson and the Registrant (incorporated by reference to Exhibit 10.10 to the Registrant's Registration Statement on Form S-1 (File No. 333- 191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.12
|
|
#
|
Amendment to Bonnie Anderson Employment Agreement, dated as of December 22, 2008, between Bonnie Anderson and the Registrant (incorporated by reference to Exhibit 10.11 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.13
|
|
#
|
Amendment No. 2 to Bonnie Anderson Employment Agreement, effective as of March 11, 2009, between Bonnie Anderson and the Registrant (incorporated by reference to Exhibit 10.12 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
10.14
|
|
#
|
Amended and Restated Change of Control and Severance Agreement, effective as of May 14, 2015, between Bonnie Anderson and the Registrant (incorporated by reference to Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2015, as amended).
|
|
|
|
|
|
|
10.15
|
|
#
|
Amended and Restated Change of Control and Severance Agreement, effective as of May 14, 2015, between Christopher Hall and the Registrant (incorporated by reference to Exhibit 10.3 to the Registrant's Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2015, as amended).
|
|
|
|
|
|
|
10.16
|
|
#
|
Amended and Restated Change of Control and Severance Agreement, effective as of May 14, 2015, between Shelly Guyer and the Registrant (incorporated by reference to Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2015, as amended).
|
|
|
|
|
|
|
10.17
|
|
#
|
Amended and Restated Change of Control and Severance Agreement, effective as of May 14, 2015, between Julie Brooks and the Registrant (incorporated by reference to Exhibit 10.4 to the Registrant's Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2015, as amended).
|
|
|
|
|
|
|
10.18
|
|
# *
|
Change of Control and Severance Agreement, effective as of February 15, 2017, between Keith Kennedy and the Registrant.
|
|
Exhibit Number
|
Description
|
||
|
10.19
|
|
#
|
Offer Letter dated as of April 8, 2013 with Shelly D. Guyer (incorporated by reference to Exhibit 10.17 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.20
|
|
#
|
Offer Letter dated as of January 28, 2010 with Christopher M. Hall (incorporated by reference to Exhibit 10.18 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.21
|
|
†
|
Pathology Services Agreement dated as of November 12, 2010 between Brazos Valley Pathology, P.A. D/B/A Reitpath and the Registrant (incorporated by reference to Exhibit 10.19 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.22
|
|
|
Approval of the Registrant to the Assignment of the Pathology Services Agreement with Brazos Valley Pathology to Thyroid Cytopathology Partners, P.A. as of May 18, 2011 (incorporated by reference to Exhibit 10.20 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.23
|
|
|
First Amendment to Pathology Services Agreement dated as of December 19, 2012 between Thyroid Cytopathology Partners, P.A. and the Registrant (incorporated by reference to Exhibit 10.21 to the Registrant's Registration Statement on Form S-1 (File No. 333-191282), as amended, declared effective on October 29, 2013).
|
|
|
|
|
|
|
10.24
|
|
|
Second Amendment to Pathology Services Agreement dated as of January 1, 2014 by and between the Registrant and Thyroid Cytopathology Partners, P.A. (incorporated by reference to Exhibit 10.23 to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2013).
|
|
|
|
|
|
|
10.25
|
|
†
|
Ex-U.S. Co-Promotion Agreement between the Registrant and Genzyme Corporation (incorporated by reference to Exhibit 10.26 to the Registrant's Annual Report Form 10-K for the year ended December 31, 2015, as amended).
|
|
10.26
|
|
|
Credit Agreement dated as of March 25, 2016 by and among Veracyte, Inc. as Borrower, Visium Healthcare Partners, LP, as Administrative Agent, the Guarantors from time to time party thereto and the Lenders from time to time party thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2016).
|
|
10.27
|
|
|
Security Agreement dated March 30, 2016 between Veracyte, Inc. and Visium Healthcare Partners, LP, as Administrative Agent (incorporated by reference to Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2016).
|
|
10.28
|
|
|
Pledge Agreement dated March 30, 2016 between Veracyte, Inc. and Visium Healthcare Partners, LP, as Administrative Agent (incorporated by reference to Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2016).
|
|
10.29
|
|
|
Letter agreement regarding potential opportunity to purchase common equity interests dated March 30, 2016 between Veracyte, Inc. and Visium Healthcare Partners, LP (incorporated by reference to Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2016).
