|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the Fiscal Year Ended December 31, 2012
|
|
|
or
|
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from
to
|
|
|
Massachusetts
|
04-3039129
|
|
(State or other jurisdiction of
incorporation or organization) |
(I.R.S. Employer
Identification No.) |
|
130 Waverly Street, Cambridge, Massachusetts
|
02139-4242
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|||
|
Common Stock, $0.01 Par Value Per Share
|
The NASDAQ Global Select Market
|
|||
|
Large accelerated filer
x
|
Accelerated filer
o
|
Non-accelerated filer
o
|
Smaller reporting company
o
|
|
(Do not check if a smaller reporting company)
|
|||
|
Page
|
||
|
ITEM 1.
|
BUSINESS
|
|
Product
|
Indication
|
Mechanism
|
Markets
|
Marketing Rights
|
|
INCIVEK (telaprevir)
|
HCV Infection (genotype 1)
|
HCV Protease Inhibitor
|
United States and Canada
|
Vertex
|
|
KALYDECO (ivacaftor)
|
CF (G551D mutation)
|
CFTR Potentiator
|
United States, Canada and Europe
|
Vertex
|
|
INCIVO (telaprevir)
|
HCV Infection (genotype 1)
|
HCV Protease Inhibitor
|
Europe and other countries in Janssen's territories
|
Janssen
|
|
TELAVIC (telaprevir)
|
HCV Infection (genotype 1)
|
HCV Protease Inhibitor
|
Japan
|
Mitsubishi Tanabe
|
|
Drug Candidate
|
Mechanism
|
Development
Stage |
|
Cystic Fibrosis
|
||
|
ivacaftor (monotherapy - label expansion trials)
|
CFTR potentiator
|
Phase 3
|
|
VX-809 (in combination with ivacaftor)
|
CFTR corrector
|
Phase 3
|
|
VX-661 (in combination with ivacaftor)
|
CFTR corrector
|
Phase 2
|
|
HCV Infection
|
||
|
VX-135 (ALS-2200)
|
HCV nucleotide analogue
|
Phase 2
|
|
VX-222
|
Non-nucleoside HCV polymerase inhibitor
|
Phase 2
|
|
Autoimmune Diseases
|
||
|
VX-509
|
JAK3 inhibitor
|
Phase 2
|
|
Influenza
|
||
|
VX-787
|
Influenza virus inhibitor
|
Phase 2
|
|
Mutation in
CFTR
Gene
|
Approximate Percentage of Patients with CF in the U.S.
|
|
G551D mutation on at least one allele
|
4%
|
|
non-G551D gating mutation on at least one allele
|
1%
|
|
R117H mutation on at least one allele
|
3%
|
|
F508del mutation on both alleles (homozygous)
|
47%
|
|
F508del mutation on one allele but not both alleles (heterozygous)
|
40%
|
|
•
|
We have completed enrollment in a Phase 3 clinical trial evaluating ivacaftor in patients six years of age and older with CF with gating mutations other than the G551D mutation.
|
|
•
|
We are continuing enrollment in a Phase 3 clinical trial evaluating ivacaftor in patients six years of age and older with CF with the R117H mutation in the
CFTR
gene on at least one allele.
|
|
•
|
We have begun dosing patients in a Phase 3 clinical trial in which we are evaluating a pediatric formulation of ivacaftor as a treatment for children two to five years of age with gating mutations in the
CFTR
gene, including the G551D mutation.
|
|
•
|
We are enrolling patients in a Phase 2 clinical trial in which we are evaluating ivacaftor in patients with CF who have clinical evidence of residual CFTR function.
