|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
|
47-1187261
(I.R.S. Employer
Identification No.)
|
|
6200 Lookout Road, Boulder, CO
(Address of principal executive offices)
|
80301
(Zip Code)
|
|
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, $0.01 par value
|
The Nasdaq Capital Market
|
|
Large accelerated filer
o
|
|
Accelerated filer
o
|
|
Non-accelerated filer
o
(do not check if a smaller reporting company)
|
|
Smaller reporting company
x
|
|
Emerging growth company
x
|
||
|
Page No.
|
||
|
PART I
|
||
|
PART II
|
|
|
|
PART III
|
||
|
PART IV
|
|
|
|
•
|
We have incurred losses since our inception, have a limited operating history on which to assess our business, and anticipate that we will continue to incur significant losses for the foreseeable future.
|
|
•
|
Raising additional capital may cause dilution to our stockholders, restrict our operations, or require us to relinquish rights.
|
|
•
|
We have never generated any revenue from product sales and may never be profitable.
|
|
•
|
We are heavily dependent on the success of our product candidates, which are in the early stages of clinical development. Some of our product candidates have produced results only in early stage or pre-clinical settings, or for other indications than those for which we contemplate conducting development and seeking U.S. Food and Drug Administration, or FDA, approval for, and we cannot give any assurance that we will generate sufficient data for any of our product candidates to receive regulatory approval in our planned indications, which will be required before they can be commercialized
|
|
•
|
Regardless of clinical trial results, the FDA and other regulatory agencies may fail to approve our product candidates for marketing.
|
|
•
|
We may be unsuccessful in maintaining orphan-drug designation for our product candidates because even after an orphan drug is approved, the FDA can subsequently approve a different drug for the same indication if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective, or makes a major contribution to patient care.
|
|
•
|
Clinical trials are costly, time consuming, and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
|
|
•
|
The approach we are taking to discover and develop novel therapeutics that target microRNAs is unproven and may never lead to marketable products.
|
|
•
|
Our microRNA-targeted therapeutic product candidates are based on a relatively novel technology, which makes it unusually difficult to predict the time and cost of development, and the time and cost, or likelihood, of obtaining regulatory approval. To date, no microRNA-targeted therapeutics have been approved for marketing in the United States.
|
|
•
|
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent regulatory approval, limit the commercial viability of an approved label, or result in significant negative consequences following marketing approval, if any.
|
|
•
|
We may use our financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
|
|
•
|
We face substantial competition, and our competitors may discover, develop, or commercialize products faster or more successfully than us.
|
|
•
|
We may be unable to realize the potential benefits of any collaboration.
|
|
•
|
We may attempt to form collaborations in the future with respect to our product candidates, but we may not be able to do so, which may cause us to alter our development and commercialization plans.
|
|
•
|
We may not be able to develop or identify technology that can effectively deliver MRG-106, or cobomarsen, MRG-201, or any other of our microRNA-targeted product candidates to the intended diseased cells or tissues, and any failure in such delivery technology could adversely affect and delay the development of cobomarsen, MRG-201, and our other product candidates.
|
|
•
|
If equity research analysts do not publish research or reports, or publish unfavorable research or reports, about us, our business, or our market, our stock price and trading volume could decline.
|

|
•
|
Presentation of additional Phase 1 CTCL data, including response rates from longer-term duration of treatment (1H 2018)
|
|
•
|
Phase 1 interim clinical data release in at least one potential expansion indication (2H 2018)
|
|
•
|
Initiation of a Phase 2 clinical trial in CTCL (2H 2018)
|
|
•
|
Presentation of Phase 2 CTCL clinical trial data (2H 2020)
|
|
•
|
Initiation of a Phase 2 clinical trial in cutaneous fibrosis (1H 2018)
|
|
•
|
Ocular fibrosis data release from preclinical models (1H 2018)
|
|
•
|
Preclinical safety and efficacy lung fibrosis data release (2H 2018)
|
|
•
|
Presentation of Phase 2 cutaneous fibrosis clinical trial data (2019)
|
|
•
|
Initiation of two Phase 1 clinical trials (1H 2018)
|
|
•
|
Continue to develop cobomarsen for blood cancers.
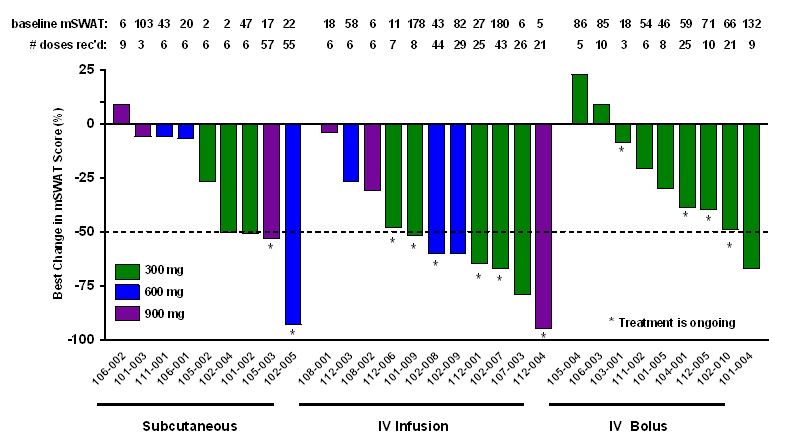
Cobomarsen is currently being developed in a Phase 1 clinical trial in multiple oncology indications. We intend to initiate a Phase 2 clinical trial for cobomarsen in patients with mycosis fungoides, or MF, the most common type of CTCL in the second half of 2018 using a 300 mg intravenous infusion, or IV infusion. This dosage and administration method demonstrated an 80% objective response rate in this cohort of five patients in the Phase 1 clinical trial. In addition to CTCL, we are also developing cobomarsen in three expansion indications where the disease process appears to correlate with an increase in miR-155 levels, the target of cobomarsen. These additional indications are adult T-cell leukemia/lymphoma, diffuse large B-cell lymphoma, and
|
|
•
|
Continue to develop MRG-201 for pathological fibrosis.
We intend to initiate a double blinded, randomized Phase 2 clinical trial to evaluate MRG-201 in subjects with a predisposition for keloid formation in the first half of 2018. In 2017, we announced results from the double-blind, placebo-controlled, single and multiple dose-escalation Phase 1 clinical trial evaluating MRG-201 in induced cutaneous fibrosis. In the trial, treatment with MRG-201 appeared to result in a reduction in fibroplasia, a histopathological marker of scar tissue deposition, while not adversely affecting wound healing. Additional indications to be studied for a miR-29 mimic could include fibrotic diseases of the lung and eye.
|
|
•
|
Utilize rare disease development pathways at the FDA and comparable programs at foreign regulatory agencies to accelerate progression to late-stage development and early approval.
For our wholly-owned programs, we intend to focus on rare and genetic diseases where RNA modulation may produce clinical benefit, so that we can potentially take advantage of regulatory programs intended to expedite drug development. In March 2017, we announced that the FDA granted orphan-drug designation to cobomarsen, for the treatment of MF. Additionally, in May 2017, we announced that the European Commission granted orphan medicinal product designation to cobomarsen for the treatment of CTCL. We plan to apply for the regulatory programs for orphan drug designation, fast track, breakthrough therapy designation, and/or priority review when available to potentially reduce clinical trial expense and decrease time to commercialization.
|
|
•
|
Collaborate with other biotechnology and pharmaceutical companies to develop additional product candidates.
We intend to seek out collaborations for the development of compounds in our pipeline for certain disease areas where the costs would exceed our resources or in other areas where we believe that leveraging a partner’s expertise or resources will allow us to accelerate development timelines. For example, we have a strategic collaboration with Servier to develop product candidates for the treatment of cardiovascular diseases.
|
|
•
|
Use our in-house research and translational expertise to further develop our product candidate pipeline.
Our in-house research team investigates microRNAs that have been identified as potential therapeutic targets through internal efforts and academic collaborations. We then seek to establish evidence that modulation of the microRNAs’ activity may provide benefit in pathological conditions or diseases in which the microRNA is implicated. We believe that this internal research and expertise could provide a foundation to develop product candidates for the treatment of a variety of diseases.
|
|
•
|
Selectively build focused commercial capabilities and establish commercial collaborations to maximize the value of our pipeline.
To date, we have retained all U.S. and Japanese rights to our product candidates in the strategic collaboration with Servier and global rights in all of our other programs. While we have not yet defined our sales, marketing, or product distribution strategy for cobomarsen, MRG-201, MRG-110, or any of our other product candidates, if approved, our commercial strategy may include the use of strategic alliances, distributors, a contract sales force, or the establishment of our own commercial and specialty sales force to maximize the value of our pipeline.
|
|
Type of Pathological Fibrosis
|
|
Description
|
|
|
Skin Fibrosis
|
|
Ÿ
|
Scarring is a result of an over production of collagen in a healing wound. Scarring may continue to thicken for up to six months or may overgrow the site of the wound, even after the wound has healed.
|
|
|
|
Ÿ
|
Hypertrophic scars and keloids are abnormal wound responses and represent an excessive connective tissue response to skin trauma, inflammation, surgery, or burns.
|
|
|
|
Ÿ
|
Hypertrophic scars and keloids are characterized by local fibroblast proliferation and overproduction of collagen. Both hypertrophic scars and keloids are diseases that tend to be painful and itchy, restrict mobility, and are resistant to treatment.
|
|
Pulmonary Fibrosis
|
|
Ÿ
|
Pulmonary fibrosis, also known as lung fibrosis, is caused by accumulation of scar tissues surrounding the air sacs (interstitial space) in the lung. As a result, the lung tissue becomes stiff and loses the ability to expand. The scar tissue also prevents normal transport of oxygen. The result is a progressive respiratory failure, with symptoms that include persistent cough, chest pain, difficulty breathing and fatigue. Pulmonary fibrosis leads to cardiac failure and death. Pulmonary fibrosis may occur as a secondary condition in various other diseases, but in many cases the underlying cause is not clear and is referred to as IPF.
|
|
|
|
Ÿ
|
IPF is a chronic, progressive lung disease which ultimately leads to death in many of the patients. This condition causes scar tissue to build up in the lungs, which makes the lungs unable to transport oxygen into the bloodstream effectively.
|
|
Liver Fibrosis
|
|
Ÿ
|
Liver fibrosis refers to the scar tissue and nodules that replace liver tissue and disrupt liver function. Major causes of liver fibrosis are alcohol, chronic hepatitis B virus, hepatitis C virus infection along with the metabolic disorders non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Liver fibrosis is a major global problem driven by increasing rates of obesity and diabetes.
|
|
Eye Fibrosis
|
|
Ÿ
|
Infection or inflammation of the eye results in impairment of visual function. Chronic inflammation can ultimately lead to fibrosis.
|
|
|
|
Ÿ
|
Eye fibrosis diseases include retinal fibrosis such as diabetic retinopathy and proliferative vitreoretinopathy, corneal fibrosis, glaucoma trabeculectomy, age-related macular degeneration, and Fuch’s endothelial corneal dystrophy.
