|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
|
47-1187261
(I.R.S. Employer
Identification No.)
|
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Stock, $0.01 par value
|
MGEN
|
The Nasdaq Capital Market
|
|
Large accelerated filer
o
|
Accelerated filer
x
|
|
|
Non-accelerated filer
o
|
Smaller reporting company
x
|
|
|
Emerging growth company
o
|
||
|
Page No.
|
||
|
PART I
|
||
|
PART II
|
|
|
|
PART III
|
||
|
PART IV
|
|
|
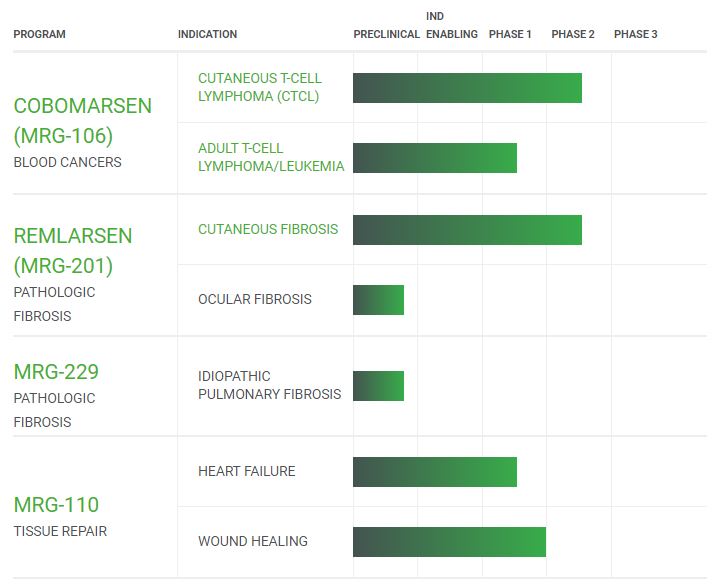
|
•
|
Report preclinical safety and efficacy data for MRG-229 in IPF (Q2-2020)
|
|
•
|
Meet with FDA to explore a potential expedited clinical development path for cobomarsen in ATLL (Q3-2020)
|
|
•
|
Report topline data from Phase 2 clinical trial of cobomarsen in CTCL (Q3-2020)
|
|
•
|
Report primary endpoint data from Phase 2 trial of remlarsen in cutaneous fibrosis (2H-2020)
|
|
•
|
Continue to develop cobomarsen for blood cancers where the disease process appears to be correlated with an increase in miR-155 levels, focusing on CTCL and
ATLL
.
Cobomarsen is currently in development for CTCL and ATLL. Our global Phase 2 clinical trial, SOLAR, is ongoing, though enrollment has been stopped to allow for interim data analysis. This study is assessing the potential benefit and safety of cobomarsen in patients with mycosis fungoides, or
MF
, the most common type of
CTCL
. In addition to CTCL, we are also developing cobomarsen in ATLL, an indication where the disease process appears to correlate with an increase in
miR-155
levels, the target of cobomarsen. In January 2020, we announced interim efficacy and safety data from the ATLL arm of our ongoing Phase 1 clinical trial of cobomarsen.
|
|
•
|
Continue to develop remlarsen and MRG-229 for pathological fibrosis, focusing on the development of MRG-229 in IPF.
Remlarsen is our most advanced product candidate in fibrosis, which is currently being evaluated in a Phase 2 clinical trial assessing its safety, tolerability, and activity in the potential prevention or reduction of keloid formation in patients with a history of keloid scars, a form of pathological scarring. In December 2019, we reported interim data from this clinical trial, which suggest that remlarsen was generally safe and well tolerated, treatment had no reported negative effect on healing, and initial volume reductions in treated keloids compared to placebo in a subset of patients were observed. In addition, we are developing MRG-229 for IPF. We believe that the clinical profile of MRG-229 in our preclinical studies positions this product candidate as a potentially differentiated approach to the treatment of IPF.
|
|
•
|
Utilize rare disease development pathways at the U.S. Food and Drug Administration, or FDA, and comparable programs at foreign regulatory agencies to accelerate progression to late-stage development and early approval.
For many of our programs, we intend to focus on rare and genetic diseases where RNA modulation may produce clinical benefit, so that we can potentially take advantage of regulatory programs intended to expedite drug development. In 2017, the FDA granted orphan drug designation to cobomarsen for the treatment of MF and the European Commission granted orphan medicinal product designation to cobomarsen for the treatment of CTCL. We plan to apply for orphan drug designation, fast track, breakthrough therapy designation, and/or priority review when available to potentially streamline clinical development and decrease time to commercialization.
|
|
•
|
Collaborate with biotechnology and pharmaceutical companies to develop additional product candidates.
We intend to seek out collaborations for the development of compounds in our pipeline for certain disease areas where the costs would exceed our resources or in other areas where we believe that leveraging a partner’s expertise or resources will allow us to accelerate development timelines.
|
|
•
|
Use our in-house research and translational expertise to further develop our product candidate pipeline.
Our in-house research team investigates microRNAs that have been identified as potential therapeutic targets through internal efforts and academic collaborations. We then seek to establish evidence that modulation of the microRNAs’ activity may provide benefit in pathological conditions or diseases in which the microRNA is implicated. We believe that this internal research and expertise could provide a foundation to develop product candidates for the treatment of a variety of diseases.
|
|
Type of Pathological Fibrosis
|
|
Description
|
|
|
Skin Fibrosis
|
|
Ÿ
|
Scarring is a result of an overproduction of collagen in a healing wound. Scarring may continue to thicken for up to six months or may overgrow the site of the wound, even after the wound has healed.
|
|
|
|
Ÿ
|
Hypertrophic scars and keloids are abnormal wound responses and represent an excessive connective tissue response to skin trauma, inflammation, surgery, or burns.
|
|
|
|
Ÿ
|
Hypertrophic scars and keloids are characterized by local fibroblast proliferation and overproduction of collagen. Both hypertrophic scars and keloids are diseases that tend to be painful and itchy, restrict mobility, and are resistant to treatment.
|
|
Pulmonary Fibrosis
|
|
Ÿ
|
Pulmonary fibrosis, also known as lung fibrosis, is caused by accumulation of scar tissue surrounding the air sacs (interstitial space) in the lung. As a result, the lung tissue becomes stiff and loses the ability to expand. The scar tissue also prevents normal transport of oxygen. The result is a progressive respiratory failure, with symptoms that include persistent cough, chest pain, difficulty breathing and fatigue. Pulmonary fibrosis leads to cardiac failure and death. Pulmonary fibrosis may occur as a secondary condition in various other diseases, but in many cases the underlying cause is not clear and is referred to as “idiopathic” pulmonary fibrosis or IPF.
|
|
|
|
Ÿ
|
IPF is a chronic, progressive lung disease which ultimately leads to death in many of the patients. This condition causes scar tissue to build up in the lungs, which makes the lungs unable to transport oxygen into the bloodstream effectively.
|
|
Liver Fibrosis
|
|
Ÿ
|
Liver fibrosis refers to the scar tissue and nodules that replace liver tissue and disrupt liver function. Major causes of liver fibrosis are alcohol consumption, chronic hepatitis B virus infection, hepatitis C virus infection, and metabolic disorders, including non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Liver fibrosis is a major global problem driven by increasing rates of obesity and diabetes.
|
|
Eye Fibrosis
|
|
Ÿ
|
Infection or inflammation of the eye results in impairment of visual function. Chronic inflammation can ultimately lead to fibrosis.