|
|
•
|
Cohort 2 -
We evaluated the 600mg once-daily (QD) dose of VX-809 in combination with ivacaftor (250mg q12h) in Cohort 2 in 21 patients with CF who are homozygous for the F508del mutation. This regimen resulted in statistically significant improvements in lung function (within group and versus placebo) during the combination dosing period, as set forth in the following table:
|
|
Mean Absolute and Relative Changes in Percent Predicted FEV
1
|
||||
|
Day 0 - 28; VX-809 Alone
|
Day 28 - 56; VX-809 + ivacaftor
|
Day 0 - 56
|
||
|
VX-809 (600mg QD) + ivacaftor (250mg q12h)
|
Within Group
|
|||
|
Absolute
|
-2.9 (p=0.07)
|
+6.1 (p<0.001)
|
+3.4 (p=0.03)
|
|
|
Relative
|
-3.5 (p=0.13)
|
+9.7 (p<0.001)
|
+5.3 (p=0.02)
|
|
|
Versus Placebo
|
||||
|
Absolute
|
-2.0 (p=0.36)
|
+8.6 (p <0.001)
|
+6.7 (p=0.002)
|
|
|
Relative
|
-3.9 (p=0.21)
|
+12.8 (p<0.001)
|
+9.2 (p=0.004)
|
|
|
•
|
Cohort 3 -
Cohort 3 was designed to evaluate the safety and pharmacokinetics of the 400mg (q12h) dose of VX-809 to support inclusion of this dose in the Phase 3 development program. We evaluated the 400mg (q12h) dose of VX-809 in combination with ivacaftor in Cohort 3 in 11 patients with CF who are homozygous for the F508del mutation. Cohort 3 also included the randomization of four patients to placebo to allow for a blinded safety assessment. Three patients completed treatment in the placebo group. A pharmacokinetic model suggested that 400mg dosing every 12 hours (q12h) of VX-809 would provide a higher total exposure area under the curve, or AUC, compared to 600mg once-daily (QD) dosing, and data from Cohort 3 were consistent with this model.
|
|
Mean Absolute and Relative Changes in Percent Predicted FEV
1
|
||||
|
Day 0 - 28; VX-809 Alone
|
Day 28 - 56; VX-809 + ivacaftor
|
Day 0 - 56
|
||
|
VX-809 (400mg q12h) + ivacaftor (250mg q12h)
|
Within Group
|
|||
|
Absolute
|
-4.3 (p=0.04)
|
+6.6 (p=0.01)
|
+1.9 (p=0.57)
|
|
|
Relative
|
-6.3 (p=0.08)
|
+8.8 (p=0.01)
|
+2.5 (p=0.67)
|
|
|
Drug Candidate
|
Mechanism(s)
|
|
VX-135 (ALS-2200)
|
|
|
VX-135 in combination with RBV
|
HCV Nucleotide Analogue/RBV
|
|
VX-135 in combination with TMC435
|
HCV Nucleotide Analogue/HCV Protease Inhibitor
|
|
VX-135 in combination with GSK2336805
|
HCV Nucleotide Analogue/HCV NS5A Inhibitor
|
|
VX-135 in combination with VX-222
|
HCV Nucleotide Analogue/HCV Polymerase Inhibitor
|
|
VX-222
|
|
|
VX-222 in combination with telaprevir and RBV
|
HCV Polymerase Inhibitor/HCV Protease Inhibitor/RBV
|
|
•
|
We are conducting Phase 2 clinical trials to evaluate VX-135 in combination with RBV.
|
|
•
|
In October 2012, we entered into a non-exclusive collaboration with Janssen to conduct a clinical trial to evaluate all-oral combinations of Janssen's investigational once-daily HCV protease inhibitor TMC435 (simeprevir), and VX-135. Janssen recently announced positive results from a Phase 3 clinical trial that evaluated TMC435 in combination with peg-IFN and RBV. We expect that Janssen will conduct a drug-drug interaction trial with VX-135 and TMC435 to support the planned initiation of a Phase 2 clinical trial in mid-2013, pending discussions with regulatory authorities. We and Janssen will share equally development costs associated with this collaboration. No further clinical development activities are covered by this agreement beyond the planned Phase 2 clinical trials.