|
|
•
|
Part A studied the expression of biomarker genes in skin at different time points following an incision and was performed without MRG-201 administration;
|
|
•
|
|
|
•
|
|
|
•
|
|
|
•
|
companies working to develop microRNA targeted products, including Regulus Therapeutics Inc., Microlin Bio, Inc., and InteRNA Technologies B.V.;
|
|
•
|
companies working to develop other types of oligonucleotide therapeutic products, including Ionis Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., Arrowhead Pharmaceuticals, Inc., Dicerna Pharmaceuticals, Inc., RaNa Therapeutics, Inc., RXi Pharmaceuticals Corporation, and Silence Therapeutics AG; and
|
|
•
|
companies with marketed products and development programs for therapeutics that treat the same diseases for which we may also be developing potential treatments.
|
|
•
|
completion of extensive preclinical laboratory tests, animal studies, and formulation studies in accordance with the FDA’s good laboratory practices, or GLP, regulations;
|
|
•
|
approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated at that site;
|
|
•
|
submission to the FDA of an investigational new drug application, or IND, for human clinical testing, which must become effective before human clinical trials may begin;
|
|
•
|
performance of adequate and well-controlled human clinical trials in accordance with good clinical practice, or GCP, requirements to establish the safety and efficacy of the drug for each proposed indication;
|
|
•
|
submission to the FDA of an NDA after completion of all pivotal clinical trials;
|
|
•
|
satisfactory completion of an FDA advisory committee review, if applicable
|
|
•
|
satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the active pharmaceutical ingredient, or API, and finished drug product are produced and tested to assess compliance with current good manufacturing practices, or cGMPs; and
|
|
•
|
FDA review and approval of the NDA prior to any commercial marketing or sale of the drug in the United States.
|
|
•
|
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market, or product recalls;
|
|
•
|
fines, warning letters, or holds on post-approval clinical trials;
|
|
•
|
refusal of the FDA to approve pending applications or supplements to approved applications or suspension or revocation of product approvals;
|
|
•
|
product seizure or detention, or refusal to permit the import or export of products, or injunctions or the imposition of civil or criminal penalties.
|
|
•
|
continue the clinical development of our product candidates;
|
|
•
|
continue efforts to discover and develop new product candidates;
|
|
•
|
undertake the manufacturing of our product candidates or increase volumes manufactured by third parties;
|
|
•
|
advance our programs into larger, more expensive clinical trials;
|
|
•
|
initiate additional preclinical, clinical, or other trials or studies for our product candidates;
|
|
•
|
seek regulatory and marketing approvals and reimbursement for our product candidates;
|
|
•
|
establish a sales, marketing, and distribution infrastructure to commercialize any products for which we may obtain marketing approval and market for ourselves;
|
|
•
|
seek to identify, assess, acquire, and/or develop other product candidates;
|
|
•
|
make milestone, royalty, or other payments under third-party license agreements;
|
|
•
|
seek to maintain, protect, and expand our intellectual property portfolio;
|
|
•
|
seek to attract and retain skilled personnel; and
|
|
•
|
experience any delays or encounter issues with the development and potential for regulatory approval of our clinical candidates such as safety issues, manufacturing delays, clinical trial accrual delays, longer follow-up for planned studies, additional major studies, or supportive studies necessary to support marketing approval.
|
|
•
|
completing research and development of our product candidates;
|
|
•
|
obtaining regulatory and marketing approvals for our product candidates;
|
|
•
|
manufacturing product candidates and establishing and maintaining supply and manufacturing relationships with third parties that are commercially feasible, meet regulatory requirements and our supply needs in sufficient quantities to meet market demand for our product candidates, if approved;
|
|
•
|
marketing, launching, and commercializing product candidates for which we obtain regulatory and marketing approval, either directly or with a collaborator or distributor;
|
|
•
|
gaining market acceptance of our product candidates as treatment options;
|
|
•
|
addressing any competing products;
|
|
•
|
protecting and enforcing our intellectual property rights, including patents, trade secrets, and know-how;
|
|
•
|
negotiating favorable terms in any collaboration, licensing, or other arrangements into which we may enter;
|
|
•
|
obtaining reimbursement or pricing for our product candidates that supports profitability; and
|
|
•
|
attracting, hiring, and retaining qualified personnel.
|
|
•
|
inability to generate satisfactory preclinical, toxicology, or other in vivo or in vitro data or diagnostics to support the initiation or continuation of clinical trials;
|
|
•
|
delays in reaching agreement on acceptable terms with contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs
|
|
•
|
delays in obtaining required institutional review board approval at each clinical trial site;
|
|
•
|
failure to permit the conduct of a clinical trial by regulatory authorities, after review of an investigational new drug or equivalent foreign application or amendment;
|
|
•
|
delays in recruiting qualified patients in our clinical trials;
|
|
•
|
failure by clinical sites or CROs or other third parties to adhere to clinical trial requirements;
|
|
•
|
failure by our clinical sites, CROs or other third parties to perform in accordance with the good clinical practices requirements of the FDA or applicable foreign regulatory guidelines;
|
|
•
|
patients dropping out of our clinical trials;
|
|
•
|
adverse events or tolerability or animal toxicology issues significant enough for the FDA or other regulatory agencies to put any or all clinical trials on hold;
|
|
•
|
occurrence of adverse events associated with our product candidates;
|
|
•
|
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols;
|
|
•
|
the cost of clinical trials of our product candidates;
|
|
•
|
negative or inconclusive results from our clinical trials, which may result in our deciding, or regulators requiring us, to conduct additional clinical trials or abandon development programs in other ongoing or planned indications for a product candidate; and
|
|
•
|
delays in reaching agreement on acceptable terms with third-party manufacturers and the time for manufacture of sufficient quantities of our product candidates for use in clinical trials.
|
|
•
|
regulatory authorities may withdraw approvals of such products;
|
|
•
|
regulatory authorities may require additional warnings on the drug label;
|
|
•
|
we may be required to create a Risk Evaluation and Mitigation Strategy, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers, and/or other elements to assure safe use;
|
|
•
|
we could be sued and held liable for harm caused to patients; and
|
|
•
|
our reputation may suffer.
|
|
•
|
withdrawal of clinical trial volunteers, investigators, patients or trial sites, or limitations on approved indications;
|
|
•
|
the inability to commercialize, or if commercialized, decreased demand for, our product candidates;
|
|
•
|
if commercialized, product recalls, labeling, marketing or promotional restrictions, or the need for product modification;
|
|
•
|
initiation of investigations by regulators;
|
|
•
|
loss of revenues;
|
|
•
|
substantial costs of litigation, including monetary awards to patients or other claimants;
|
|
•
|
liabilities that substantially exceed our product liability insurance, which we would then be required to pay ourselves;
|
|
•
|
an increase in our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable terms, if at all;
|
|
•
|
the diversion of management’s attention from our business; and
|
|
•
|
damage to our reputation and the reputation of our products and our technology.
|
|
•
|
issue warning letters;
|
|
•
|
impose civil or criminal penalties;
|
|
•
|
suspend or withdraw regulatory approval;
|
|
•
|
suspend any of our ongoing clinical trials;
|
|
•
|
refuse to approve pending applications or supplements to approved applications submitted by us;
|
|
•
|
impose restrictions on our operations, including closing our contract manufacturers’ facilities; or
|
|
•
|
require a product recall.
|
|
•
|
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs;
|
|
•
|
federal civil and criminal false claims laws and civil monetary penalties law, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
|
|
•
|
the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their implementing regulations, which imposes specified obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information without the appropriate authorization, on entities subject to the law, such as healthcare providers, health plans, and healthcare clearinghouses and their respective business associates that perform services for them that involve the creation, use, maintenance, or disclosure of individually identifiable health information;
|
|
•
|
the federal Physician Payment Sunshine Act under the Affordable Care Act requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services
|
|
•
|
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including governmental and private payors, to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and state laws governing the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
|
|
•
|
require repayment of all or a portion of the grant proceeds, in specified cases with interest, in the event we violate specified covenants pertaining to various matters that include a failure to achieve;
|
|
•
|
specify milestones or terms relating to use of grant proceeds, or to comply with specified laws;
|
|
•
|
terminate agreements, in whole or in part, for any reason or no reason;
|
|
•
|
reduce or modify the government’s obligations under such agreements without the consent of the other party;
|
|
•
|
claim rights, including intellectual property rights, in products and data developed under such agreements;
|
|
•
|
audit contract related costs and fees, including allocated indirect costs;
|
|
•
|
suspend the contractor or grantee from receiving new contracts pending resolution of alleged violations of procurement laws or regulations;
|
|
•
|
impose U.S. manufacturing requirements for products that embody inventions conceived or first reduced to practice
|
|
•
|
impose qualifications for the engagement of manufacturers, suppliers, and other contractors as well as other criteria for reimbursements;
|
|
•
|
suspend or debar the contractor or grantee from doing future business with the government;
|
|
•
|
control and potentially prohibit the export of products;
|
|
•
|
pursue criminal or civil remedies under the False Claims Act, False Statements Act, and similar remedy provisions specific to government agreements; and
|
|
•
|
limit the government’s financial liability to amounts appropriated by the U.S. Congress on a fiscal year basis, thereby leaving some uncertainty about the future availability of funding for a program even after we have been funded for an initial period.
|
|
•
|
specialized accounting systems unique to government contracts and grants;
|
|
•
|
mandatory financial audits and potential liability for price adjustments or recoupment of government funds after such funds have been spent;
|
|
•
|
public disclosures of some contract and grant information, which may enable competitors to gain insights into our research program; and
|
|
•
|
mandatory socioeconomic compliance requirements, including labor standards, non-discrimination and affirmative action programs, and environmental compliance requirements.
|
|
•
|
We may be unable to identify manufacturers on acceptable terms or at all.
|
|
•
|
Our third-party manufacturers might be unable to timely formulate and manufacture our product or produce the quantity and quality required to meet our clinical and commercial needs, if any.
|
|
•
|
Contract manufacturers may not be able to execute our manufacturing procedures appropriately.
|
|
•
|
Our future third-party manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store, and distribute our products.
|
|
•
|
Manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMPs and other government regulations and corresponding foreign standards. We do not have control over third-party manufacturers’ compliance with these regulations and standards.
|
|
•
|
We may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our product candidates.
|
|
•
|
Our third-party manufacturers could breach or terminate their agreement with us.
|
|
•
|
collaborators often have significant discretion in determining the efforts and resources that they will apply to the collaboration and may not commit sufficient resources to the development, marketing, or commercialization of the product or products that are subject to the collaboration;
|
|
•
|
collaborators may not perform their obligations as expected;
|
|
•
|
any such collaboration may significantly limit our share of potential future profits from the associated program and may require us to relinquish potentially valuable rights to our current product candidates, potential products, proprietary technologies, or grant licenses on terms that are not favorable to us;
|
|
•
|
collaborators may cease to devote resources to the development or commercialization of our product candidates if the collaborators view our product candidates as competitive with their own products or product candidates;
|
|
•
|
disagreements with collaborators, including disagreements over proprietary rights, contract interpretation, or the course of development, might cause delays or termination of the development or commercialization of product candidates, and might result in legal proceedings, which would be time consuming, distracting, and expensive;
|
|
•
|
collaborators may be impacted by changes in their strategic focus or available funding, or business combinations involving them, which could cause them to divert resources away from the collaboration;
|
|
•
|
collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability;
|
|
•
|
the collaborations may not result in us achieving revenues to justify such transactions; and
|
|
•
|
collaborations may be terminated and, if terminated, may result in a need for us to raise additional capital to pursue further development or commercialization of the applicable product candidate.