|
|
|
|
Ÿ
|
Eye fibrosis diseases include retinal fibrosis (such as diabetic retinopathy, diabetic vitreoretinopathy, age macular degeneration), corneal fibrosis (such as post-traumatic or infections corneal fibrosis and Fuch’s endothelial corneal dystrophy), and other indications (such as prevention of fibrosis complications in glaucoma trabeculotomy).
|
|
•
|
companies working to develop microRNA-targeted products, including Regulus Therapeutics Inc. and InteRNA Technologies B.V.;
|
|
•
|
companies working to develop other types of oligonucleotide therapeutic products, including Ionis Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., Arrowhead Pharmaceuticals, Inc., Dicerna Pharmaceuticals, Inc., STELLAS Life Sciences Group, Inc., Silence Therapeutics AG, and Translate Bio, Inc.; and
|
|
•
|
companies with marketed products and development programs for therapeutics that treat the same diseases for which we may also be developing potential treatments.
|
|
•
|
completion of extensive preclinical laboratory tests, animal studies, and formulation studies in accordance with the FDA’s good laboratory practices, or GLP, regulations;
|
|
•
|
approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated at that site;
|
|
•
|
submission to the FDA of an investigational new drug application, or IND, for human clinical testing, which must become effective before human clinical trials may begin;
|
|
•
|
performance of adequate and well-controlled human clinical trials in accordance with good clinical practice, or GCP, requirements to establish the safety and efficacy of the drug for each proposed indication;
|
|
•
|
submission to the FDA of an NDA after completion of all pivotal clinical trials;
|
|
•
|
satisfactory completion of an FDA advisory committee review, if applicable
|
|
•
|
satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the active pharmaceutical ingredient, or API, and finished drug product are produced and tested to assess compliance with current good manufacturing practices, or cGMPs; and
|
|
•
|
FDA review and approval of the NDA prior to any commercial marketing or sale of the drug in the United States.
|
|
•
|
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market, or product recalls;
|
|
•
|
fines, warning letters, or holds on post-approval clinical trials;
|
|
•
|
refusal of the FDA to approve pending applications or supplements to approved applications or suspension or revocation of product approvals;
|
|
•
|
product seizure or detention, or refusal to permit the import or export of products, or injunctions or the imposition of civil or criminal penalties.
|
|
•
|
significantly delay, scale back, or discontinue the development or commercialization of our product candidates;
|
|
•
|
seek strategic alliances, or amend existing alliances, for research and development programs at an earlier stage than otherwise would be desirable or that we otherwise would have sought to develop independently, or on terms that are less favorable than might otherwise be available in the future;
|
|
•
|
dispose of technology assets, or relinquish or license on unfavorable terms, our rights to technologies or any of our product candidates that we otherwise would seek to develop or commercialize ourselves;
|
|
•
|
pursue the sale of our company to a third party at a price that may result in a loss on investment for our stockholders; or
|
|
•
|
file for bankruptcy or cease operations altogether.
|
|
•
|
continue the clinical development of our product candidates;
|
|
•
|
continue efforts to discover and develop new product candidates;
|
|
•
|
continue the manufacturing of our product candidates or increase volumes manufactured by third parties;
|
|
•
|
advance our programs into larger, more expensive clinical trials;
|
|
•
|
initiate additional preclinical studies or clinical trials for our product candidates;
|
|
•
|
seek regulatory and marketing approvals and reimbursement for our product candidates;
|
|
•
|
establish a sales, marketing, and distribution infrastructure to commercialize any products for which we may obtain marketing approval and market for ourselves;
|
|
•
|
seek to identify, assess, acquire, and/or develop other product candidates;
|
|
•
|
make milestone, royalty, or other payments under third-party license agreements;
|
|
•
|
seek to maintain, protect, and expand our intellectual property portfolio;
|
|
•
|
seek to attract and retain skilled personnel; and
|
|
•
|
experience any delays or encounter issues with the development and potential for regulatory approval of our clinical and product candidates such as safety issues, manufacturing delays, clinical trial accrual delays, longer follow-up for planned studies or trials, additional major studies or trials, or supportive trials necessary to support marketing approval.
|
|
•
|
completing research and development of our product candidates;
|
|
•
|
obtaining regulatory and marketing approvals for our product candidates;
|
|
•
|
manufacturing product candidates and establishing and maintaining supply and manufacturing relationships with third parties that are commercially feasible, meet regulatory requirements and our supply needs in sufficient quantities to meet market demand for our product candidates, if approved;
|
|
•
|
marketing, launching, and commercializing product candidates for which we obtain regulatory and marketing approval, either directly or with a collaborator or distributor;
|
|
•
|
gaining market acceptance of our product candidates as treatment options;
|
|
•
|
addressing any competing products;
|
|
•
|
protecting and enforcing our intellectual property rights, including patents, trade secrets, and know-how;
|
|
•
|
negotiating favorable terms in any collaboration, licensing, or other arrangements into which we may enter;
|
|
•
|
obtaining coverage and adequate reimbursement from third party payors and maintaining pricing for our product candidates that supports profitability; and
|
|
•
|
attracting, hiring, and retaining qualified personnel.
|
|
•
|
inability to generate satisfactory preclinical, toxicology, or other in vivo or in vitro data or diagnostics to support the initiation or continuation of clinical trials;
|
|
•
|
delays in reaching agreement on acceptable terms with CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites;
|
|
•
|
delays in obtaining required approvals from institutional review boards or independent ethics committees at each clinical trial site;
|
|
•
|
failure to permit the conduct of a clinical trial by regulatory authorities;
|
|
•
|
delays in recruiting eligible patients and/or subjects in our clinical trials;
|
|
•
|
failure by clinical sites or CROs or other third parties to adhere to clinical trial requirements;
|
|
•
|
failure by our clinical sites, CROs or other third parties to perform in accordance with the good clinical practices requirements of the FDA or applicable foreign regulatory guidelines;
|
|
•
|
patients and/or subjects dropping out of our clinical trials;
|
|
•
|
adverse events or tolerability or animal toxicology issues significant enough for the FDA or other regulatory agencies to put any or all clinical trials on hold;
|
|
•
|
occurrence of adverse events associated with our product candidates;
|
|
•
|
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols;
|
|
•
|
the cost of clinical trials of our product candidates, including manufacturing costs;
|
|
•
|
negative or inconclusive results from our clinical trials, which may result in our deciding, or regulators requiring us, to conduct additional clinical trials or abandon development programs in other ongoing or planned indications for a product candidate; and
|
|
•
|
delays in reaching agreement on acceptable terms with third-party manufacturers and the time to manufacture sufficient quantities of our product candidates acceptable for use in clinical trials.
|
|
•
|
regulatory authorities may withdraw approvals of such products;
|
|
•
|
regulatory authorities may require additional warnings on the drug label;
|
|
•
|
we may be required to create a Risk Evaluation and Mitigation Strategy, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers, and/or other elements to assure safe use;
|
|
•
|
we could be sued and held liable for harm caused to patients or subjects; and
|
|
•
|
our reputation may suffer.