|
|
•
|
In October 2012, we entered into a non-exclusive collaboration with GlaxoSmithKline plc to evaluate all-oral combinations of GlaxoSmithKline's investigational once-daily NS5A inhibitor GSK2336805 and VX-135. We expect to initiate the Phase 2 clinical trial to evaluate VX-135 and GSK2336805 in the first half of 2013, pending discussions with regulatory authorities. We and GSK will share equally development costs associated with this collaboration. No further clinical development activities are covered by this agreement beyond the planned Phase 2 clinical trial.
|
|
•
|
We are planning to conduct a Phase 2 clinical trial to evaluate VX-135 in combination with our HCV polymerase inhibitor VX-222.
|
|
•
|
We are conducting a Phase 2 clinical trial that enrolled approximately 60 patients with genotype 1a HCV infection to evaluate a treatment regimen of telaprevir, VX-222 and RBV.
|
|
Drug/Drug Candidate
|
Status of United States Patent
(Anticipated Expiration, Subject to Potential Extensions) |
Status of European Union Patent
(Anticipated Expiration, Subject to Potential Extensions) |
|
INCIVEK/INCIVO (telaprevir)
|
Granted (2025)
|
Granted (2026)
|
|
KALYDECO (ivacaftor)
|
Granted (2027)
|
Application Pending (2025)
|
|
VX-135
|
Application Pending (2031)
|
Application Pending (2031)
|
|
VX-222
|
Granted (2030)
|
Application Pending (2027)
|
|
VX-809
|
Application Pending (2026)
|
Application Pending (2026)
|
|
VX-661
|
Granted (2027)
|
Application Pending (2027)
|
|
VX-509
|
Granted (2026)
|
Application Pending (2025)
|
|
VX-787
|
Application Pending (2030)
|
Application Pending (2030)
|
|
•
|
U.S. and foreign patents and patent applications covering telaprevir, VX-222 and other HCV protease and polymerase inhibitors and the use of these compounds to treat HCV infection.
|
|
•
|
U.S. and foreign patent applications licensed from Alios covering VX-135 and other HCV nucleotide inhibitors and the use of these compounds to treat HCV infection.
|
|
•
|
U.S. and foreign patent applications covering potentiator compounds and corrector compounds for the CFTR protein, including ivacaftor, VX-809 and VX-661 and many other related compounds, and the use of those potentiators and correctors to treat CF.
|
|
•
|
U.S. and foreign patents and patent applications covering inhibitors of a variety of kinase proteins, including VX-509, and the use of those inhibitors to treat autoimmune disease, including rheumatoid arthritis.
|
|
•
|
U.S. and foreign patents and patent applications covering influenza virus inhibitors, including VX-787.
|
|
•
|
U.S. and foreign patent applications covering the manufacture, pharmaceutical compositions, related solid forms, formulations, dosing regimens and methods of use of these compounds, including our two marketed products telaprevir and ivacaftor.