|
|
•
|
the efficacy of the product as demonstrated in clinical trials and potential advantages over competing treatments;
|
|
•
|
the prevalence and severity of the disease and any side effects;
|
|
•
|
the clinical indications for which approval is granted, including any limitations or warnings contained in a product’s approved labeling;
|
|
•
|
the convenience and ease of administration;
|
|
•
|
the cost of treatment;
|
|
•
|
the willingness of the patients and physicians to accept these therapies;
|
|
•
|
the perceived ratio of risk and benefit of these therapies by physicians and the willingness of physicians to recommend these therapies to patients based on such risks and benefits;
|
|
•
|
the marketing, sales, and distribution support for the product;
|
|
•
|
the publicity concerning our products or competing products and treatments; and
|
|
•
|
the pricing and availability of third-party insurance coverage and reimbursement.
|
|
•
|
our research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates;
|
|
•
|
we may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates;
|
|
•
|
our product candidates may not succeed in preclinical or clinical testing;
|
|
•
|
our potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval;
|
|
•
|
competitors may develop alternatives that render our product candidates obsolete or less attractive;
|
|
•
|
product candidates we develop may be covered by third parties’ patents or other exclusive rights;
|
|
•
|
the market for a product candidate may change during our program so that such a product may become unreasonable to continue to develop;
|
|
•
|
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
|
|
•
|
a product candidate may not be accepted as safe and effective by patients, the medical community, or third-party payors.
|
|
•
|
our ability to obtain regulatory approvals for cobomarsen, MRG-201, MRG-110, or other product candidates, and delays or failures to obtain such approvals;
|
|
•
|
failure of any of our product candidates, if approved, to achieve commercial success;
|
|
•
|
failure to maintain our existing third-party license and supply agreements;
|
|
•
|
changes in laws or regulations applicable to our product candidates;
|
|
•
|
any inability to obtain adequate supply of our product candidates or the inability to do so at acceptable prices;
|
|
•
|
adverse regulatory authority decisions;
|
|
•
|
introduction of new products, services, or technologies by our competitors;
|
|
•
|
failure to meet or exceed financial and development projections we may provide to the public;
|
|
•
|
failure to meet or exceed the financial and development projections of the investment community;
|
|
•
|
the perception of the pharmaceutical industry by the public, legislatures, regulators, and the investment community;
|
|
•
|
announcements of significant acquisitions, strategic collaborations, joint ventures, or capital commitments by us or our competitors;
|
|
•
|
disputes or other developments relating to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies;
|
|
•
|
additions or departures of key personnel;
|
|
•
|
significant lawsuits, including patent or stockholder litigation;
|
|
•
|
if securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our business and stock;
|
|
•
|
changes in the market valuations of similar companies;
|
|
•
|
general market or macroeconomic conditions;
|
|
•
|
sales of our common stock by us our stockholders in the future;
|
|
•
|
trading volume of our common stock;
|
|
•
|
announcements by commercial partners or competitors of new commercial products, clinical progress or the lack thereof, significant contracts, commercial relationships, or capital commitments;
|
|
•
|
adverse publicity relating to microRNA-targeted therapeutics generally, including with respect to other products and potential products in such markets;
|
|
•
|
the introduction of technological innovations or new therapies that compete with our potential products;
|
|
•
|
changes in the structure of health care payment systems; and
|
|
•
|
period-to-period fluctuations in our financial results.
|
|
|
High
|
|
Low
|
||||
|
Year ended December 31, 2017
|
|
|
|
|
|
||
|
Fourth quarter
|
$
|
10.72
|
|
|
$
|
6.02
|
|
|
Third quarter
|
15.91
|
|
|
7.67
|
|
||
|
Second quarter
|
13.50
|
|
|
7.39
|
|
||
|
First quarter
|
18.00
|
|
|
4.76
|
|
||
|
Year ended December 31, 2016
|
|
|
|
|
|
||
|
Fourth quarter
|
$
|
15.11
|
|
|
$
|
1.80
|
|
|
Third quarter
|
9.45
|
|
|
6.00
|
|
||
|
Second quarter
|
11.10
|
|
|
6.00
|
|
||
|
First quarter
|
12.45
|
|
|
6.15
|
|
||
|
Year Ended
December 31, |
|||||||
|
2017
|
2016
|
||||||
|
|
(in thousands,
except share and per share data)
|
||||||
|
Revenue:
|
|||||||
|
Collaboration revenue
|
$
|
3,097
|
|
$
|
2,814
|
|
|
|
Grant revenue
|
906
|
|
663
|
|
|||
|
Total revenue
|
4,003
|
|
3,477
|
|
|||
|
Operating expenses:
|
|||||||
|
Research and development
|
19,623
|
|
13,692
|
|
|||
|
General and administrative
|
10,912
|
|
6,772
|
|
|||
|
Total operating expenses
|
30,535
|
|
20,464
|
|
|||
|
Loss from operations
|
(26,532
|
)
|
(16,987
|
)
|
|||
|
Other income (expense):
|
|||||||
|
Interest and other income
|
403
|
|
39
|
|
|||
|
Interest and other expense
|
(383
|
)
|
(326
|
)
|
|||
|
Net loss
|
(26,512
|
)
|
(17,274
|
)
|
|||
|
Accretion of redeemable convertible preferred stock to redemption value
|
(5
|
)
|
(49
|
)
|
|||
|
Net loss available to common stockholders
|
$
|
(26,517
|
)
|
$
|
(17,323
|
)
|
|
|
Net loss per share, basic and diluted
|
$
|
(1.38
|
)
|
$
|
(28.21
|
)
|
|
|
Weighted-average shares used to compute basic and diluted net loss per share
|
19,244,605
|
|
614,017
|
|
|||
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
(in thousands)
|
|||||||
|
Cash and cash equivalents
|
$
|
47,441
|
|
$
|
22,104
|
|
|
|
Total assets
|
$
|
52,481
|
|
$
|
24,760
|
|
|
|
Notes payable, inclusive of current portion
|
$
|
9,922
|
|
$
|
4,789
|
|
|
|
Total liabilities
|
$
|
13,971
|
|
$
|
9,705
|
|
|
|
Redeemable convertible preferred stock
|
$
|
—
|
|
$
|
76,976
|
|
|
|
Total stockholders’ equity (deficit)
|
$
|
38,510
|
|
$
|
(61,921
|
)
|
|
|
•
|
employee-related expenses, including salaries, benefits, travel, and share-based compensation expense;
|
|
•
|
external research and development expenses incurred under arrangements with third parties, such as CROs, contract manufacturing organizations, or CMOs, other clinical trial-related vendors, consultants, and our scientific advisors;
|
|
•
|
license fees; and
|
|
•
|
facilities, depreciation, and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities, depreciation of leasehold improvements and equipment, and laboratory and other supplies.
|
|
Year Ended
December 31, |
|||||||
|
2017
|
2016
|
||||||
|
(in thousands)
|
|||||||
|
Revenue
|
$
|
4,003
|
|
$
|
3,477
|
|
|
|
Research and development expenses
|
(19,623
|
)
|
(13,692
|
)
|
|||
|
General and administrative expenses
|
(10,912
|
)
|
(6,772
|
)
|
|||
|
Other income (expense), net
|
20
|
|
(287
|
)
|
|||
|
Net loss
|
$
|
(26,512
|
)
|
$
|
(17,274
|
)
|
|
|
•
|
increased clinical and outsourced manufacturing expenses of $3.3 million, primarily related to expanded clinical development of cobomarsen in 2017; and
|
|
•
|
increased salaries, wages, and benefits of
$3.2 million
, due primarily to an increase in our research and development team to support and expand our research and development capabilities; partially offset by
|
|
•
|
decreases in other research and development expenses, including preclinical outsourced studies, of approximately $0.4 million, and fees associated with third party licenses of $0.3 million.
|
|
•
|
an increase in costs generally associated with becoming a public company of $1.8 million, which included accounting and tax support, consulting, insurance, general corporate legal expenses, and board of director compensation;
|
|
•
|
increased salaries, wages, and benefits of $1.2 million, due primarily to an increase in share-based compensation charges following the merger as well as an increase in general and administrative employees during 2017; and
|
|
•
|
increased costs related to patent filings, prosecution, and enforcement of approximately $0.6 million.
|
|
Year Ended
December 31, |
|||||||||||
|
2017
|
2016
|
Change
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Net cash used in operating activities
|
$
|
(28,167
|
)
|
$
|
(14,755
|
)
|
$
|
(13,412
|
)
|
||
|
Net cash provided by (used in) investing activities
|
1,034
|
|
(250
|
)
|
1,284
|
|
|||||
|
Net cash provided by financing activities
|
52,470
|
|
15,874
|
|
36,596
|
|
|||||
|
Net increase in cash and cash equivalents
|
$
|
25,337
|
|
$
|
869
|
|
$
|
24,468
|
|
||
|
Name
|
|
Age
|
|
Position(s)
|
|
Executive Officers
|
|
|
|
|
|
William S. Marshall, Ph.D.
|
|
54
|
|
President, Chief Executive Officer, and Director
|
|
Jason A. Leverone
|
|
44
|
|
Chief Financial Officer, Secretary, and Treasurer
|
|
Adam S. Levy
|
|
39
|
|
Chief Business Officer
|
|
Paul D. Rubin, M.D.
|
|
64
|
|
Executive Vice President, Research and Development
|
|
|
|
|
|
|
|
Non-Employee Directors
|
|
|
|
|
|
Bruce L. Booth, Ph.D.
|
|
43
|
|
Director
|
|
Christopher J. Bowden, M.D.
|
|
56
|
|
Director
|
|
Jeffrey S. Hatfield
|
60
|
Director
|
||
|
Thomas E. Hughes, Ph.D.
|
|
58
|
|
Director
|
|
Kevin Koch, Ph.D.
|
|
57
|
|
Director
|
|
Arlene M. Morris
|
66
|
Director
|
||
|
Joseph L. Turner
|
|
66
|
|
Director
|
|
Officer
|
Title
|
|
|
William S. Marshall, Ph.D.
|
Chief Executive Officer and Director (Principal Executive Officer)
|
|
|
Adam S. Levy
|
Chief Business Officer
|
|
|
Paul D. Rubin, M.D.
|
Executive Vice President, Research and Development
|
|
|
Samuel D. Riccitelli (1)
|
Chief Executive Officer and President
|
|
|
(1)
|
Mr. Riccitelli resigned as our chief executive officer and president in February 2017 in connection with the closing of the Merger. Thereafter Dr. Marshall was appointed as our chief executive officer.
|
|
Name
|
Principal Position
|
Fiscal Year
|
Salary
|
Bonus
|
Stock Award(s)(1)
|
Option Award(s)(1)
|
All Other Compensation
|
Total
|
|||||||||||||||||||||
|
William S. Marshall, Ph.D.
|
Chief Executive Officer and President
|
2017
|
$
|
400,000
|
|
$
|
180,000
|
|
$
|
—
|
|
$
|
1,609,483
|
|
$
|
—
|
|
$
|
2,189,483
|
|
|||||||||
|
2016
|
$
|
347,086
|
|
$
|
200,056
|
|
$
|
—
|
|
$
|
109,754
|
|
$
|
—
|
|
$
|
656,896
|
|
|||||||||||
|
Adam S. Levy
|
Chief Business Officer
|
2017
|
$
|
300,000
|
|
$
|
108,000
|
|
$
|
—
|
|
$
|
704,149
|
|
$
|
—
|
|
$
|
1,112,149
|
|
|||||||||
|
2016
|
$
|
187,500
|
|
$
|
150,000
|
|
$
|
—
|
|
$
|
113,633
|
|
$
|
20,385
|
|
(4)
|
$
|
471,518
|
|
||||||||||
|
Paul D. Rubin, M.D.