|
|
•
|
withdrawal of clinical trial volunteers, investigators, patients or subjects, or trial sites, or limitations on approved indications;
|
|
•
|
the inability to commercialize, or if commercialized, decreased demand for, our product candidates;
|
|
•
|
if commercialized, product recalls, labeling, marketing or promotional restrictions, or the need for product modification;
|
|
•
|
initiation of investigations by regulators;
|
|
•
|
loss of revenue;
|
|
•
|
substantial costs of litigation, including monetary awards to patients or other claimants;
|
|
•
|
liabilities that substantially exceed our product liability insurance, which we would then be required to pay ourselves;
|
|
•
|
an increase in our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable terms, if at all;
|
|
•
|
the diversion of management’s attention from our business; and
|
|
•
|
damage to our reputation and the reputation of our products and our technology.
|
|
•
|
issue warning letters;
|
|
•
|
impose civil or criminal penalties;
|
|
•
|
suspend or withdraw regulatory approval;
|
|
•
|
suspend any of our ongoing clinical trials;
|
|
•
|
refuse to approve pending applications or supplements to approved applications submitted by us;
|
|
•
|
impose restrictions on our operations, including closing our contract manufacturers’ facilities; or
|
|
•
|
require a product recall.
|
|
•
|
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs;
|
|
•
|
federal civil and criminal false claims laws, including the federal False Claims Act which can be enforced by individuals through civil whistleblower or qui tams actions, and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
|
|
•
|
the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their implementing regulations, which imposes specified obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information without the appropriate authorization, on entities subject to the law, such as certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, and their respective business associates, individuals, and entities that perform services for them that involve the creation, use, maintenance, or disclosure of individually identifiable health information;
|
|
•
|
the federal Physician Payments Sunshine Act under the Affordable Care Act which requires manufacturers of drugs, devices, biologics, and medical supplies, with certain exceptions, to report annually to the Center for Medicare and Medicaid Services, or CMS, information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers, as well as their immediate family members and applicable group purchasing organizations;
|
|
•
|
the GDPR and other EU member state data protection legislation as well as that of the United Kingdom, which requires data controllers and processors, to adopt administrative, physical, and technical safeguards designed to protect personal data, including health-related data, including mandatory contractual terms with third-party providers, requirements for establishing an appropriate legal basis for processing personal data, transparency requirements related to communications with data subjects regarding the processing of their personal data, standards for obtaining consent from individuals to process their personal data, notification requirements to individuals about the processing of their personal data, an individual data rights regime, mandatory data breach notifications, limitations on the retention of personal data, increased requirements pertaining to health data, and strict rules and restrictions on the transfer of personal data outside of the EU, including to the United States; and
|
|
•
|
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including governmental and private payors, to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or drug pricing; state and local laws that require
|
|
•
|
require repayment of all or a portion of the grant proceeds, in specified cases with interest, in the event we violate specified covenants pertaining to various matters that include a failure to achieve;
|
|
•
|
specify milestones or terms relating to use of grant proceeds, or to comply with specified laws;
|
|
•
|
terminate agreements, in whole or in part, for any reason or no reason;
|
|
•
|
reduce or modify the government’s obligations under such agreements without the consent of the other party;
|
|
•
|
claim rights, including intellectual property rights, in products and data developed under such agreements;
|
|
•
|
audit contract related costs and fees, including allocated indirect costs;
|
|
•
|
suspend the contractor or grantee from receiving new contracts pending resolution of alleged violations of procurement laws or regulations;
|
|
•
|
impose U.S. manufacturing requirements for products that embody inventions conceived or first reduced to practice under such agreements;
|
|
•
|
impose qualifications for the engagement of manufacturers, suppliers, and other contractors as well as other criteria for reimbursements;
|
|
•
|
suspend or debar the contractor or grantee from doing future business with the government;
|
|
•
|
control and potentially prohibit the export of products;
|
|
•
|
pursue criminal or civil remedies under the federal False Claims Act, False Statements Act, and similar remedy provisions specific to government agreements; and
|
|
•
|
limit the government’s financial liability to amounts appropriated by the U.S. Congress on a fiscal year basis, thereby leaving some uncertainty about the future availability of funding for a program even after we have been funded for an initial period.
|
|
•
|
specialized accounting systems unique to government contracts and grants;
|
|
•
|
mandatory financial audits and potential liability for price adjustments or recoupment of government funds after such funds have been spent;
|
|
•
|
public disclosures of some contract and grant information, which may enable competitors to gain insights into our research program; and
|
|
•
|
mandatory socioeconomic compliance requirements, including labor standards, non-discrimination and affirmative action programs, and environmental compliance requirements.
|
|
•
|
We may be unable to identify manufacturers on acceptable terms or at all.
|
|
•
|
Our third-party manufacturers might be unable to timely formulate and manufacture our product or produce the quantity and quality required to meet our clinical and commercial needs, if any.
|
|
•
|
Contract manufacturers may not be able to execute our manufacturing procedures appropriately.
|
|
•
|
Our future third-party manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store, and distribute our products.
|
|
•
|
Manufacturers are subject to ongoing periodic unannounced inspection by the FDA and some state agencies to ensure strict compliance with cGMPs and other government regulations and corresponding foreign standards. We do not have control over third-party manufacturers’ compliance with these regulations and standards.
|
|
•
|
We may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our product candidates.
|
|
•
|
Our third-party manufacturers could breach or terminate their agreement with us.
|
|
•
|
Labor disputes or shortages, including from the effects of health emergencies (such as novel viruses or pandemics) and natural disasters.
|
|
•
|
collaborators often have significant discretion in determining the efforts and resources that they will apply to the collaboration and may not commit sufficient resources to the development, marketing, or commercialization of the product or products that are subject to the collaboration;
|
|
•
|
collaborators may not perform their obligations as expected;
|
|
•
|
any such collaboration may significantly limit our share of potential future profits from the associated program and may require us to relinquish potentially valuable rights to our current product candidates, potential products, proprietary technologies, or grant licenses on terms that are not favorable to us;
|
|
•
|
collaborators may cease to devote resources to the development or commercialization of our product candidates if the collaborators view our product candidates as competitive with their own products or product candidates;
|
|
•
|
disagreements with collaborators, including disagreements over proprietary rights, contract interpretation, or the course of development, might cause delays or termination of the development or commercialization of product candidates, and might result in legal proceedings, which would be time consuming, distracting, and expensive;
|
|
•
|
collaborators may be impacted by changes in their strategic focus or available funding, or business combinations involving them, which could cause them to divert resources away from the collaboration;
|
|
•
|
collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability;
|
|
•
|
the collaborations may not result in us achieving revenue to justify such transactions; and
|
|
•
|
collaborations may be terminated and, if terminated, may result in a need for us to raise additional capital to pursue further development or commercialization of the applicable product candidate.
|
|
•
|
the efficacy of the product as demonstrated in clinical trials and potential advantages over competing treatments;
|
|
•
|
the prevalence and severity of the disease and any side effects;
|
|
•
|
the clinical indications for which approval is granted, including any limitations or warnings contained in a product’s approved labeling;
|
|
•
|
the convenience and ease of administration;
|
|
•
|
the cost of treatment;
|
|
•
|
the willingness of the patients and physicians to accept these therapies;
|
|
•
|
the perceived ratio of risk and benefit of these therapies by physicians and the willingness of physicians to recommend these therapies to patients based on such risks and benefits;
|
|
•
|
the marketing, sales, and distribution support for the product;
|
|
•
|
the publicity concerning our products or competing products and treatments; and
|
|
•
|
the pricing and availability of third-party payor coverage and adequate reimbursement.