|
|
10.30
|
|
*
|
First Amendment to Credit Agreement dated as of December 14, 2016 by and among Veracyte, Inc. as Borrower, Visium Healthcare Partners, LP, as Administrative Agent, the Guarantors from time to time party thereto and the Lenders from time to time party thereto.
|
|
12.1
|
|
*
|
Statement Regarding Computation of Ratios.
|
|
|
|
|
|
|
23.1
|
|
*
|
Consent of independent registered public accounting firm.
|
|
|
|
|
|
|
24.1
|
|
*
|
Power of Attorney (see the signature page of this Annual Report on Form 10-K).
|
|
Exhibit Number
|
Description
|
||
|
|
|
|
|
|
31.1
|
|
*
|
Principal Executive Officer's Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
|
31.2
|
|
*
|
Principal Financial Officer's Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
|
32.1
|
|
* **
|
Certification Pursuant to 18 U.S.C. § 1350 (Section 906 of the Sarbanes-Oxley Act of 2002).
|
|
|
|
|
|
|
32.2
|
|
* **
|
Certification Pursuant to 18 U.S.C. § 1350 (Section 906 of the Sarbanes-Oxley Act of 2002).
|
|
|
|
|
|
|
101.INS
|
|
|
XBRL Instance Document
|
|
|
|
|
|
|
101.SCH
|
|
|
XBRL Taxonomy Extension Schema
|
|
|
|
|
|
|
101.CAL
|
|
|
XBRL Taxonomy Extension Calculation Linkbase
|
|
|
|
|
|
|
101.DEF
|
|
|
XBRL Taxonomy Extension Definition Linkbase
|
|
|
|
|
|
|
101.LAB
|
|
|
XBRL Taxonomy Extension Label Linkbase
|
|
|
|
|
|
|
101.PRE
|
|
|
XBRL Taxonomy Extension Presentation Linkbase
|
|
†
|
Confidential treatment has been granted with respect to certain portions of this Exhibit.
|
|
|
#
|
Indicates management contract or compensatory plan or arrangement.
|
|
|
*
|
Filed herewith.
|
|
|
**
|
In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release No. 34-47986, the certifications furnished in Exhibits 32.1 and 32.2 hereto are deemed to accompany this Form 10-K and will not be deemed "filed" for purposes of Section 18 of the Securities Exchange Act of 1934 (the "Exchange Act") or deemed to be incorporated by reference into any filing under the Exchange Act or the Securities Act of 1933 except to the extent that the registrant specifically incorporates it by reference.
|
|
|
(c)
|
Financial Statement Schedules
|
|
|
|
VERACYTE, INC.
|
|
|
By:
|
/s/ BONNIE H. ANDERSON
|
||
|
|
Bonnie H. Anderson
Chairman and Chief Executive Officer
|
||
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ BONNIE H. ANDERSON
|
Chairman and Chief Executive Officer (Principal Executive Officer)
|
March 1, 2017
|
||
|
Bonnie H. Anderson
|
|
|
||
|
/s/ KEITH S. KENNEDY
|
Chief Financial Officer (Principal Financial and Accounting Officer)
|
March 1, 2017
|
||
|
Keith S. Kennedy
|
|
|
||
|
/s/ JOHN L. BISHOP
|
Lead Independent Director
|
March 1, 2017
|
||
|
John L. Bishop
|
|
|
||
|
/s/ FRED E. COHEN, M.D., D.PHIL.
|
Director
|
|
March 1, 2017
|
|
|
Fred E. Cohen, M.D., D.Phil.
|
|
|||
|
/s/ KARIN EASTHAM
|
Director
|
|
March 1, 2017
|
|
|
Karin Eastham
|
|
|||
|
/s/ ROBERT S. EPSTEIN
|
Director
|
|
March 1, 2017
|
|
|
Robert S. Epstein
|
|
|||
|
/s/ KEVIN K. GORDON
|
Director
|
|
March 1, 2017
|
|
|
Kevin K. Gordon
|
|
|||
|
/s/ EVAN JONES
|
Director
|
|
March 1, 2017
|
|
|
Evan Jones
|
|
|||
|
/s/ TINA S. NOVA, PH.D.
|
Director
|
|
March 1, 2017
|
|
|
Tina S. Nova, Ph.D.
|
|
|||
|
/s/ JESSE I. TREU, PH.D.
|
Director
|
|
March 1, 2017
|
|
|
Jesse I. Treu, Ph.D.
|
|
|||