|
Executive Vice President, Research and Development
|
2017
|
$
|
395,000
|
|
$
|
142,200
|
|
$
|
—
|
|
$
|
704,149
|
|
$
|
—
|
|
$
|
1,241,349
|
|
|||||||||
|
2016
|
$
|
124,375
|
|
$
|
33,575
|
|
$
|
—
|
|
$
|
809,639
|
|
$
|
—
|
|
$
|
967,589
|
|
|||||||||||
|
Samuel D. Riccitelli
|
Former Chief Executive Officer
|
2017
|
$
|
56,495
|
|
$
|
144,450
|
|
$
|
—
|
|
$
|
—
|
|
$
|
499,543
|
|
(3)
|
$
|
700,488
|
|
||||||||
|
2016
|
$
|
450,000
|
|
$
|
135,000
|
|
(2)
|
$
|
102,000
|
|
$
|
—
|
|
$
|
—
|
|
$
|
687,000
|
|
||||||||||
|
(1)
|
Represents the aggregate grant date fair value of stock awards or options for common stock computed in accordance with FASB ASC Topic 718.
|
|
(2)
|
Represents discretionary bonus for services rendered in our year ended December 31, 2016 granted in January 2017, which was not made pursuant to any contractual arrangement.
|
|
(3)
|
Includes a severance payment of $450,000 paid in February 2017 and the remainder relates to paid vacation accruals at the date of termination.
|
|
(4)
|
Includes reimbursement for travel and relocation expenses.
|
|
•
|
“cause” means (i) Dr. Marshall’s commission of any felony or any crime involving fraud, dishonesty or moral turpitude under the laws of the United States or any state thereof; (ii) Dr. Marshall’s attempted commission of, or participation in, a fraud or act of dishonesty against us; (iii) Dr. Marshall’s intentional, material violation of any contract or agreement between Dr. Marshall and us or any statutory duty Dr. Marshall owes to us, in each case, which remains uncured for 30 days after we provide written notice of such action or conduct to Dr. Marshall; (iv) Dr. Marshall’s unauthorized use or disclosure of our confidential information or trade secrets; or (v) Dr. Marshall’s gross misconduct which remains uncured for 30 days after we provide written notice of such action or conduct to Dr. Marshall.
|
|
•
|
“good reason” means the occurrence, without Dr. Marshall’s consent, of any one or more of the following: (i) a material reduction in his base salary of 10% or more (unless such reduction is pursuant to a salary reduction program applicable generally to our similarly situated executives); (ii) a material reduction in Dr. Marshall’s authority, duties or responsibilities; (iii) a relocation of Dr. Marshall’s principal place of employment to a place that increases Dr. Marshall’s one-way commute by more than 25 miles; or (iv) material breach by us of any material provision of Dr. Marshall’s employment agreement.
|
|
•
|
“cause” means (i) Mr. Leverone’s commission of any felony or any crime involving fraud, dishonesty or moral turpitude under the laws of the United States or any state thereof; (ii) Mr. Leverone’s attempted commission of, or participation in, a fraud or act of dishonesty against us; (iii) Mr. Leverone’s intentional, material violation of any contract or agreement between Mr. Leverone and us or any statutory duty Mr. Leverone owes to us, in each case, which remains uncured for 30 days after we provide written notice of such action or conduct to Mr. Leverone; (iv) Mr. Leverone’s unauthorized use or disclosure of our confidential information or trade secrets; or (v) Mr. Leverone’s gross misconduct which remains uncured for 30 days after we provide written notice of such action or conduct to Mr. Leverone.
|
|
•
|
“good reason” means the occurrence, without Mr. Leverone’s consent, of any one or more of the following: (i) a material reduction in his base salary of 10% or more (unless such reduction is pursuant to a salary reduction program applicable generally to our similarly situated executives); (ii) a material reduction in Mr. Leverone’s authority, duties or responsibilities; (iii) a relocation of Mr. Leverone’s principal place of employment to a place that increases Mr. Leverone’s one-way commute by more than 25 miles; or (iv) material breach by us of any material provision of Mr. Leverone’s employment agreement.
|
|
•
|
“cause” means (i) Mr. Levy’s commission of any felony or any crime involving fraud, dishonesty or moral turpitude under the laws of the United States or any state thereof; (ii) Mr. Levy’s attempted commission of, or participation in, a fraud or act of dishonesty against us; (iii) Mr. Levy’s intentional, material violation of any contract or agreement between Mr. Levy and us or any statutory duty Mr. Levy owes to us, in each case, which remains uncured for 30 days after we provide written notice of such action or conduct to Mr. Levy; (iv) Mr. Levy’s unauthorized use or disclosure of our confidential information or trade secrets; or (v) Mr. Levy’s gross misconduct which remains uncured for 30 days after we provide written notice of such action or conduct to Mr. Levy.
|
|
•
|
“good reason” means the occurrence, without Mr. Levy’s consent, of any one or more of the following: (i) a material reduction in his base salary of 10% or more (unless such reduction is pursuant to a salary reduction program
|
|
•
|
“cause” means (i) Dr. Rubin’s commission of any felony or any crime involving fraud, dishonesty or moral turpitude under the laws of the United States or any state thereof; (ii) Dr. Rubin’s attempted commission of, or participation in, a fraud or act of dishonesty against us; (iii) Dr. Rubin’s intentional, material violation of any contract or agreement between Dr. Rubin and us or any statutory duty Dr. Rubin owes to us, in each case, which remains uncured for 30 days after we provide written notice of such action or conduct to Dr. Rubin; (iv) Dr. Rubin’s unauthorized use or disclosure of our confidential information or trade secrets; or (v) Dr. Rubin’s gross misconduct which remains uncured for 30 days after we provide written notice of such action or conduct to Dr. Rubin.
|
|
•
|
“good reason” means the occurrence, without Dr. Rubin’s consent, of any one or more of the following: (i) a material reduction in his base salary of 10% or more (unless such reduction is pursuant to a salary reduction program applicable generally to our similarly situated executives); (ii) a material reduction in Dr. Rubin’s authority, duties or responsibilities; (iii) a relocation of Dr. Rubin’s principal place of employment to a place that increases Dr. Rubin’s one-way commute by more than 25 miles; or (iv) material breach by us of any material provision of Dr. Rubin’s employment agreement.
|
|
Name
|
Grant Date
|
Vesting Commencement Date
|
Number of securities underlying unexercised options exercisable
|
Number of securities underlying unexercised options unexercisable
|
Option
exercise
price
|
Option Expiration Date
|
|||||||
|
William S. Marshall, Ph.D.
|
7/31/2008
|
5/16/2008
|
(1)
|
115,818
|
—
|
$0.57
|
7/30/2018
|
||||||
|
6/15/2012
|
6/15/2012
|
(2)
|
230,968
|
—
|
$1.22
|
6/13/2022
|
|||||||
|
2/22/2016
|
2/22/2016
|
(2)
|
71,862
|
84,929
|
$1.05
|
2/19/2026
|
|||||||
|
2/16/2017
|
2/16/2017
|
(2)
|
41,666
|
158,334
|
$11.01
|
2/16/2027
|
|||||||
|
Adam S. Levy
|
6/15/2016
|
5/16/2016
|
(1)
|
64,257
|
98,076
|
$1.05
|
6/13/2026
|
||||||
|
2/16/2017
|
2/16/2017
|
(2)
|
18,229
|
69,271
|
$11.01
|
2/16/2027
|
|||||||
|
Paul D. Rubin, M.D.
|
11/30/2016
|
11/16/2016
|
(1)
|
54,956
|
147,961
|
$5.69
|
11/30/2026
|
||||||
|
2/16/2017
|
2/16/2017
|
(2)
|
18,229
|
69,271
|
$11.01
|
2/16/2027
|
|||||||
|
(1)
|
Twenty-five percent of the shares subject to the option vest on the first anniversary of the vesting commencement date, and the remainder vest thereafter in 36 equal installments.
|
|
(2)
|
The option vests as to 1/48 of the shares in monthly installments measured from vesting commencement date.
|
|
•
|
the exercise price of the ISO must be at least 110% of the fair market value of the common stock subject to the ISO on the date of grant; and
|
|
•
|
the term of the ISO must not exceed five years from the date of grant.
|
|
•
|
arrange for the surviving or acquiring corporation (or its parent company) to assume or continue the stock award or to substitute a similar stock award for the stock award (including an award to acquire the same consideration paid to our stockholders pursuant to the corporate transaction);
|
|
•
|
arrange for the assignment of any reacquisition or repurchase rights held by us in respect of common stock issued pursuant to the stock award to the surviving or acquiring corporation (or its parent company);
|
|
•
|
accelerate the vesting (and, if applicable, the exercisability) of the stock award to a date prior to the effective time of the corporate transaction as determined by the Plan Administrator (or, if the Plan Administrator does not determine such a date, to the date that is five days prior to the effective date of the corporate transaction), with the stock award terminating if not exercised (if applicable) at or prior to the effective time of the corporate transaction; provided,
|
|
•
|
arrange for the lapse of any reacquisition or repurchase rights held by us with respect to the stock award;
|
|
•
|
cancel or arrange for the cancellation of the stock award, to the extent not vested or not exercised prior to the effective time of the corporate transaction, and pay such cash consideration (including no consideration) as the Plan Administrator may consider appropriate; and
|
|
•
|
cancel or arrange for the cancellation of the stock award, to the extent not vested or not exercised prior to the effective time of the corporate transaction, in exchange for a payment, in such form as may be determined by our board of directors equal to the excess, if any, of (i) the per share amount payable to holders of common stock in connection with the corporate transaction, over (ii) the per share exercise price under the applicable award. For clarity, this payment may be zero if the value of the property is equal to or less than the exercise price. In addition, any escrow, holdback, earnout or similar provisions in the definitive agreement for the corporate transaction may apply to such payment to the same extent and in the same manner as such provisions apply to the holders of common stock.
|
|
Member
Annual Service (1) |
Chairperson Annual Service (1) (2)
|
|||||||
|
Board of directors
|
$
|
35,000
|
|
$
|
—
|
|
||
|
Non-executive chairperson
|
30,000
|
|
N/A
|
|
||||
|
Audit committee
|
7,500
|
|
15,000
|
|
||||
|
Compensation committee
|
5,000
|
|
10,000
|
|
||||
|
Nominating and corporate governance committee
|
3,750
|
|
7,500
|
|
||||
|
(1)
|
Each non-employee director has the right to elect to receive all or a portion of annual cash compensation under the policy in the form of either cash, quarterly restricted common stock based on the closing price of our common stock on The Nasdaq Capital Market on the date of grant, or stock options to purchase common stock based on the Black-Scholes option-pricing model as of the date of grant. Any such election will be made before the start of the fiscal year or within thirty days of first becoming eligible to receive compensation under this policy and with any such stock options or restricted common stock elected by the directors to vest on a quarterly basis in arrears, with stock options to expire ten years from the date of grant.
|
|
(2)
|
Chairpersons will not receive cash compensation for being a member of the applicable committee.