|
|
•
|
our research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates;
|
|
•
|
we may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates;
|
|
•
|
our product candidates may not succeed in preclinical or clinical testing;
|
|
•
|
our potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval;
|
|
•
|
competitors may develop alternatives that render our product candidates obsolete or less attractive;
|
|
•
|
product candidates we develop may be covered by third parties’ patents or other exclusive rights;
|
|
•
|
the market for a product candidate may change during our program so that such a product may become unreasonable to continue to develop;
|
|
•
|
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
|
|
•
|
a product candidate may not be accepted as safe and effective by patients, the medical community, or third-party payors.
|
|
•
|
our ability to obtain regulatory approvals for cobomarsen, remlarsen, MRG-110, or other product candidates, and delays or failures to obtain such approvals;
|
|
•
|
failure of any of our product candidates, if approved, to achieve commercial success;
|
|
•
|
failure to maintain our existing third-party license and supply agreements;
|
|
•
|
changes in laws or regulations applicable to our product candidates;
|
|
•
|
any inability to obtain adequate supply of our product candidates or the inability to do so at acceptable prices;
|
|
•
|
adverse regulatory authority decisions;
|
|
•
|
introduction of new products, services, or technologies by our competitors;
|
|
•
|
failure to meet or exceed financial and development projections we may provide to the public and the investment community;
|
|
•
|
the perception of the pharmaceutical industry by the public, legislatures, regulators, and the investment community;
|
|
•
|
announcements of significant acquisitions, strategic collaborations, joint ventures, or capital commitments by us or our competitors;
|
|
•
|
disputes or other developments relating to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies;
|
|
•
|
additions or departures of key personnel;
|
|
•
|
significant lawsuits, including patent or stockholder litigation;
|
|
•
|
if securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our business and stock;
|
|
•
|
changes in the market valuations of similar companies;
|
|
•
|
general market or macroeconomic conditions;
|
|
•
|
sales of our common stock by us or our stockholders in the future;
|
|
•
|
trading volume of our common stock;
|
|
•
|
announcements by commercial partners or competitors of new commercial products, clinical progress or the lack thereof, significant contracts, commercial relationships, or capital commitments;
|
|
•
|
adverse publicity relating to microRNA-targeted therapeutics generally, including with respect to other products and potential products in such markets;
|
|
•
|
the introduction of technological innovations or new therapies that compete with our potential products;
|
|
•
|
changes in the structure of health care payment systems; and
|
|
•
|
period-to-period fluctuations in our financial results.
|
|
•
|
Cutaneous T-Cell Lymphoma:
In December 2019, we announced plans to stop the enrollment of new patients in the SOLAR trial and conduct an analysis of topline clinical response. This analysis will provide controlled data to assess the benefit of cobomarsen based on disease response in the skin in comparison to vorinostat. A total of 37 patients have been enrolled and will continue to be evaluated for safety and clinical response in the coming months. We plan to assess the rate of an objective response in the skin, that is durable for four months, defined as 50% or greater improvement in the severity of a patient’s skin disease over the entire body (mSWAT). This change from assessing overall response to skin response was driven by the fact that patients allowed into the study only have skin disease and are verified not to have blood, nodes, or visceral involvement at study entry. Improvements in skin disease are thus intended to reflect efficacy of the drug whereas progression in skin disease reflect lack of efficacy. Follow up analysis for blood, nodes or visceral disease may be conducted based on the results obtained using mSWAT. We believe that obtaining controlled clinical data from this cohort of patients may allow for a better assessment of the clinical potential of cobomarsen as compared to data from the Phase 1 trial. We intend for this controlled clinical data to form the basis of determining what additional clinical investigation of cobomarsen in CTCL is warranted, if any, and what would be required to potentially obtain regulatory approval. Topline data from this amended trial is expected to be announced in the third quarter of 2020.
|
|
•
|
Adult T-Cell Leukemia/Lymphoma:
In January 2020, we announced positive data for cobomarsen in ATLL patients with residual disease from this first-in-human Phase 1 clinical trial. In this trial, cobomarsen was observed to improve disease stabilization and reduce cellular proliferation and activation biomarker expression associated with ATLL cellular proliferation and activation in patients with persistent residual disease after chemotherapy and other therapies. Based on these results, we announced that we are focusing our cobomarsen expansion indication efforts on ATLL and expect to meet with the FDA in the third quarter of 2020 to explore a potential expedited development path for cobomarsen in ATLL.
|
|
•
|
Cutaneous Fibrosis (Remlarsen):
During the fourth quarter of 2019, we reported interim data from a Phase 2 clinical trial assessing remlarsen for safety, tolerability, and activity in the potential prevention or reduction of keloid formation in patients with a history of keloid scars, a form of pathological scarring. These data suggest that remlarsen was generally safe and well tolerated and treatment had no negative effect on healing reported. In addition, we observed initial volume reductions in treated keloids compared to placebo in a subset of patients. Based on these data, we decided to continue our analysis of patient data at the one-year primary endpoint of the clinical trial. With these data, we may seek a collaboration partner for the future development of remlarsen.
|
|
•
|
Ocular Fibrosis (Remlarsen):
We are also evaluating remlarsen in ocular fibrotic indications, such as corneal injury and keratitis. In April 2019, we presented data in preclinical studies testing ability of remlarsen to penetrate injured corneas and reduce fibrosis after a corneal injury. Topical administration of remlarsen to an injured rat cornea resulted in faster healing of the cornea and reduced scarring/hazing. Remlarsen has also been observed in in vitro studies to regulate miR-29 pharmacodynamic biomarkers in the cornea.
|
|
•
|
Idiopathic Pulmonary Fibrosis (MRG-229):
In December 2019, we announced that our preclinical pipeline development efforts will be primarily focused on the development of MRG-229 as a potential treatment for patients with IPF. We believe that the efficacy and safety profile of MRG-229 positions it as a potentially differentiated approach for IPF. This program is supported in part by a grant in collaboration with the National Institutes of Health and Yale University. We expect to report additional preclinical safety and efficacy data for MRG-229 during the second quarter of 2020.
|
|
•
|
Tissue Repair:
During the fourth quarter of 2019, we announced data from two Phase 1 clinical trials of MRG-110 in normal human volunteers, in which administration of MRG-110 was observed to increase angiogenesis, as demonstrated by increased perfusion and histological markers of neoangiogenesis, as well as reduce alpha-smooth muscle actin (α-SMA) expression, which has been shown to correlate with activation of myofibroblasts. A total of 65 subjects were exposed for up to three weeks. MRG-110 was shown to be generally safe and well tolerated, with no evidence of unwanted distal angiogenesis, acute inflammatory toxicities, or significant abnormalities in liver, kidney, or blood, and no injection site
|
|
•
|
employee-related expenses, including salaries, severance, retention, benefits, insurance, and share-based compensation expense;
|
|
•
|
expenses incurred under agreements with contract research organizations, or CROs, investigative sites that conduct our clinical trials, and other clinical trial-related vendors, and consultants;
|
|
•
|
the costs of acquiring, developing, and manufacturing and testing clinical and preclinical materials, including costs incurred under agreements with contract manufacturing organizations, or CMOs;
|
|
•
|
costs associated with non-clinical activities and regulatory operations;
|
|
•
|
license fees and milestone payments related to the acquisition and retention of certain licensed technology and intellectual property rights; and
|
|
•
|
facilities, depreciation, market research, and other expenses, which include allocated expenses for rent and maintenance of facilities, depreciation of leasehold improvements and equipment, and laboratory supplies.