|
|
Drug Candidate
|
Mechanism
|
Development Phase
|
|
Gilead
|
||
|
sofosbuvir (GS-7977)
|
HCV Nucleotide Analogue
|
Phase 3
|
|
GS-9451
|
HCV Protease Inhibitor
|
Phase 2
|
|
tegobuvir (GS-9190)
|
Non-nucleoside HCV Polymerase Inhibitor
|
Phase 2
|
|
GS-5885
|
HCV NS5A Inhibitor
|
Phase 2
|
|
Janssen/Medivir AB
|
||
|
simeprevir (TMC435)
|
HCV Protease Inhibitor
|
Phase 3
|
|
TMC647055
|
Non-nucleoside HCV Polymerase Inhibitor
|
Phase 2
|
|
Abbvie
|
||
|
ABT-450
|
HCV Protease Inhibitor
|
Phase 3
|
|
ABT-333
|
Non-nucleoside HCV Polymerase Inhibitor
|
Phase 3
|
|
ABT-267
|
HCV NS5A Inhibitor
|
Phase 3
|
|
Vertex
|
||
|
VX-135
|
HCV Nucleotide Analogue
|
Phase 2
|
|
VX-222
|
Non-nucleoside HCV Polymerase Inhibitor
|
Phase 2
|
|
Boehringer Ingelheim
|
||
|
faldaprevir (BI 201335)
|
HCV Protease Inhibitor
|
Phase 3
|
|
BI 207127
|
Non-nucleoside HCV Polymerase Inhibitor
|
Phase 3
|
|
Merck
|
||
|
vaniprevir (MK-7009)
|
HCV Protease Inhibitor
|
Phase 2
|
|
Bristol-Myers Squibb
|
||
|
daclatasvir
|
HCV NS5A Inhibitor
|
Phase 3
|
|
BMS-650032
|
HCV Protease Inhibitor
|
Phase 2
|
|
Achillion
|
||
|
Sovaprevir
|
HCV Protease Inhibitor
|
Phase 2
|
|
ACH-3102
|
HCV NS5A Inhibitor
|
Phase 2
|
|
Roche
|
||
|
danoprevir / RG7227
|
HCV Protease Inhibitor
|
Phase 2
|
|
setrobuvir
|
Non-nucleoside HCV Polymerase Inhibitor
|
Phase 2
|
|
GlaxoSmithKline
|
||
|
GSK2336805
|
HCV NS5A Inhibitor
|
Phase 2
|
|
Idenix
|
||
|
IDX719
|
HCV NS5A Inhibitor
|
Phase 2
|
|
•
|
refusal to approve pending applications;
|
|
•
|
withdrawal of an approval;
|
|
•
|
imposition of a clinical hold;
|
|
•
|
warning letters;
|
|
•
|
product seizures;
|
|
•
|
total or partial suspension of production or distribution; or
|
|
•
|
injunctions, fines, disgorgement, or civil or criminal penalties.
|
|
•
|
completion of preclinical laboratory tests, animal studies and formulation studies conducted according to Good Laboratory Practices, or GLP, and other applicable regulations;
|
|
•
|
submission to the FDA of an investigational new drug, or IND, application, which must become effective before clinical trials in the United States may begin;
|
|
•
|
performance of adequate and well-controlled clinical trials according to Good Clinical Practices, or GCP, to establish the safety and efficacy of the proposed drug for its intended use;
|
|
•
|
submission to the FDA of an NDA;
|
|
•
|
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product will be produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; and
|
|
•
|
FDA review and approval of the NDA.
|
|
•
|
Phase 1.
The drug initially is introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and elimination. In the case of some drug candidates for severe or life-threatening diseases, such as cancer, especially when the drug candidate may be inherently too toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
|
|
•
|
Phase 2.
Clinical trials are initiated in a limited patient population intended to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the drug candidate for specific targeted diseases and to determine dosage tolerance and optimal dosage.
|
|
•
|
Phase 3.
Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk-benefit ratio of the drug candidate and provide an adequate basis for regulatory approval and product labeling.
|
|
Phase
|
Estimated Duration
|
|
|
Discovery
|
2 to 4 years
|
|
|
Preclinical
|
1 to 2 years
|
|
|
Phase 1
|
1 to 2 years
|
|
|
Phase 2
|
2 to 4 years
|
|
|
Phase 3
|
2 to 4 years
|
|
|
FDA approval
|
6 months to 2 years
|
|
|
•
|
record-keeping requirements;
|
|
•
|
reporting of adverse experiences with the product;
|
|
•
|
providing the FDA with updated safety and efficacy information;
|
|
•
|
drug sampling and distribution requirements;
|
|
•
|
notifying the FDA and gaining its approval of specified manufacturing or labeling changes;
|
|
•
|
complying with certain electronic records and signature requirements; and
|
|
•
|
complying with FDA promotion and advertising requirements.