|
|
•
|
each person, or group of affiliated persons, who are known by us to beneficially own more than 5% of the outstanding shares of our capital stock;
|
|
•
|
each of our directors as of March 1, 2018;
|
|
•
|
each of our named executive officers as of March 1, 2018; and
|
|
•
|
all of our current directors and executive officers of as a group.
|
|
Name
|
Number of Shares Beneficially Owned
|
Percentage Ownership
|
||||
|
5% or Greater Stockholders
|
||||||
|
Entities affiliated with Atlas Venture VII, L.P.
|
4,493,670
|
|
(1)
|
14.9%
|
||
|
FMR, LLC
|
3,124,888
|
|
(2)
|
10.4%
|
||
|
Remeditex Ventures LLC
|
2,706,563
|
|
(3)
|
9.0%
|
||
|
Directors and Named Executive Officers
|
||||||
|
Samuel D. Riccitelli
|
11,274
|
|
(4)
|
*
|
||
|
William S. Marshall, Ph.D.
|
656,437
|
|
(5)
|
2.2%
|
||
|
Adam S. Levy
|
120,094
|
|
(6)
|
*
|
||
|
Paul D. Rubin, M.D.
|
102,698
|
|
(7)
|
*
|
||
|
Bruce L. Booth, Ph.D.
|
4,502,944
|
|
(1)
|
14.9%
|
||
|
Christopher J. Bowden, M.D.
|
5,333
|
|
(8)
|
*
|
||
|
Jeffrey S. Hatfield
|
5,333
|
|
(9)
|
*
|
||
|
Thomas E. Hughes, Ph.D.
|
25,243
|
|
(10)
|
*
|
||
|
Kevin Koch, Ph.D.
|
14,624
|
|
(11)
|
*
|
||
|
Arlene M. Morris
|
2,000
|
|
(12)
|
*
|
||
|
Joseph L. Turner
|
9,333
|
|
(13)
|
*
|
||
|
All directors and officers as a group (11 persons)
|
5,582,066
|
|
(14)
|
18.5%
|
||
|
(1)
|
Includes 3,142,580 shares of common stock held directly by Atlas Venture VII, L.P. (“Atlas Venture VII”) and 1,351,090 shares of common stock held directly by Atlas Venture Fund X, L.P. (“Atlas Venture X”). Atlas Venture Associates VII, L.P. (“AVA VII LP”) is the general partner of Atlas Venture VII, and Atlas Venture Associates VII,
|
|
(2)
|
Based solely upon a Schedule 13G filed with the SEC on March 10, 2017. The address for FMR, LLC is 245 Summer Street, Boston, MA 02110.
|
|
(3)
|
Based solely upon a Schedule 13G filed with the SEC on February 17, 2017. The principal business address of Remeditex is 2727 N. Harwood Street, Suite 200, Dallas, TX 75201.
|
|
(4)
|
Mr. Riccitelli’s service to Miragen terminated in February 2017.
|
|
(5)
|
Includes 266,896 shares of common stock and 389,541 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(6)
|
Includes 11,790 shares of common stock and 108,304 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(7)
|
Includes 102,698 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(8)
|
Includes 5,333 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(9)
|
Includes 5,333 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(10)
|
Includes 2,827 shares of common stock and 22,416 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(11)
|
Includes 14,624 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(12)
|
Includes 2,000 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(13)
|
Includes 9,333 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018.
|
|
(14)
|
Includes 4,776,390 shares of common stock and 805,676 shares of common stock issuable upon exercise of options to purchase our common stock within 60 days of March 1, 2018 held by our current directors and executive officers, including William S. Marshall, Ph.D., Jason A. Leverone, Adam S. Levy, Paul D. Rubin, M.D., Bruce L. Booth, Ph.D., Christopher J. Bowden, M.D., Jeffrey S. Hatfield, Thomas E. Hughes, Ph.D., Kevin Koch, Ph.D., Arlene M. Morris and Joseph L. Turner, and their affiliates. Samuel D. Riccitelli and Tamara A. Seymour are not included among our directors and executive officers, as neither is a director or officer of our company as of March 1, 2018.
|
|
Plan Category
|
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
|
|
Weighted average exercise price of outstanding options, warrants and rights
|
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in first column)
|
|||||
|
Equity compensation plans approved by stockholders (1)
|
|
2,862,559
|
|
(2)
|
|
$
|
4.85
|
|
|
742,058
|
|
|
Equity compensation plans not approved by stockholders
|
|
—
|
|
|
|
$
|
—
|
|
|
—
|
|
|
Total
|
|
2,862,559
|
|
|
|
$
|
4.85
|
|
|
742,058
|
|
|
(1)
|
The 2016 Plan includes an “evergreen” feature, which provides that an additional number of shares will automatically be added to the shares reserved for issuance under the 2016 Plan on January 1st of each year, beginning on January 1, 2018 and ending on (and including) January 1, 2026. The number of shares added each calendar year will equal the lesser of: (i) 4% of the total number of shares of our common stock outstanding on December 31st of the preceding calendar year or (ii) a lesser number of shares determined by the board of directors.
|
|
(2)
|
Represents outstanding options or warrants to purchase shares of common stock.
|
|
•
|
the risks, costs, and benefits to us;
|
|
•
|
the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated;
|
|
•
|
the availability of other sources for comparable services or products; and
|
|
•
|
the terms available to or from, as the case may be, unrelated third parties or to or from employees generally.
|
|
•
|
the amounts involved exceeded or will exceed the lesser of (x) $120,000 or (y) 1% of the average of our total assets at year end for the last two completed fiscal years; and
|
|
•
|
any of the directors, executive officers, holders of more than 5% of our capital stock (sometimes refer to as 5% stockholders below) or any member of their immediate family had or will have a direct or indirect material interest.
|
|
Name of Purchaser
|
Shares of
Common Stock
|
Purchase Price
|
||||
|
Atlas Venture Fund X, L.P. (1)
|
545,454
|
$
|
2,999,997
|
|
||
|
Adam Levy
|
9,090
|
$
|
49,995
|
|
||
|
(1)
|
The Atlas Venture Funds, together, hold more than 5% of our outstanding capital stock. Dr. Booth is a member of our board of directors and a director of Atlas Venture Associates VII, Inc. and Atlas Venture Associates X, Inc., which are affiliated with the Atlas Venture Funds.
|
|
Name of Purchaser
|
Shares of
Common Stock PreMerger
|
Shares of Common Stock PostMerger
|
Purchase Price
|
|||||||
|
Fidelity Select Portfolios: Biotechnology Portfolio (1)
|
3,507,819
|
|
2,466,347
|
|
$
|
15,785,186
|
|
|||
|
Fidelity Advisor Series VII: Fidelity Advisor Biotechnology Fund (1)
|
936,625
|
|
658,541
|
|
$
|
4,214,813
|
|
|||
|
Atlas Venture Fund X, L.P. (2)
|
1,145,835
|
|
805,636
|
|
$
|
5,156,258
|
|
|||
|
Boulder Ventures VI, L.P. (3)
|
147,419
|
|
103,650
|
|
$
|
663,386
|
|
|||
|
MRL Ventures Fund, LLC (4)
|
412,774
|
|
290,221
|
|
$
|
1,857,483
|
|
|||
|
JAFCO SV4 Investment Limited Partnership (5)
|
353,806
|
|
248,760
|
|
$
|
1,592,127
|
|
|||
|
Remeditex Ventures LLC (6)
|
797,308
|
|
560,587
|
|
$
|
3,587,886
|
|
|||
|
BraMira LLC (7)
|
1,111,111
|
|
781,222
|
|
$
|
5,000,000
|
|
|||
|
(1)
|
Fidelity Select Portfolios: Biotechnology Portfolio and Fidelity Advisor Series VII: Fidelity Advisor Biotechnology Fund, together, held more than 5% of our outstanding capital stock.
|
|
(2)
|
The Atlas Venture Funds, together, hold more than 5% of our outstanding capital stock. Dr. Booth is a member of our board of directors and a director of Atlas Venture Associates VII, Inc. and Atlas Venture Associates X, Inc., which are affiliated with the Atlas Venture Funds.
|
|
(3)
|
Boulder Ventures held more than 5% of our outstanding capital stock. At the time of this transaction, Mr. Lefkoff was a member of our board of directors and a managing member of BV Partners V, L.L.C. and BV Partners VI, L.L.C., which are each affiliated with Boulder Ventures.
|
|
(4)
|
MRL Ventures Fund, LLC holds more than 5% of our outstanding capital stock.
|
|
(5)
|
JAFCO SV4 Investment Limited Partnership, or JAFCO, holds more than 5% of our outstanding capital stock.
|
|
(6)
|
Remeditex Ventures LLC holds more than 5% of our outstanding capital stock. At the time of the transaction, Mr. Creecy was a member of our board of directors and the chief executive officer of Remeditex Ventures LLC.
|
|
(7)
|
BraMira LLC, together with its affiliates, holds more than 5% of our outstanding capital stock.
|
|
Name of Purchaser
|
Shares of Series C Convertible Preferred Stock PreMerger
|
Shares of Common Stock PostMerger
|
Purchase Price
|
|||||||
|
Atlas Venture Fund VII, L.P. (1)
|
1,245,502
|
|
875,712
|
|
$
|
5,517,574
|
|
|||
|
Boulder Ventures V, L.P. (2)
|
233,089
|
|
163,884
|
|
$
|
1,032,584
|
|
|||
|
Boulder Ventures VI, L.P. (2)
|
564,334
|
|
396,783
|
|
$
|
2,500,000
|
|
|||
|
MRL Ventures Fund, LLC (3)
|
1,580,135
|
|
1,110,992
|
|
$
|
6,999,998
|
|
|||
|
JAFCO SV4 Investment Limited Partnership (4)
|
1,354,402
|
|
952,280
|
|
$
|
6,000,001
|
|
|||
|
Remeditex Ventures LLC (5)
|
1,968,830
|
|
1,384,284
|
|
$
|
8,721,917
|
|
|||
|
BraMira LLC (6)
|
1,128,668
|
|
793,566
|
|
$
|
4,999,999
|
|
|||
|
William S. Marshall, Ph.D. (7)
|
17,263
|
|
12,137
|
|
$
|
76,475
|
|
|||
|
(1)
|
Atlas Venture Fund VII, L.P. holds more than 5% of our outstanding capital stock. Dr. Booth is a member of our board of directors and a director of Atlas Venture Associates VII, Inc., which is affiliated with the Atlas Venture Fund VII, L.P.
|
|
(2)
|
Boulder Ventures held more than 5% of our outstanding capital stock. At the time of the transaction, Mr. Lefkoff was a member of our board of directors and a managing member of BV Partners V, L.L.C. and BV Partners VI, L.L.C., which are each affiliated with Boulder Ventures.
|
|
(3)
|
MRL Ventures Fund, LLC holds more than 5% of our outstanding capital stock.
|
|
(4)
|
JAFCO holds more than 5% of our outstanding capital stock.
|
|
(5)
|
Remeditex Ventures LLC holds more than 5% of our outstanding capital stock. At the time of the transaction, Mr. Creecy was a member of our board of directors and the chief executive officer of Remeditex Ventures LLC.
|
|
(6)
|
BraMira LLC, together with its affiliates, holds more than 5% of our outstanding capital stock.
|
|
(7)
|
Dr. Marshall is a member of our board of directors and serves as our president and chief executive officer.