|
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Revenue
|
$
|
4,461
|
|
$
|
8,386
|
|
|
|
Research and development expenses
|
34,794
|
|
30,421
|
|
|||
|
General and administrative expenses
|
11,646
|
|
11,049
|
|
|||
|
Other income, net
|
106
|
|
381
|
|
|||
|
Net loss
|
$
|
(41,873
|
)
|
$
|
(32,703
|
)
|
|
|
•
|
increased clinical development and related manufacturing expenses of $3.4 million, primarily related to expenses incurred in connection with the clinical development of cobomarsen;
|
|
•
|
increased personnel-related costs of $2.5 million, including restructuring costs, share-based compensation charges, consulting and contract labor costs; partially offset by
|
|
•
|
decreased technology license fees of $0.8 million, primarily related to a milestone payment under one of our license agreements related to the initiation of clinical development of MRG-110 during 2018 that did not recur in 2019; and
|
|
•
|
decreased other miscellaneous expenses of $0.3 million associated with the reduction in headcount, travel, and other research activities.
|
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Net cash provided by (used in):
|
|||||||
|
Operating activities
|
$
|
(36,056
|
)
|
$
|
(26,844
|
)
|
|
|
Investing activities
|
28,226
|
|
(29,907
|
)
|
|||
|
Financing activities
|
70
|
|
41,916
|
|
|||
|
Total
|
$
|
(7,760
|
)
|
$
|
(14,835
|
)
|
|
|
Incorporated by Reference
|
||||||
|
Exhibit
Number
|
Description of Exhibit
|
Form
|
Filing Date
|
Number
|
Filed Herewith
|
|
|
10-Q
|
08/14/2014
|
3.1
|
||||
|
S-4
|
12/02/2016
|
3.3
|
||||
|
8-K
|
02/13/2017
|
3.1
|
||||
|
8-K
|
02/13/2017
|
3.2
|
||||
|
10-Q
|
08/15/2016
|
3.1
|
||||
|
8-K
|
02/13/2017
|
3.3
|
||||
|
8-K
|
02/13/2017
|
3.4
|
||||
|
S-1
|
03/19/2014
|
4.1
|
||||
|
10-K
|
03/14/2019
|
4.2
|
||||
|
8-K
|
11/15/2017
|
10.2
|
||||
|
8-K
|
02/07/2020
|
4.1
|
||||
|
Ÿ
|
||||||
|
S-1
|
03/19/2014
|
10.14
|
||||
|
S-4
|
12/02/2016
|
10.32
|
||||
|
S-4
|
12/02/2016
|
10.37
|
||||
|
S-4
|
12/02/2016
|
10.38
|
||||
|
10-Q
|
05/11/2017
|
10.12
|
||||
|
S-4
|
12/02/2016
|
10.48
|
||||
|
S-4
|
12/02/2016
|
10.49
|
||||
|
S-4
|
12/02/2016
|
10.39
|
||||
|
10-Q
|
05/11/2017
|
10.13
|
||||
|
S-4
|
12/02/2016
|
10.33
|
||||
|
S-4
|
12/02/2016
|
10.34
|
||||
|
Ÿ
|
||||||
|
S-4
|
12/02/2016
|
10.36
|
||||
|
S-4
|
12/02/2016
|
10.40
|
||||
|
S-4
|
12/02/2016
|
10.40.1
|
||||
|
S-4
|
12/02/2016
|
10.40.2
|
||||
|
Ÿ
|
||||||
|
S-4
|
12/02/2016
|
10.41
|
||||
|
S-4
|
12/02/2016
|
10.43
|
||||
|
S-4
|
12/02/2016
|
10.43.1
|
||||
|
S-4
|
12/02/2016
|
10.44
|
||||
|
10-Q
|
11/08/2019
|
10.3
|
||||
|
S-4
|
12/02/2016
|
10.45
|
||||
|
S-4
|
12/02/2016
|
10.45.1
|
||||
|
S-4
|
12/02/2016
|
10.45.2
|
||||
|
S-4
|
12/02/2016
|
10.45.3
|
||||
|
S-4
|
12/02/2016
|
10.45.4
|
||||
|
10-Q
|
08/11/2017
|
10.1
|
||||
|
10-Q
|
11/09/2017
|
10.1
|
||||
|
10-K
|
05/09/2018
|
10.1
|
||||
|
10-K
|
03/14/2019
|
10.17.8
|
||||
|
S-4
|
12/02/2016
|
10.51
|
||||
|
10-Q/A
|
06/07/2017
|
10.14
|
||||
|
10-Q
|
11/09/2017
|
10.2
|
||||
|
10-Q
|
08/08/2018
|
10.2
|
||||
|
10-Q
|
08/08/2018
|
10.3
|
||||
|
10-Q
|
05/09/2019
|
10.1
|
||||
|
10-Q
|
11/08/2019
|
10.1
|
||||
|
S-4
|
12/02/2016
|
10.47
|
||||
|
S-4
|
01/04/2017
|
10.47.1
|
||||
|
8-K
|
11/15/2017
|
10.1
|
||||
|
8-K
|
03/31/2017
|
10.1
|
||||
|
10-Q
|
11/07/2018
|
10.2
|
||||
|
8-K
|
10/31/2019
|
10.1
|
||||
|
8-K
|
12/11/2019
|
10.1
|
||||
|
8-K
|
12/11/2019
|
4.1
|
||||
|
Ÿ
|
||||||
|
Ÿ
|
||||||
|
Ÿ
|
||||||
|
Ÿ
|
||||||
|
Ÿ
|
||||||
|
Ÿ
|
||||||
|
101.INS**
|
XBRL Instance Document
|
Ÿ
|
||||
|
101.SCH**
|
XBRL Taxonomy Extension Schema Document
|
Ÿ
|
||||
|
101.CAL**
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
Ÿ
|
||||
|
101.DEF**
|
XBRL Taxonomy Extension Definition Linkbase Document
|
Ÿ
|
||||
|
101.LAB**
|
XBRL Taxonomy Extension Label Linkbase Document
|
Ÿ
|
||||
|
101.PRE**
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
Ÿ
|
||||
|
^
|
Certain portions of the exhibit, identified by the mark, “[*]”, have been omitted because such portions contained information that is both (i) not material and (ii) would likely cause competitive harm if publicly disclosed.
|
|
*
|
This certification is being furnished pursuant to 18 U.S.C. Section 1350 and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the Registrant, whether made before or after the date hereof.
|
|
**
|
In accordance with Rule 406T of Regulation S-T, the Interactive Data Files in Exhibit 101 are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Exchange Act of 1934, as amended, and otherwise are not subject to liability under these sections.