|
|
Name
|
Age
|
Position
|
|
|
Jeffrey M. Leiden, M.D., Ph.D.
|
57
|
Chairman of the Board, President and Chief Executive Officer
|
|
|
Stuart A. Arbuckle
|
47
|
Executive Vice President and Chief Commercial Officer
|
|
|
Kenneth L. Horton
|
46
|
Executive Vice President and Chief Legal Officer
|
|
|
Peter Mueller, Ph.D.
|
56
|
Executive Vice President, Global Research and Development, and Chief Scientific Officer
|
|
|
Ian F. Smith
|
47
|
Executive Vice President and Chief Financial Officer
|
|
|
Megan Pace
|
40
|
Senior Vice President, Corporate Communications
|
|
|
Amit K. Sachdev, J.D.
|
45
|
Senior Vice President, Global Government Strategy, Market Access and Value
|
|
|
Christiana Stamoulis, M.B.A.
|
42
|
Senior Vice President, Corporate Strategy and Business Development
|
|
|
Paul M. Silva
|
47
|
Senior Vice President and Corporate Controller
|
|
|
David Altshuler, M.D., Ph.D.
|
48
|
Director
|
|
|
Joshua S. Boger, Ph.D.
|
61
|
Director
|
|
|
Matthew W. Emmens
|
61
|
Director
|
|
|
Terrence C. Kearney
|
58
|
Director
|
|
|
Yuchun Lee
|
47
|
Director
|
|
|
Margaret G. McGlynn
|
53
|
Director
|
|
|
Wayne J. Riley, M.D., M.B.A.
|
53
|
Director
|
|
|
Bruce I. Sachs
|
53
|
Director
|
|
|
Elaine S. Ullian
|
65
|
Director
|
|
|
•
|
the efficacy, safety, tolerability and other characteristics of any combination therapy including INCIVEK, VX-135 and/or VX-222 relative to existing and future treatments for HCV infection;
|
|
•
|
our ability to establish VX-135 and/or VX-222, if approved, or INCIVEK as a significant component of any commercially competitive all-oral therapy for the treatment of HCV infection; and
|
|
•
|
the clinical data obtained and timing of marketing approvals for drug candidates being developed by our competitors, including any all-oral therapy or shorter duration therapy for the treatment of HCV infection.
|
|
•
|
lower demonstrated efficacy, safety and/or tolerability compared to other drugs;
|
|
•
|
prevalence and severity of adverse side-effects;
|
|
•
|
lack of cost-effectiveness;
|
|
•
|
lack of reimbursement availability from third-party payors;
|
|
•
|
a decision to wait for the approval of other therapies in development that have significant perceived advantages over our applicable drug;
|
|
•
|
convenience and ease of administration;
|
|
•
|
other potential advantages of alternative treatment methods; and
|
|
•
|
ineffective marketing and/or distribution support.
|
|
•
|
offer therapeutic or other improvement over existing competitive drugs;
|
|
•
|
be proven safe and effective in clinical trials;
|
|
•
|
meet applicable regulatory standards;
|
|
•
|
be capable of being produced in commercial quantities at acceptable costs; or
|
|
•
|
if approved for commercial sale, be successfully marketed as pharmaceutical products.
|
|
•
|
ongoing discussions with the FDA or comparable foreign authorities regarding the scope or design of our clinical trials and the number of clinical trials we must conduct;
|
|
•
|
delays in enrolling volunteers or patients into clinical trials, including as a result of low numbers of patients that meet the eligibility criteria for the trial;
|
|
•
|
a lower than anticipated retention rate of volunteers or patients in clinical trials;
|
|
•
|
the need to repeat clinical trials as a result of inconclusive results, unforeseen complications in testing or clinical investigator error;
|
|
•
|
inadequate supply or deficient quality of drug candidate materials or other materials necessary for the conduct of our clinical trials;
|
|
•
|
unfavorable FDA or foreign regulatory authority inspection and review of a manufacturing facility that supplied clinical trial materials or its relevant manufacturing records or a clinical trial site or records of any clinical or preclinical investigation;
|
|
•
|
unfavorable scientific results from clinical trials;
|
|
•
|
serious and unexpected drug-related side-effects experienced by participants in our clinical trials or by participants in clinical trials being conducted by our competitors to evaluate drug candidates with similar mechanisms of action or structures to drug candidates that we are developing;
|
|
•
|
favorable results in testing of our competitors’ drug candidates, or FDA or foreign regulatory authority approval of our competitors’ drug candidates; or
|
|
•
|
action by the FDA or a foreign regulatory authority to place a clinical hold on a trial.