|
|
Year Ended
|
|||||||
|
December 31,
2017 |
December 31,
2016 |
||||||
|
(in thousands)
|
|||||||
|
Audit fees (1)
|
$
|
300
|
|
$
|
279
|
|
|
|
Audit-related fees (2)
|
—
|
|
—
|
|
|||
|
Tax fees (3)
|
—
|
|
—
|
|
|||
|
All other fees (4)
|
—
|
|
—
|
|
|||
|
Total fees
|
$
|
300
|
|
$
|
279
|
|
|
|
(1)
|
Audit fees consist of fees billed for professional services for audit and quarterly review of our financial statements and review of our registration statement for the Merger, and related services that are normally provided in connection with statutory and regulatory filings or engagements.
|
|
(2)
|
Audit-related fees include services relating to accounting consultations and reviews and due diligence services.
|
|
(3)
|
Tax fees include services relating to tax compliance, tax advice, and tax planning in the United States.
|
|
(4)
|
All other fees include the aggregate of the fees billed for products and services provided by the principal accountant other than the products and services disclosed as audit fees, audit-related fees, and tax fees.
|
|
Year Ended
|
|||||||
|
December 31,
2017 |
December 31,
2016 |
||||||
|
(in thousands)
|
|||||||
|
Audit fees (1)
|
$
|
56
|
|
$
|
205
|
|
|
|
Audit-related fees (2)
|
—
|
|
—
|
|
|||
|
Tax fees (3)
|
—
|
|
—
|
|
|||
|
All other fees (4)
|
—
|
|
—
|
|
|||
|
Total fees
|
$
|
56
|
|
$
|
205
|
|
|
|
(1)
|
Audit fees consist of fees billed for professional services for audit and quarterly review of our financial statements and review of our registration statement for the Merger, and related services that are normally provided in connection with statutory and regulatory filings or engagements.
|
|
(2)
|
Audit-related fees include services relating to accounting consultations and reviews and due diligence services.
|
|
(3)
|
Tax fees include services relating to tax compliance, tax advice, and tax planning in the United States.
|
|
(4)
|
All other fees include the aggregate of the fees billed for products and services provided by the principal accountant other than the products and services disclosed as audit fees, audit-related fees, and tax fees.
|
|
Incorporated by Reference
|
||||||
|
Exhibit
Number
|
|
Description of Exhibit
|
Form
|
Filing Date
|
Number
|
Filed Herewith
|
|
8-K
|
11/01/2016
|
2.1
|
||||
|
S-4
|
12/02/2016
|
2.4
|
||||
|
S-4
|
12/02/2016
|
2.5
|
||||
|
|
10-Q
|
08/14/2014
|
3.1
|
|||
|
S-4
|
12/02/2016
|
3.3
|
||||
|
8-K
|
02/13/2017
|
3.1
|
||||
|
8-K
|
02/13/2017
|
3.2
|
||||
|
10-Q
|
08/15/2016
|
3.1
|
||||
|
|
8-K
|
02/13/2017
|
3.3
|
|||
|
8-K
|
02/13/2017
|
3.4
|
||||
|
|
S-1
|
03/19/2014
|
4.1
|
|||
|
10-K
|
03/15/2018
|
4.2
|
Ÿ
|
|||
|
8-K
|
11/15/2017
|
10.2
|
||||
|
S-1
|
03/19/2014
|
10.14
|
||||
|
S-4
|
12/02/2016
|
10.32
|
||||
|
S-4
|
12/02/2016
|
10.37
|
||||
|
S-4
|
12/02/2016
|
10.38
|
||||
|
10-Q
|
05/11/2017
|
10.12
|
||||
|
S-4
|
12/02/2016
|
10.48
|
||||
|
S-4
|
12/02/2016
|
10.49
|
||||
|
S-4
|
12/02/2016
|
10.39
|
||||
|
10-Q
|
05/11/2017
|
10.13
|
||||
|
10-Q
|
08/14/2014
|
10.4
|
||||
|
8-K
|
07/23/2014
|
10.2
|
||||
|
8-K
|
07/23/2014
|
10.1
|
||||
|
S-4
|
12/02/2016
|
10.33
|
||||
|
S-4
|
12/02/2016
|
10.34
|
||||
|
S-4
|
12/02/2016
|
10.35
|
||||
|
S-4
|
12/02/2016
|
10.36
|
||||
|
S-4
|
12/02/2016
|
10.40
|
||||
|
S-4
|
12/02/2016
|
10.40.1
|
||||
|
S-4
|
12/02/2016
|
10.40.2
|
||||
|
S-4
|
12/02/2016
|
10.41
|
||||
|
S-4
|
12/02/2016
|
10.42
|
||||
|
S-4
|
12/02/2016
|
10.43
|
||||
|
S-4
|
12/02/2016
|
10.43.1
|
||||
|
S-4
|
12/02/2016
|
10.44
|
||||
|
S-4
|
12/02/2016
|
10.45
|
||||
|
S-4
|
12/02/2016
|
10.45.1
|
||||
|
S-4
|
12/02/2016
|
10.45.2
|
||||
|
S-4
|
12/02/2016
|
10.45.3
|
||||
|
S-4
|
12/02/2016
|
10.45.4
|
||||
|
10-Q
|
08/11/2017
|
10.1
|
||||
|
10-Q
|
11/09/2017
|
10.1
|
||||
|
S-4
|
12/02/2016
|
10.46
|
||||
|
S-4
|
12/02/2016
|
10.51
|
||||
|
10-Q/A
|
06/07/2017
|
10.14
|
||||
|
10-Q
|
11/09/2017
|
10.2
|
||||
|
S-4
|
12/02/2016
|
10.47
|
||||
|
S-4
|
01/04/2017
|
10.47.1
|
||||
|
8-K
|
11/15/2017
|
10.1
|
||||
|
8-K
|
03/31/2017
|
10.1
|
||||
|
|
10-K
|
03/15/2018
|
21.1
|
Ÿ
|
||
|
|
10-K
|
03/15/2018
|
23.1
|
Ÿ
|
||
|
10-K
|
03/15/2018
|
24.1
|
Ÿ
|
|||
|
10-K
|
03/15/2018
|
31.1
|
Ÿ
|
|||
|
10-K
|
03/15/2018
|
31.2
|
Ÿ
|
|||
|
10-K
|
03/15/2018
|
32.1
|
Ÿ
|
|||
|
101.INS***
|
XBRL Instance Document
|
10-K
|
03/15/2018
|
Ÿ
|
||
|
101.SCH***
|
XBRL Taxonomy Extension Schema Document
|
10-K
|
03/15/2018
|
Ÿ
|
||
|
101.CAL***
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
10-K
|
03/15/2018
|
Ÿ
|
||
|
101.DEF***
|
XBRL Taxonomy Extension Definition Linkbase Document
|
10-K
|
03/15/2018
|
Ÿ
|
||
|
101.LAB***
|
XBRL Taxonomy Extension Label Linkbase Document
|
10-K
|
03/15/2018
|
Ÿ
|
||
|
101.PRE***
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
10-K
|
03/15/2018
|
Ÿ
|
||
|
^
|
The schedules and exhibits to this exhibit have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request.
|
|
†
|
Confidential treatment granted as to portions of the exhibit. Confidential materials omitted and filed separately with the SEC.
|
|
*
|
Management contract or compensatory plans or arrangements.
|
|
**
|
This certification is being furnished pursuant to 18 U.S.C. Section 1350, and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the Registrant, whether made before or after the date hereof.
|
|
***
|
In accordance with Rule 406T of Regulation S-T, the Interactive Data Files in Exhibit 101 are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Exchange Act of 1934, as amended, and otherwise are not subject to liability under these sections.
|
|
|
|
MIRAGEN THERAPEUTICS, INC.
|
|
|
|
|
||
|
Date: March 15, 2018
|
|
|
|
|
|
|
|
|
|
By:
|
/s/ William S. Marshall
|
||
|
William S. Marshall, Ph.D.
|
|||
|
Chief Executive Officer
|
|||
|
(Principal Executive Officer)
|
|||
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ William S. Marshall, Ph.D.
|
|
Chief Executive Officer and Director (Principal Executive Officer)
|
|
March 15, 2018
|
|
William S Marshall, Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Jason A. Leverone
|
|
Chief Financial Officer, Treasurer, and Secretary (Principal Financial Officer; Principal Accounting Officer)
|
|
March 15, 2018
|
|
Jason A. Leverone
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Bruce L. Booth, Ph.D.
|
|
Chairman of the Board
|
|
March 15, 2018
|
|
Bruce L. Booth, Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Christopher Bowden, M.D.
|
|
Director
|
|
March 15, 2018
|
|
Christopher Bowden, M.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Jeffrey S. Hatfield
|
|
Director
|
|
March 15, 2018
|
|
Jeffrey S. Hatfield
|
|
|
|
|
|
/s/ Thomas E. Hughes, Ph.D.
|
|
Director
|
|
March 15, 2018
|
|
Thomas E. Hughes, Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Kevin Koch, Ph.D.