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
24,846
|
|
$
|
32,606
|
|
|
|
Short-term investments
|
1,999
|
|
29,875
|
|
|||
|
Accounts receivable
|
108
|
|
24
|
|
|||
|
Prepaid expenses and other current assets
|
2,786
|
|
2,865
|
|
|||
|
Total current assets
|
29,739
|
|
65,370
|
|
|||
|
Property and equipment, net
|
523
|
|
727
|
|
|||
|
Other assets
|
—
|
|
50
|
|
|||
|
Total assets
|
$
|
30,262
|
|
$
|
66,147
|
|
|
|
Liabilities and Stockholders’ Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
1,096
|
|
$
|
571
|
|
|
|
Accrued liabilities
|
5,108
|
|
3,868
|
|
|||
|
Current portion of note payable
|
3,976
|
|
2,294
|
|
|||
|
Total current liabilities
|
10,180
|
|
6,733
|
|
|||
|
Note payable, net of current portion
|
4,328
|
|
8,004
|
|
|||
|
Other liabilities
|
—
|
|
66
|
|
|||
|
Total liabilities
|
14,508
|
|
14,803
|
|
|||
|
Commitments and contingencies
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Common stock, $0.01 par value; 100,000,000 shares authorized; 34,861,876 and 30,839,463 shares issued and outstanding at December 31, 2019 and 2018, respectively
|
349
|
|
308
|
|
|||
|
Additional paid-in capital
|
183,574
|
|
177,335
|
|
|||
|
Accumulated other comprehensive loss
|
—
|
|
(3
|
)
|
|||
|
Accumulated deficit
|
(168,169
|
)
|
(126,296
|
)
|
|||
|
Total stockholders’ equity
|
15,754
|
|
51,344
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
30,262
|
|
$
|
66,147
|
|
|
|
Year Ended
December 31, |
||||||||
|
2019
|
2018
|
|||||||
|
Revenue:
|
||||||||
|
Collaboration revenue
|
$
|
4,308
|
|
$
|
7,373
|
|
||
|
Grant revenue
|
153
|
|
1,013
|
|
||||
|
Total revenue
|
4,461
|
|
8,386
|
|
||||
|
Operating expenses:
|
||||||||
|
Research and development
|
34,794
|
|
30,421
|
|
||||
|
General and administrative
|
11,646
|
|
11,049
|
|
||||
|
Total operating expenses
|
46,440
|
|
41,470
|
|
||||
|
Loss from operations
|
(41,979
|
)
|
(33,084
|
)
|
||||
|
Other income (expense):
|
||||||||
|
Interest and other income
|
941
|
|
1,254
|
|
||||
|
Interest and other expense
|
(835
|
)
|
(873
|
)
|
||||
|
Net loss
|
(41,873
|
)
|
(32,703
|
)
|
||||
|
Change in unrealized gain (loss) on investments
|
3
|
|
(3
|
)
|
||||
|
Comprehensive loss
|
$
|
(41,870
|
)
|
$
|
(32,706
|
)
|
||
|
Net loss
|
$
|
(41,873
|
)
|
$
|
(32,703
|
)
|
||
|
Net loss per share, basic and diluted
|
$
|
(1.34
|
)
|
$
|
(1.10
|
)
|
||
|
Weighted-average shares used to compute basic and diluted net loss per share
|
31,336,409
|
|
29,600,332
|
|
||||
|
Common Stock
|
Additional
Paid-in Capital |
Accumulated Other Comprehensive Gain (Loss)
|
Accumulated
Deficit |
Total
Stockholders’ Equity |
|||||||||||||||||||
|
Shares
|
Amount
|
||||||||||||||||||||||
|
Balance as of Balance at December 31, 2017
|
22,568,006
|
|
$
|
226
|
|
$
|
131,877
|
|
$
|
—
|
|
$
|
(93,593
|
)
|
$
|
38,510
|
|
||||||
|
Issuance of common stock in a public offering, net of issuance costs
|
7,414,996
|
|
74
|
|
37,771
|
|
—
|
|
—
|
|
37,845
|
|
|||||||||||
|
Issuance of common stock under the 2017 ATM, net of issuance costs
|
372,852
|
|
4
|
|
2,653
|
|
—
|
|
—
|
|
2,657
|
|
|||||||||||
|
Issuance of common stock pursuant to a 2018 stock purchase agreement, net of issuance costs
|
150,987
|
|
2
|
|
933
|
|
—
|
|
—
|
|
935
|
|
|||||||||||
|
Shares issued for cash upon the exercise of stock options under an equity incentive plan
|
280,178
|
|
2
|
|
180
|
|
—
|
|
—
|
|
182
|
|
|||||||||||
|
Issuance of common stock for cash under employee stock purchase plan
|
52,444
|
|
—
|
|
242
|
|
—
|
|
—
|
|
242
|
|
|||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
3,679
|
|
—
|
|
—
|
|
3,679
|
|
|||||||||||
|
Change in unrealized loss on investments
|
(3
|
)
|
(3
|
)
|
|||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(32,703
|
)
|
(32,703
|
)
|
|||||||||||
|
Balance as of December 31, 2018
|
30,839,463
|
|
308
|
|
177,335
|
|
(3
|
)
|
(126,296
|
)
|
51,344
|
|
|||||||||||
|
Issuance of common stock pursuant to a 2019 stock purchase agreement, net of issuance costs
|
2,557,544
|
|
26
|
|
864
|
|
—
|
|
—
|
|
890
|
|
|||||||||||
|
Issuance of common stock under the 2017 ATM, net of issuance costs
|
658,000
|
|
7
|
|
751
|
|
—
|
|
—
|
|
758
|
|
|||||||||||
|
Issuance of common stock pursuant to a 2018 stock purchase agreement, net of issuance costs
|
606,364
|
|
6
|
|
477
|
|
—
|
|
—
|
|
483
|
|
|||||||||||
|
Shares issued for cash upon the exercise of stock options under an equity incentive plan
|
144,252
|
|
1
|
|
86
|
|
—
|
|
—
|
|
87
|
|
|||||||||||
|
Issuance of common stock for cash under employee stock purchase plan
|
56,253
|
|
1
|
|
109
|
|
—
|
|
—
|
|
110
|
|
|||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
3,970
|
|
—
|
|
—
|
|
3,970
|
|
|||||||||||
|
Reclassification of warrant liability from equity
|
—
|
|
—
|
|
(18
|
)
|
—
|
|
—
|
|
(18
|
)
|
|||||||||||
|
Change in unrealized gain on investments
|
—
|
|
—
|
|
—
|
|
3
|
|
—
|
|
3
|
|
|||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(41,873
|
)
|
(41,873
|
)
|
|||||||||||
|
Balance as of December 31, 2019
|
34,861,876
|
|
$
|
349
|
|
$
|
183,574
|
|
$
|
—
|
|
$
|
(168,169
|
)
|
$
|
15,754
|
|
||||||
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
Cash flows from operating activities:
|
|||||||
|
Net loss
|
$
|
(41,873
|
)
|
$
|
(32,703
|
)
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||
|
Share-based compensation expense
|
3,970
|
|
3,679
|
|
|||
|
Non-cash interest expense
|
340
|
|
376
|
|
|||
|
Depreciation and amortization
|
288
|
|
281
|
|
|||
|
Amortization of premiums and discounts on available-for-sale securities
|
(432
|
)
|
(416
|
)
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Accounts receivable
|
(84
|
)
|
1,432
|
|
|||
|
Prepaid expenses and other assets
|
126
|
|
51
|
|
|||
|
Accounts payable
|
525
|
|
(335
|
)
|
|||
|
Accrued and other liabilities
|
1,084
|
|
791
|
|
|||
|
Net cash used in operating activities
|
(36,056
|
)
|
(26,844
|
)
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Purchases of short-term investments
|
(32,690
|
)
|
(62,462
|
)
|
|||
|
Maturities of short-term investments
|
61,000
|
|
33,000
|
|
|||
|
Purchases of property and equipment
|
(84
|
)
|
(445
|
)
|
|||
|
Net cash provided by (used in) investing activities
|
28,226
|
|
(29,907
|
)
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Payments of principal on note payable
|
(2,333
|
)
|
—
|
|
|||
|
Proceeds from the sale of common stock
|
2,283
|
|
44,529
|
|
|||
|
Payment of issuance costs associated with the sale of common stock
|
(77
|
)
|
(3,037
|
)
|
|||
|
Proceeds from stock purchases under employee stock purchase plan
|
110
|
|
242
|
|
|||
|
Proceeds from the exercise of stock options
|
87
|
|
182
|
|
|||
|
Net cash provided by financing activities
|
70
|
|
41,916
|
|
|||
|
Net decrease in cash and cash equivalents
|
(7,760
|
)
|
(14,835
|
)
|
|||
|
Cash and cash equivalents at beginning of period
|
32,606
|
|
47,441
|
|
|||
|
Cash and cash equivalents at end of period
|
$
|
24,846
|
|
$
|
32,606
|
|
|
|
Supplemental disclosure of cash flow information
|
|||||||
|
Cash paid for interest
|
$
|
511
|
|
$
|
489
|
|
|
|
Supplemental disclosure of non-cash investing and financing activities
|
|||||||
|
Change in unrealized gain (loss) on investments
|
$
|
3
|
|
$
|
(3
|
)
|
|
|
Amortization of public offering costs
|
$
|
3
|
|
$
|
55
|
|
|
|
December 31, 2019
|
December 31, 2018
|
||||||||||||||
|
Level 1
|
Level 3
|
Level 1
|
Level 3
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Assets:
|
|||||||||||||||
|
Money market funds (included in cash and cash equivalents)
(1)
|
$
|
25,263
|
|
$
|
—
|
|
$
|
32,936
|
|
$
|
—
|
|
|||
|
U.S. treasury securities (included in short-term investments)
|
1,999
|
|
—
|
|
29,875
|
|
—
|
|
|||||||
|
Total assets
|
$
|
27,262
|
|
$
|
—
|
|
$
|
62,811
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Common Stock warrants (included in accrued and other liabilities) (2)
|
$
|
—
|
|
$
|
100
|
|
$
|
—
|
|
$
|
82
|
|
|||
|
(1)
|
Amounts presented for each period above differ from cash and cash equivalents reported in the
consolidated
balance sheets due to uninvested cash balances and outstanding disbursements and deposits.