|
|
•
|
data from clinical trials involving compounds evaluated separately may not predict possible outcomes, such as unforeseen drug interactions from compounds dosed in combination, which could negatively affect the efficacy and safety profile of the combination therapy;
|
|
•
|
positive results in small clinical trials and nonclinical studies may not be predictive of results in clinical trials involving large numbers of patients; and
|
|
•
|
favorable results of testing or earlier FDA or foreign regulatory approval of competitors’ products with a better product profile.
|
|
•
|
Our collaborators may change the focus of their development and commercialization efforts or may have insufficient resources to effectively develop our drug candidates. The ability of some of our products and drug candidates to reach their potential could be limited if collaborators decrease or fail to increase development or commercialization efforts related to those products or drug candidates.
|
|
•
|
Any future collaboration agreements may have the effect of limiting the areas of research and development that we may pursue, either alone or in collaboration with third parties.
|
|
•
|
Collaborators may develop and commercialize, either alone or with others, drugs that are similar to or competitive with the drugs or drug candidates that are the subject of their collaborations with us. For example, Janssen is dedicating significant development resources and planning to seek approval to market TMC435, a potentially competitive HCV protease inhibitor, which could increase the likelihood that Janssen would terminate our collaboration or apply fewer resources to marketing INCIVO.
|
|
•
|
Our collaboration agreements are subject to termination under various circumstances, including, as in the case of our agreement with Janssen, termination without cause. Any such termination by Janssen could have a material adverse effect on our financial condition and/or disrupt the commercial sale of INCIVO in Janssen’s territories.
|
|
•
|
implement and clearly communicate our corporate-wide strategies;
|
|
•
|
enhance our operational and financial infrastructure, including our controls over records and information;
|
|
•
|
enhance our operational, financial and management processes, including our cross-functional decision-making processes and our portfolio management systems;
|
|
•
|
train and manage our global employee base;
|
|
•
|
transition from a U.S.-centric company into an organization capable of developing and commercializing multiple drug candidates in international markets; and
|
|
•
|
enhance our compliance and legal resources.
|
|
•
|
differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries;
|
|
•
|
collectibility of accounts receivable;
|
|
•
|
unexpected changes in tariffs, trade barriers and regulatory requirements;
|
|
•
|
economic weakness, including inflation, or political instability in particular foreign economies and markets;
|
|
•
|
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
|
|
•
|
foreign taxes, including withholding of payroll taxes;
|
|
•
|
foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country;
|
|
•
|
workforce uncertainty in countries where labor unrest is more common than in the United States;
|
|
•
|
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
|
|
•
|
business interruptions resulting from geo-political actions, including war and terrorism.
|
|
•
|
the information contained in our quarterly earnings releases, including our net product revenues, royalty revenues and operating expenses for completed periods and guidance regarding future periods;
|
|
•
|
prescription data and other information disclosed by third-parties regarding our business or products;
|
|
•
|
announcements of FDA actions with respect to our drugs or our competitors’ drugs, or regulatory filings for our drug candidates or those of our competitors or of results of clinical trials or nonclinical studies relating to our drugs, drug candidates or those of our competitors;
|
|
•
|
technological innovations or the introduction of new drugs by our competitors;
|
|
•
|
government regulatory action;
|
|
•
|
public concern as to the safety of drugs developed by us or our competitors;
|
|
•
|
developments in patent or other intellectual property rights or announcements relating to these matters;
|
|
•
|
developments in domestic and international governmental policy or regulation, for example relating to intellectual property rights;
|
|
•
|
developments relating specifically to other companies and market conditions for pharmaceutical and biotechnology stocks or stocks in general;
|
|
•
|
business development, capital structuring or financing activities; and
|
|
•
|
general worldwide or national economic, political and capital market conditions.