|
|
Director
|
|
March 15, 2018
|
|
Kevin Koch, Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Joseph L. Turner
|
|
Director
|
|
March 15, 2018
|
|
Joseph L. Turner
|
|
|
|
|
|
/s/ Arlene M. Morris
|
|
Director
|
|
March 15, 2018
|
|
Arlene M. Morris
|
|
|
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
47,441
|
|
$
|
22,104
|
|
|
|
Accounts receivable
|
1,456
|
|
20
|
|
|||
|
Prepaid expenses and other current assets
|
2,971
|
|
1,753
|
|
|||
|
Total current assets
|
51,868
|
|
23,877
|
|
|||
|
Property and equipment, net
|
563
|
|
625
|
|
|||
|
Other assets
|
50
|
|
258
|
|
|||
|
Total assets
|
$
|
52,481
|
|
$
|
24,760
|
|
|
|
Liabilities, Preferred Stock, and Stockholders’ Equity (Deficit)
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
906
|
|
$
|
1,007
|
|
|
|
Accrued liabilities
|
2,991
|
|
3,909
|
|
|||
|
Current portion of notes payable
|
—
|
|
1,969
|
|
|||
|
Total current liabilities
|
3,897
|
|
6,885
|
|
|||
|
Notes payable, less current portion
|
9,922
|
|
2,820
|
|
|||
|
Other liabilities
|
152
|
|
—
|
|
|||
|
Total liabilities
|
13,971
|
|
9,705
|
|
|||
|
Commitments and contingencies
|
|
|
|||||
|
Series A redeemable convertible preferred stock, $0.001 par value; 7,169,176 shares authorized; 7,149,176 shares issued and outstanding and liquidation preference of $21,448 at December 31, 2016
|
—
|
|
23,124
|
|
|||
|
Series B redeemable convertible preferred stock, $0.001 par value; 2,183,318 shares authorized; 2,166,651 shares issued and outstanding and liquidation preference of $13,000 at December 31, 2016; stated at accreted redemption value
|
—
|
|
12,975
|
|
|||
|
Series C redeemable convertible preferred stock, $0.001 par value; 9,303,000 shares authorized; 9,268,563 shares issued and outstanding and liquidation preference of $41,060 at December 31, 2016; stated at accreted redemption value
|
—
|
|
40,877
|
|
|||
|
Stockholders’ equity (deficit):
|
|||||||
|
Common stock, $0.01 par value; 100,000,000 shares authorized; 22,568,006 and 833,744 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively
|
226
|
|
8
|
|
|||
|
Additional paid-in capital
|
131,877
|
|
5,147
|
|
|||
|
Accumulated deficit
|
(93,593
|
)
|
(67,076
|
)
|
|||
|
Total stockholders’ equity (deficit)
|
38,510
|
|
(61,921
|
)
|
|||
|
Total liabilities, preferred stock, and stockholders’ equity (deficit)
|
$
|
52,481
|
|
$
|
24,760
|
|
|
|
Year Ended
December 31, |
|||||||
|
2017
|
2016
|
||||||
|
Revenue:
|
|||||||
|
Collaboration revenue
|
$
|
3,097
|
|
$
|
2,814
|
|
|
|
Grant revenue
|
906
|
|
663
|
|
|||
|
Total revenue
|
4,003
|
|
3,477
|
|
|||
|
Operating expenses:
|
|||||||
|
Research and development
|
19,623
|
|
13,692
|
|
|||
|
General and administrative
|
10,912
|
|
6,772
|
|
|||
|
Total operating expenses
|
30,535
|
|
20,464
|
|
|||
|
Loss from operations
|
(26,532
|
)
|
(16,987
|
)
|
|||
|
Other income (expense):
|
|||||||
|
Interest and other income
|
403
|
|
39
|
|
|||
|
Interest and other expense
|
(383
|
)
|
(326
|
)
|
|||
|
Net loss
|
(26,512
|
)
|
(17,274
|
)
|
|||
|
Accretion of redeemable convertible preferred stock to redemption value
|
(5
|
)
|
(49
|
)
|
|||
|
Net loss available to common stockholders
|
$
|
(26,517
|
)
|
$
|
(17,323
|
)
|
|
|
Net loss per share, basic and diluted
|
$
|
(1.38
|
)
|
$
|
(28.21
|
)
|
|
|
Weighted-average shares used to compute basic and diluted net loss per share
|
19,244,605
|
|
614,017
|
|
|||
|
Redeemable Convertible
Preferred Stock |
Common Stock
|
Additional
Paid-in Capital |
Accumulated
Deficit |
Total
Stockholders’ Equity (Deficit) |
|||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||
|
Balance at December 31, 2015
|
14,952,053
|
|
$
|
60,850
|
|
601,667
|
|
$
|
6
|
|
$
|
4,457
|
|
$
|
(49,753
|
)
|
$
|
(45,290
|
)
|
||||||
|
Issuance of Series C redeemable convertible preferred stock
|
3,632,337
|
|
16,077
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Issuance of common stock under subscription agreement
|
—
|
|
—
|
|
49,374
|
|
—
|
|
281
|
|
—
|
|
281
|
|
|||||||||||
|
Exercises of stock options
|
—
|
|
—
|
|
182,703
|
|
2
|
|
211
|
|
—
|
|
213
|
|
|||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
198
|
|
—
|
|
198
|
|
|||||||||||
|
Accretion of preferred stock to current redemption value
|
—
|
|
49
|
|
—
|
|
—
|
|
—
|
|
(49
|
)
|
(49
|
)
|
|||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(17,274
|
)
|
(17,274
|
)
|
|||||||||||
|
Balance at December 31, 2016
|
18,584,390
|
|
76,976
|
|
833,744
|
|
8
|
|
5,147
|
|
(67,076
|
)
|
(61,921
|
)
|
|||||||||||
|
Issuance of common stock, net of issuance cost; private financing
|
—
|
|
—
|
|
6,359,628
|
|
64
|
|
39,092
|
|
—
|
|
39,156
|
|
|||||||||||
|
Issuance of common stock, net of issuance cost; at the market
|
—
|
|
—
|
|
840,534
|
|
8
|
|
7,595
|
|
—
|
|
7,603
|
|
|||||||||||
|
Accretion of redeemable convertible preferred stock to redemption value
|
—
|
|
5
|
|
—
|
|
—
|
|
—
|
|
(5
|
)
|
(5
|
)
|
|||||||||||
|
Conversion of preferred stock to common stock
|
(18,584,390
|
)
|
(76,981
|
)
|
13,066,666
|
|
131
|
|
76,850
|
|
—
|
|
76,981
|
|
|||||||||||
|
Issuance of common stock upon reverse merger
|
—
|
|
—
|
|
1,024,960
|
|
10
|
|
194
|
|
—
|
|
204
|
|
|||||||||||
|
Reclassification of warrant liability to equity
|
—
|
|
—
|
|
—
|
|
—
|
|
51
|
|
—
|
|
51
|
|
|||||||||||
|
Issuance of common stock upon cashless exercise of warrant
|
—
|
|
—
|
|
16,387
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Exercises of stock options and issuance of restricted stock awards
|
—
|
|
—
|
|
412,894
|
|
5
|
|
302
|
|
—
|
|
307
|
|
|||||||||||
|
Issuance of common stock for cash under employee stock purchase plan
|
—
|
|
—
|
|
13,193
|
|
—
|
|
110
|
|
—
|
|
110
|
|
|||||||||||
|
Issuance of warrant classified as equity
|
—
|
|
—
|
|
—
|
|
—
|
|
127
|
|
127
|
|
|||||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
2,409
|
|
—
|
|
2,409
|
|
|||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(26,512
|
)
|
(26,512
|
)
|
|||||||||||
|
Balance at December 31, 2017
|
—
|
|
$
|
—
|
|
22,568,006
|
|
$
|
226
|
|
$
|
131,877
|
|
$
|
(93,593
|
)
|
$
|
38,510
|
|
||||||
|
Year Ended
December 31, |
|||||||
|
2017
|
2016
|
||||||
|
Cash flows from operating activities:
|
|||||||
|
Net loss
|
$
|
(26,512
|
)
|
$
|
(17,274
|
)
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||
|
Depreciation and amortization
|
308
|
|
341
|
|
|||
|
Issuance of common stock under subscription agreement
|
—
|
|
281
|
|
|||
|
Share-based compensation expense
|
2,409
|
|
198
|
|
|||
|
Non-cash interest expense
|
94
|
|
158
|
|
|||
|
Change in fair value of preferred stock warrants
|
—
|
|
3
|
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Accounts receivable
|
(1,436
|
)
|
(20
|
)
|
|||
|
Prepaid expenses and other assets
|
(724
|
)
|
(426
|
)
|
|||
|
Deferred revenue
|
—
|
|
(519
|
)
|
|||
|
Accounts payable
|
(101
|
)
|
292
|
|
|||
|
Accrued and other liabilities
|
(2,205
|
)
|
2,211
|
|
|||
|
Net cash used in operating activities
|
(28,167
|
)
|
(14,755
|
)
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Cash acquired in reverse merger
|
1,280
|
|
—
|
|
|||
|
Purchases of property and equipment
|
(246
|
)
|
(250
|
)
|
|||
|
Purchases of short-term investments
|
—
|
|
(1,000
|
)
|
|||
|
Maturity of short-term investments
|
—
|
|
1,000
|
|
|||
|
Net cash provided by (used in) investing activities
|
1,034
|
|
(250
|
)
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Proceeds from the sale of common stock - private financing
|
40,703
|
|
—
|
|
|||
|
Payment of issuance costs associated with the sale of common stock - private financing
|
(1,216
|
)
|
—
|
|
|||
|
Proceeds from the sale of common stock - at the market
|
7,862
|
|
—
|
|
|||
|
Payment of issuance costs associated with the sale of common stock - at the market
|
(330
|
)
|
—
|
|
|||
|
Payment of issuance costs associated with the shelf registration
|
(299
|
)
|
—
|
|
|||
|
Proceeds from issuance of notes payable
|
10,000
|
|
—
|
|
|||
|
Payments of principal on notes payable
|
(4,667
|
)
|
(333
|
)
|
|||
|
Proceeds from the exercise of stock options
|
307
|
|
213
|
|
|||
|
Proceeds from stock purchases under employee stock purchase plan
|
110
|
|
—
|
|
|||
|
Proceeds from issuance of redeemable convertible preferred stock
|
—
|
|
16,091
|
|
|||
|
Payment of redeemable convertible preferred stock issuance costs
|
|
|
(91
|
)
|
|||
|
Payment of notes payable issuance costs
|
—
|
|
(6
|
)
|
|||
|
Net cash provided by financing activities
|
52,470
|
|
15,874
|
|
|||
|
Net increase in cash and cash equivalents
|
25,337
|
|
869
|
|
|||
|
Cash and cash equivalents at beginning of period
|
22,104
|
|
21,235
|
|
|||
|
Cash and cash equivalents at end of period
|
$
|
47,441
|
|
$
|
22,104
|
|
|
|
Supplemental disclosure of cash flow information
|
|||||||
|
Interest paid
|
$
|
446
|
|
$
|
164
|
|
|
|
Supplemental disclosure of non-cash investing and financing activities
|
|||||||
|
Conversion of preferred stock to common stock
|
$
|
76,981
|
|
$
|
—
|
|
|
|
Liabilities assumed, net of non-cash assets received in reverse merger
|
$
|
1,076
|
|
$
|
—
|
|
|
|
Transfer of common stock issuance costs from prepaid expenses and other current assets to equity (private financing and at the market sales)
|
$
|
331
|
|
$
|
—
|
|
|
|
Transfer of common stock issuance costs from prepaid expenses and other current assets to equity (At the market / shelf costs)
|
$
|
23
|
|
$
|
—
|
|
|
|
Issuance of warrant classified as equity
|
$
|
127
|
|
$
|
—
|
|
|
|
Reclassification of preferred stock warrant (accrued liability) to common stock warrant (equity)
|
$
|
51
|
|
$
|
—
|
|
|
|
Accretion of redeemable convertible preferred stock to redemption value
|
$
|
5
|
|
$
|
49
|
|
|
|
December 31,
2017 |
December 31,
2016 |
||||||||||||||
|
Level 1
|
Level 3
|
Level 1
|
Level 3
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Assets:
|
|||||||||||||||
|
Money market funds (included in cash and cash equivalents)
(1)
|
$
|
47,653
|
|
$
|
—
|
|
$
|
22,189
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Preferred and common stock warrants (included in accrued and other liabilities)
|
$
|
—
|
|
$
|
82
|
|
$
|
—
|
|
$
|
133
|
|
|||
|
(1)
|
Amounts presented for each period above differ from cash and cash equivalents reported in the consolidated balance sheets due to outstanding disbursements and deposits.