|
|
(2)
|
The change in the balance was due to changes in fair value. This adjustment was reflected in interest expense and other related expenses.
|
|
December 31, 2018
|
Additions
|
Cash Payments
|
December 31, 2019
|
||||||||||||
|
Retention
|
$
|
—
|
|
$
|
1,013
|
|
$
|
(78
|
)
|
$
|
935
|
|
|||
|
Severance and severance-related expenses
|
—
|
|
1,021
|
|
(441
|
)
|
580
|
|
|||||||
|
Total restructuring liability
|
$
|
—
|
|
$
|
2,034
|
|
$
|
(519
|
)
|
$
|
1,515
|
|
|||
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Milestone payments
|
$
|
—
|
|
$
|
3,690
|
|
|
|
Research and development reimbursable costs
|
4,308
|
|
3,683
|
|
|||
|
Total collaboration revenue
|
$
|
4,308
|
|
$
|
7,373
|
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Lab equipment
|
$
|
2,507
|
|
$
|
2,489
|
|
|
|
Leasehold improvements
|
741
|
|
741
|
|
|||
|
Computer hardware and software
|
457
|
|
428
|
|
|||
|
Furniture and fixtures
|
166
|
|
159
|
|
|||
|
Property and equipment, gross
|
3,871
|
|
3,817
|
|
|||
|
Less: accumulated depreciation and amortization
|
(3,348
|
)
|
(3,090
|
)
|
|||
|
Property and equipment, net
|
$
|
523
|
|
$
|
727
|
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Accrued outsourced clinical trials and preclinical studies
|
$
|
2,259
|
|
$
|
1,129
|
|
|
|
Restructuring liability
|
1,515
|
|
—
|
|
|||
|
Accrued employee compensation and related taxes
|
508
|
|
1,704
|
|
|||
|
Accrued legal fees and expenses
|
284
|
|
376
|
|
|||
|
Accrued other professional service fees
|
254
|
|
246
|
|
|||
|
Value of liability-classified stock purchase warrants
|
100
|
|
82
|
|
|||
|
Deferred and accrued facility lease obligations
|
66
|
|
124
|
|
|||
|
Accrued equipment and lab materials
|
1
|
|
33
|
|
|||
|
Other accrued liabilities
|
121
|
|
174
|
|
|||
|
Total accrued liabilities
|
$
|
5,108
|
|
$
|
3,868
|
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Principal amount outstanding
|
$
|
7,667
|
|
$
|
10,000
|
|
|
|
Unamortized debt discount
|
(32
|
)
|
(69
|
)
|
|||
|
Accreted final payment fee
|
669
|
|
367
|
|
|||
|
Total note payable
|
8,304
|
|
10,298
|
|
|||
|
Less: current maturities
|
(3,976
|
)
|
(2,294
|
)
|
|||
|
Note payable, net of current portion
|
$
|
4,328
|
|
$
|
8,004
|
|
|
|
2020
|
$
|
4,000
|
|
|
2021
|
3,667
|
|
|
|
Total
|
$
|
7,667
|
|
|
Number of Underlying Shares
|
Exercise Price
|
Expiration Date
|
||
|
10,707
|
$52.50
|
2020
|
||
|
11,718
|
$8.53
|
2025
|
||
|
24,097
|
$7.15
|
2024
|
||
|
46,522
|
||||
|
Common Stock Warrants
|
||||||
|
Number
|
Weighted Average Exercise Price
|
|||||
|
Outstanding at December 31, 2018
|
49,349
|
|
$
|
27.65
|
|
|
|
Expired
|
(2,827
|
)
|
$
|
187.50
|
|
|
|
Outstanding at December 31, 2019
|
46,522
|
|
$
|
17.93
|
|
|
|
Number of Options
(in thousands) |
Weighted Average Exercise Price
|
Weighted Average Remaining Contractual Term
(years) |
Aggregate Intrinsic Value
(in thousands) |
|||||||||
|
Outstanding at December 31, 2017
|
2,863
|
|
$
|
4.85
|
|
|||||||
|
Granted
|
1,108
|
|
$
|
7.37
|
|
|||||||
|
Exercised
|
(280
|
)
|
$
|
0.65
|
|
|||||||
|
Forfeited or expired
|
(164
|
)
|
$
|
9.52
|
|
|||||||
|
Outstanding at December 31, 2018
|
3,527
|
|
$
|
5.76
|
|
|||||||
|
Granted
|
1,078
|
|
$
|
2.89
|
|
|||||||
|
Exercised
|
(143
|
)
|
$
|
0.60
|
|
|||||||
|
Forfeited or expired
|
(965
|
)
|
$
|
5.39
|
|
|||||||
|
Outstanding at December 31, 2019
|
3,497
|
|
$
|
5.19
|
|
5.91
|
$
|
—
|
|
|||
|
Vested or expected to vest at December 31, 2019
|
3,497
|
|
$
|
5.19
|
|
5.91
|
$
|
—
|
|
|||
|
Exercisable as of December 31, 2019
|
2,388
|
|
$
|
5.06
|
|
4.68
|
$
|
—
|
|
|||
|
Vested as of December 31, 2019
|
2,388
|
|
$
|
5.06
|
|
4.68
|
$
|
—
|
|
|||
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
Expected term, in years
|
6.35
|
|
6.35
|
|
|||
|
Expected volatility
|
98.8
|
%
|
85.2
|
%
|
|||
|
Risk-free interest rate
|
2.5
|
%
|
2.6
|
%
|
|||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
|||
|
Weighted-average exercise price
|
$
|
2.97
|
|
$
|
7.37
|
|
|
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Research and development
|
$
|
1,677
|
|
$
|
1,286
|
|
|
|
General and administrative
|
2,293
|
|
2,393
|
|
|||
|
Total share-based compensation expense
|
$
|
3,970
|
|
$
|
3,679
|
|
|
|
December 31,
|
|||||
|
2019
|
2018
|
||||
|
(in thousands)
|
|||||
|
Options to purchase Common Stock
|
3,497
|
|
3,527
|
|
|
|