|
|
•
|
making it more difficult to obtain additional financing for working capital, capital expenditures, debt service requirements or other purposes;
|
|
•
|
shortening the duration of available revolving credit because lenders may seek to avoid conflicting maturity dates;
|
|
•
|
constraining our ability to react quickly in an unfavorable economic climate or to changes in our business or the pharmaceutical industry; or
|
|
•
|
potentially requiring the dedication of substantial amounts to service the repayment of outstanding debt, including periodic interest payments, thereby reducing the amount of cash available for other purposes.
|
|
•
|
expectations regarding the amount of, timing of and trends with respect to our revenues, costs and expenses and other gains and losses, including those related to net product revenues from sales of INCIVEK and KALYDECO and royalty revenues from net sales of INCIVO and to the intangible assets associated with the ViroChem acquisition and the Alios collaboration;
|
|
•
|
our expectations regarding clinical trials, development timelines and regulatory authority filings and submissions for telaprevir, ivacaftor, VX-135, VX-222, VX-509, VX-661, VX-787 and VX-809;
|
|
•
|
our expectations regarding the timing of data from our clinical trials of ivacaftor monotherapy and VX-809 in combination with ivacaftor, the possibility of using that data to support regulatory submissions and the timing of those potential submissions;
|
|
•
|
our ability to successfully market INCIVEK and/or KALYDECO or any of our other drug candidates if we obtain regulatory approval;
|
|
•
|
our expectations regarding the timing and structure of clinical trials of our drugs and drug candidates, including telaprevir, ivacaftor, VX-135, VX-222, VX-509, VX-661, VX-787 and VX-809, and the expected timing of our receipt of data from our ongoing and planned clinical trials;
|
|
•
|
the data that will be generated by ongoing and planned clinical trials and the ability to use that data to support regulatory filings;
|
|
•
|
our beliefs regarding the support provided by clinical trials and preclinical and nonclinical studies of our drug candidates for further investigation, clinical trials or potential use as a treatment;
|
|
•
|
the focus of our drug development efforts and our financial and management resources and our plan to continue investing in our research and development programs and our strategy to develop our drug candidates, alone or with third party-collaborators;
|
|
•
|
the establishment, development and maintenance of collaborative relationships;
|
|
•
|
potential business development activities;
|
|
•
|
our ability to use our research programs to identify and develop new drug candidates to address serious diseases and significant unmet medical needs;
|
|
•
|
our estimates regarding obligations associated with a lease of a facility in Kendall Square, Cambridge, Massachusetts; and
|
|
•
|
our liquidity and our expectations regarding the possibility of raising additional capital.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Year Ended December 31, 2012:
|
High
|
Low