|
|
Balance of liability as of December 31, 2015
|
$
|
169
|
|
|
Other
|
(39
|
)
|
|
|
Change in estimated value of warrants
|
3
|
|
|
|
Balance of liability as of December 31, 2016
|
133
|
|
|
|
Reclassification of warrant liability to equity
|
(51
|
)
|
|
|
Balance of liability as of December 31, 2017
|
$
|
82
|
|
|
Cash and cash equivalents
|
$
|
1,280
|
|
|
Prepaid and other assets
|
248
|
|
|
|
Accrued liabilities
|
(1,324
|
)
|
|
|
Net acquired tangible assets
|
$
|
204
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
(in thousands)
|
|||||||
|
Lab equipment
|
$
|
2,229
|
|
$
|
2,163
|
|
|
|
Leasehold improvements
|
737
|
|
688
|
|
|||
|
Computer hardware and software
|
355
|
|
281
|
|
|||
|
Furniture and fixtures
|
77
|
|
51
|
|
|||
|
Property and equipment, gross
|
3,398
|
|
3,183
|
|
|||
|
Less: accumulated depreciation and amortization
|
(2,835
|
)
|
(2,558
|
)
|
|||
|
Property and equipment, net
|
$
|
563
|
|
$
|
625
|
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
(in thousands)
|
|||||||
|
Accrued employee compensation and related taxes
|
$
|
1,538
|
|
$
|
928
|
|
|
|
Accrued outsourced clinical and preclinical studies
|
581
|
|
1,684
|
|
|||
|
Accrued other professional service fees
|
232
|
|
124
|
|
|||
|
Accrued equipment and lab materials
|
197
|
|
—
|
|
|||
|
Accrued legal fees and expenses
|
185
|
|
759
|
|
|||
|
Value of liability-classified stock purchase warrants
|
82
|
|
133
|
|
|||
|
Deferred and accrued facility lease obligations
|
74
|
|
221
|
|
|||
|
Other accrued liabilities
|
102
|
|
60
|
|
|||
|
Total accrued liabilities
|
$
|
2,991
|
|
$
|
3,909
|
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
(in thousands)
|
|||||||
|
Principal amount outstanding
|
$
|
10,000
|
|
$
|
4,667
|
|
|
|
Unamortized debt discount
|
(119
|
)
|
(14
|
)
|
|||
|
Unamortized debt issuance costs
|
—
|
|
(31
|
)
|
|||
|
Accreted final payment fee
|
41
|
|
167
|
|
|||
|
Total notes payable
|
9,922
|
|
4,789
|
|
|||
|
Less: current maturities
|
—
|
|
(1,969
|
)
|
|||
|
Long-term notes payable, net of current portion
|
$
|
9,922
|
|
$
|
2,820
|
|
|
|
2018
|
$
|
—
|
|
|
2019
|
2,333
|
|
|
|
2020
|
4,000
|
|
|
|
2021
|
3,667
|
|
|
|
Total
|
$
|
10,000
|
|
|
2018
|
$
|
391
|
|
|
2019
|
404
|
|
|
|
2020
|
277
|
|
|
|
Total
|
$
|
1,072
|
|
|
Series A
|
Series B
|
Series C
|
Total
|
||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||
|
Balance at December 31, 2016
|
7,149,176
|
|
$
|
23,124
|
|
2,166,651
|
|
$
|
12,975
|
|
9,268,563
|
|
$
|
40,877
|
|
18,584,390
|
|
$
|
76,976
|
|
|||||||
|
Accretion of redeemable convertible preferred stock to redemption value
|
—
|
|
1
|
|
—
|
|
1
|
|
—
|
|
3
|
|
—
|
|
5
|
|
|||||||||||
|
Conversion of preferred stock to common stock
|
(7,149,176
|
)
|
(23,125
|
)
|
(2,166,651
|
)
|
(12,976
|
)
|
(9,268,563
|
)
|
(40,880
|
)
|
(18,584,390
|
)
|
(76,981
|
)
|
|||||||||||
|
Balance at February 13, 2017
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
|||||||
|
Common Stock Warrants
|
Preferred Stock Warrants
|
||||||||||||
|
Number
|
Weighted Average Exercise Price
|
Number
|
Weighted Average Exercise Price
|
||||||||||
|
Outstanding at December 31, 2015
|
7,031
|
|
$
|
0.57
|
|
25,779
|
|
$
|
6.21
|
|
|||
|
Granted
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
|||
|
Outstanding at December 31, 2016
|
7,031
|
|
$
|
0.57
|
|
25,779
|
|
$
|
6.21
|
|
|||
|
Warrants acquired in Merger
|
13,534
|
|
$
|
80.70
|
|
—
|
|
$
|
—
|
|
|||
|
Preferred stock warrants converted into Common Stock warrants
|
25,779
|
|
$
|
6.21
|
|
(25,779
|
)
|
$
|
6.21
|
|
|||
|
Granted
|
24,097
|
|
$
|
7.15
|
|
—
|
|
$
|
—
|
|
|||
|
Exercised
|
(21,092
|
)
|
$
|
3.04
|
|
—
|
|
$
|
—
|
|
|||
|
Outstanding at December 31, 2017
|
49,349
|
|
$
|
27.65
|
|
—
|
|
$
|
—
|
|
|||
|
Number of Underlying Shares
|
Exercise Price
|
Expiration Date
|
||
|
13,534
|
$80.70
|
2019 & 2020
|
||
|
11,718
|
$8.53
|
2025
|
||
|
24,097
|
$7.15
|
2024
|
||
|
49,349
|
||||
|
Number of Options
(in thousands) |
Weighted Average Exercise Price
|
Weighted Average Remaining Contractual Term
(years) |
Aggregate Intrinsic Value
(in thousands) |
|||||||||
|
Outstanding at December 31, 2015
|
1,884
|
|
$
|
0.95
|
|
|||||||
|
Granted
|
793
|
|
$
|
2.45
|
|
|||||||
|
Exercised
|
(183)
|
|
$
|
(1.17
|
)
|
|||||||
|
Forfeited
|
(173)
|
|
$
|
(1.04
|
)
|
|||||||
|
Outstanding at December 31, 2016
|
2,321
|
|
$
|
1.44
|
|
|||||||
|
Granted
|
989
|
|
$
|
11.37
|
|
|||||||
|
Exercised
|
(412
|
)
|
$
|
(0.74
|
)
|
|||||||
|
Forfeited
|
(35
|
)
|
$
|
(11.36
|
)
|
|||||||
|
Outstanding at December 31, 2017
|
2,863
|
|
$
|
4.85
|
|
6.64
|
$
|
17,021
|
|
|||
|
Vested or expected to vest at December 31, 2017
|
2,863
|
|
$
|
4.85
|
|
6.64
|
$
|
17,021
|
|
|||
|
Exercisable as of December 31, 2017
|
1,616
|
|
$
|
2.17
|
|
4.84
|
$
|
13,495
|
|
|||
|
Year Ended
December 31, |
|||||||
|
2017
|
2016
|
||||||
|
Expected term, in years
|
6.43
|
|
5.00
|
|
|||
|
Expected volatility
|
83.8
|
%
|
85.8
|
%
|
|||
|
Risk-free interest rate
|
2.1
|
%
|
1.3
|
%
|
|||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
|||
|
Weighted-average grant date fair value of underlying Common Stock
|
$
|
11.37
|
|
$
|
2.45
|
|
|
|
Year Ended
December 31, |
||||||||
|
2017
|
2016
|
|||||||
|
(in thousands)
|
||||||||
|
Research and development
|
$
|
917
|
|
$
|
53
|
|
||
|
General and administrative
|
1,492
|
|
145
|
|
||||
|
Total share-based compensation expense
|
$
|
2,409
|
|
$
|
198
|
|
||
|
Year Ended December 31,
|
|||||
|
2017
|
2016
|
||||
|
Federal statutory income tax rate
|
35.0
|
%
|
35.0
|
%
|
|
|
State income taxes, net of federal benefit
|
3.1
|
|
3.2
|
|
|
|
Federal and state tax credits
|
(4.8
|
)
|
5.2
|
|
|
|
Transaction costs
|
(2.9
|
)
|
—
|
|
|
|
Amortization of interest and related charges
|
—
|
|
(0.1
|
)
|
|
|
Other permanent items
|
1.9
|
|
(0.4
|
)
|
|
|
Change in valuation allowance
|
14.5
|
|
(43.0
|
)
|
|
|
Change in tax rate
|
(40.7
|
)
|
—
|
|
|
|
Net operating loss reduction
|
(6.7
|
)
|
—
|
|
|
|
Other, net
|
0.6
|
|
0.1
|
|
|
|
Effective income tax rate
|
—
|
%
|
—
|
%
|
|
|
Year Ended December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
(in thousands)
|
|||||||
|
Net operating loss carryforwards
|
$
|
18,257
|
|
$
|
20,961
|
|
|
|
Tax credits
|
2,287
|
|
2,785
|
|
|||
|
Accruals and reserves
|
697
|
|
1,182
|
|
|||
|
Start-up costs
|
829
|
|
1,414
|
|
|||
|
Stock-based expense
|
439
|
|
—
|
|
|||
|
Gross deferred tax assets
|
22,509
|
|
26,342
|
|
|||
|
Valuation allowance
|
(22,509
|
)
|
(26,342
|
)
|
|||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
|
|
December 31,
|
|||||
|
2017
|
2016
|
||||
|
(in thousands)
|
|||||
|
Options to purchase Common Stock
|
2,863
|
|
2,321
|
|
|
|
Warrants to purchase Common Stock
|
49
|
|
7
|
|
|
|
Redeemable convertible preferred stock (1)
|
—
|
|
13,067
|
|
|
|
Warrants to purchase redeemable convertible preferred stock
|
—
|
|
26
|
|
|
|
Total
|
2,912
|
|
15,421
|
|
|
|
(1)
|
In February 2017, in conjunction with the Merger, all of the outstanding redeemable convertible preferred stock of Private Miragen converted into Private Miragen common stock at a ratio of
1
:1 and was immediately exchanged for the Company’s Common Stock at an exchange ratio of
0.7031
as a result of the Merger. Share amounts in the table above reflect this conversion.
|
|
For the Quarters Ended
|
|||||||||||||||
|
March
|
June
|
September
|
December
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Revenue
|
$
|
462
|
|
$
|
718
|
|
$
|
1,631
|
|
$
|
1,192
|
|
|||
|
Research and development expenses
|
(4,120
|
)
|
(5,487
|
)
|
(5,018
|
)
|
(4,998
|
)
|
|||||||
|
General and administrative expenses
|
(3,281
|
)
|
(2,581
|
)
|
(2,502
|
)
|
(2,548
|
)
|
|||||||
|
Other income (expense), net
|
(41
|
)
|
38
|
|
55
|
|
(32
|
)
|
|||||||
|
Net loss
|
$
|
(6,980
|
)
|
$
|
(7,312
|
)
|
$
|
(5,834
|
)
|
$
|
(6,386
|
)
|
|||
|
Net loss available to common stockholders
|
$
|
(6,985
|
)
|
$
|
(7,312
|
)
|
$
|
(5,834
|
)
|
$
|
(6,386
|
)
|
|||
|
Net loss per share, basic and diluted
|
$
|
(0.60
|
)
|
$
|
(0.34
|
)
|
$
|
(0.27
|
)
|
$
|
(0.29
|
)
|
|||
|
For the Quarters Ended
|
|||||||||||||||
|
March
|
June
|
September
|
December
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Revenue
|
$
|
917
|
|
$
|
1,115
|
|
$
|
936
|
|
$
|
509
|
|
|||
|
Research and development expenses
|
(3,466
|
)
|
(3,355
|
)
|
(2,965
|
)
|
(3,906
|
)
|
|||||||
|
General and administrative expenses
|
(992
|
)
|
(1,210
|
)
|
(2,053
|
)
|
(2,517
|
)
|
|||||||
|
Other expense, net
|
(82
|
)
|
(74
|
)
|
(71
|
)
|
(60
|
)
|
|||||||
|
Net loss
|
$
|
(3,623
|
)
|
$
|
(3,524
|
)
|
$
|
(4,153
|
)
|
$
|
(5,974
|
)
|
|||
|
Net loss available to common stockholders
|
$
|
(3,635
|
)
|
$
|
(3,536
|
)
|
$
|
(4,166
|
)
|
$
|
(5,986
|
)
|
|||
|
Net loss per share, basic and diluted
|
$
|
(5.58
|
)
|
$
|
(5.88
|
)
|
$
|
(6.92
|
)
|
$
|
(9.20
|
)
|
|||