Warrants to purchase Common Stock
|
47
|
|
49
|
|
|
|
Total
|
3,544
|
|
3,576
|
|
|
|
Year Ended December 31,
|
|||||
|
2019
|
2018
|
||||
|
Federal statutory income tax rate
|
21.0
|
%
|
21.0
|
%
|
|
|
Federal and state tax credits
|
10.1
|
|
10.3
|
|
|
|
State income taxes, net of federal benefit
|
3.7
|
|
3.0
|
|
|
|
Change in valuation allowance
|
(31.3
|
)
|
(32.5
|
)
|
|
|
Other permanent items
|
(3.9
|
)
|
(1.0
|
)
|
|
|
Change in tax rate
|
—
|
|
(0.7
|
)
|
|
|
Other, net
|
0.4
|
|
(0.1
|
)
|
|
|
Effective income tax rate
|
—
|
%
|
—
|
%
|
|
|
Year Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Net operating loss carryforwards
|
$
|
32,792
|
|
$
|
24,137
|
|
|
|
Tax credits
|
10,812
|
|
6,571
|
|
|||
|
Accruals and reserves
|
829
|
|
854
|
|
|||
|
Stock-based expense
|
1,202
|
|
876
|
|
|||
|
Start-up costs
|
605
|
|
698
|
|
|||
|
Gross deferred tax assets
|
46,240
|
|
33,136
|
|
|||
|
Valuation allowance
|
(46,240
|
)
|
(33,136
|
)
|
|||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
|
|
For the Quarters Ended
|
|||||||||||||||||
|
March
|
June
|
September
|
December
|
||||||||||||||
|
(unaudited, in thousands)
|
|||||||||||||||||
|
Revenue
|
$
|
372
|
|
$
|
2,514
|
|
$
|
695
|
|
$
|
880
|
|
|||||
|
Research and development expenses
|
(8,751
|
)
|
(8,599
|
)
|
(9,027
|
)
|
(1)
|
(8,417
|
)
|
(2
|
)
|
||||||
|
General and administrative expenses
|
(3,357
|
)
|
(2,857
|
)
|
(2,898
|
)
|
(1)
|
(2,534
|
)
|
(2
|
)
|
||||||
|
Other income (expense), net
|
107
|
|
46
|
|
—
|
|
(47
|
)
|
|||||||||
|
Net loss
|
$
|
(11,629
|
)
|
$
|
(8,896
|
)
|
$
|
(11,230
|
)
|
$
|
(10,118
|
)
|
|||||
|
Comprehensive loss
|
$
|
(11,624
|
)
|
$
|
(8,887
|
)
|
$
|
(11,238
|
)
|
$
|
(10,121
|
)
|
|||||
|
Net loss per share, basic and diluted
|
$
|
(0.38
|
)
|
$
|
(0.29
|
)
|
$
|
(0.36
|
)
|
$
|
(0.31
|
)
|
|||||
|
(1)
|
Restructuring charges of
$1.1 million
were recorded during the quarter, of which
$0.9 million
was recorded in research and development expenses and
$0.2 million
recorded in general and administrative expenses.
|
|
(2)
|
Restructuring charges of
$0.9 million
were recorded during the quarter, of which
$0.8 million
was recorded in research and development expenses and
$0.1 million
recorded in general and administrative expenses.
|
|
For the Quarters Ended
|
||||||||||||||||
|
March
|
June
|
September
|
December
|
|||||||||||||
|
(unaudited, in thousands)
|
||||||||||||||||
|
Revenue
|
$
|
4,784
|
|
$
|
2,182
|
|
$
|
944
|
|
$
|
476
|
|
||||
|
Research and development expenses
|
(6,413
|
)
|
(8,375
|
)
|
(7,399
|
)
|
(8,234
|
)
|
||||||||
|
General and administrative expenses
|
(2,990
|
)
|
(2,668
|
)
|
(2,696
|
)
|
(2,695
|
)
|
||||||||
|
Other income (expense), net
|
(42
|
)
|
147
|
|
140
|
|
136
|
|
||||||||
|
Net loss
|
$
|
(4,661
|
)
|
$
|
(8,714
|
)
|
$
|
(9,011
|
)
|
$
|
(10,317
|
)
|
||||
|
Comprehensive loss
|
$
|
(4,661
|
)
|
$
|
(8,710
|
)
|
$
|
(9,021
|
)
|
$
|
(10,314
|
)
|
||||
|
Net loss per share, basic and diluted
|
$
|
(0.18
|
)
|
$
|
(0.29
|
)
|
$
|
(0.29
|
)
|
$
|
(0.33
|
)
|
||||
|
|
|
MIRAGEN THERAPEUTICS, INC.
|
|
|
|
|
|
|
|
Date: March 13, 2020
|
By:
|
/s/ William S. Marshall
|
|
|
William S. Marshall, Ph.D.
|
|||
|
Chief Executive Officer
|
|||
|
(Principal Executive Officer)
|
|||
|
Date: March 13, 2020
|
|
By:
|
/s/ Jason A. Leverone
|
|
|
|
|
Jason A. Leverone
|
|
|
|
|
Chief Financial Officer
|
|
(Principal Financial Officer; Principal Accounting Officer)
|
|||
|
Signature
|
Title
|
Date
|
||
|
|
|
|
||
|
/s/ William S. Marshall
|
President, Chief Executive Officer, and Director
|
March 13, 2020
|
||
|
William S. Marshall, Ph.D.
|
(Principal Executive Officer)
|
|
||
|
|
|
|
||
|
/s/ Jason A. Leverone
|
Chief Financial Officer, Treasurer, and Secretary
|
March 13, 2020
|
||
|
Jason A. Leverone
|
(Principal Financial Officer; Principal Accounting Officer)
|
|
||
|
/s/ Jeffrey S. Hatfield
|
Chairman of the Board
|
March 13, 2020
|
||
|
Jeffrey S. Hatfield
|
|
|
||
|
/s/ Christopher Bowden
|
Director
|
March 13, 2020
|
||
|
Christopher Bowden, M.D.
|
|
|
||
|
|
|
|
||
|
/s/ Thomas E. Hughes
|
Director
|
March 13, 2020
|
||
|
Thomas E. Hughes, Ph.D.
|
|
|
||
|
/s/ Kevin Koch
|
Director
|
March 13, 2020
|
||
|
Kevin Koch, Ph.D.
|
|
|
||
|
/s/ Arlene M. Morris
|
Director
|
March 13, 2020
|
||
|
Arlene M. Morris
|
|
|
||
|
/s/ Joseph L. Turner
|
Director
|
March 13, 2020
|
||
|
Joseph L. Turner
|
|
|
||