|
|||||
|
First quarter
|
$
|
43.13
|
|
$
|
32.04
|
|
|
|
Second quarter
|
66.10
|
|
35.26
|
|
|||
|
Third quarter
|
59.98
|
|
46.03
|
|
|||
|
Fourth quarter
|
60.00
|
|
38.44
|
|
|||
|
Year Ended December 31, 2011:
|
|||||||
|
First quarter
|
$
|
52.13
|
|
$
|
35.19
|
|
|
|
Second quarter
|
58.87
|
|
44.57
|
|
|||
|
Third quarter
|
54.38
|
|
39.06
|
|
|||
|
Fourth quarter
|
45.26
|
|
26.50
|
|
|||
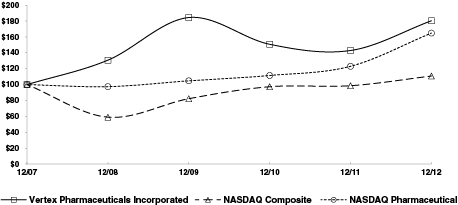
|
Period
|
Total Number
of Shares Purchased |
Average Price
Paid per Share |
Total Number of Shares
Purchased as Part of Publicly Announced Plans or Programs |
Maximum Number of
Shares that May Yet be Purchased Under the Plans or Programs |
||||
|
Oct. 1, 2012 to Oct. 31, 2012
|
14,425
|
|
$
|
0.01
|
|
—
|
—
|
|
|
Nov. 1, 2012 to Nov. 30, 2012
|
27,159
|
|
$
|
0.01
|
|
—
|
—
|
|
|
Dec. 1, 2012 to Dec. 31, 2012
|
23,573
|
|
$
|
0.01
|
|
—
|
—
|
|
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
2012
|
2011
|
2010
|
2009
|
2008
|
|||||||||||||||
|
(in thousands, except per share amounts)
|
|||||||||||||||||||
|
Consolidated Statements of Operations Data:
|
|||||||||||||||||||
|
Product revenues, net
|
$
|
1,333,458
|
|
$
|
950,889
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Royalty revenues
|
141,498
|
|
50,015
|
|
30,244
|
|
28,320
|
|
37,483
|
|
|||||||||
|
Collaborative revenues
|
52,086
|
|
409,722
|
|
113,126
|
|
73,569
|
|
138,021
|
|
|||||||||
|
Total revenues
|
1,527,042
|
|
1,410,626
|
|
143,370
|
|
101,889
|
|
175,504
|
|
|||||||||
|
Total costs and expenses (1)
|
1,524,710
|
|
1,296,806
|
|
839,447
|
|
715,901
|
|
638,212
|
|
|||||||||
|
Income (loss) from operations
|
2,332
|
|
113,820
|
|
(696,077
|
)
|
(614,012
|
)
|
(462,708
|
)
|
|||||||||
|
Net loss (income) attributable to noncontrolling interest (Alios) (2)
|
(55,897
|
)
|
(11,605
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income (loss) attributable to Vertex
|
$
|
(107,032
|
)
|
$
|
29,574
|
|
$
|
(754,626
|
)
|
$
|
(642,178
|
)
|
$
|
(459,851
|
)
|
||||
|
Net income (loss) per diluted share attributable to Vertex common shareholders
|
$
|
(0.50
|
)
|
$
|
0.14
|
|
$
|
(3.77
|
)
|
$
|
(3.71
|
)
|
$
|
(3.27
|
)
|
||||
|
Shares used in per diluted share calculations
|
211,946
|
|
208,807
|
|
200,402
|
|
173,259
|
|
140,556
|
|
|||||||||
|
As of December 31,
|
|||||||||||||||||||
|
2012
|
2011
|
2010
|
2009
|
2008
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
|||||||||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
1,321,215
|
|
$
|
968,922
|
|
$
|
1,031,411
|
|
$
|
1,284,913
|
|
$
|
832,101
|
|
||||
|
Total assets
|
2,759,288
|
|
2,204,280
|
|
1,725,446
|
|
1,955,488
|
|
980,479
|
|
|||||||||
|
Total current liabilities
|
432,624
|
|
392,348
|
|
474,783
|
|
284,883
|
|
216,564
|
|
|||||||||
|
Long-term debt obligations (3)
|
400,000
|
|
400,000
|
|
400,000
|
|
159,972
|
|
287,500
|
|
|||||||||
|
Construction financing lease obligation (4)
|
268,031
|
|
55,950
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Other long-term obligations
|
424,251
|
|
390,470
|
|
346,690
|
|
414,287
|
|
237,541
|
|
|||||||||
|
(1)
|
In 2012, total costs and expenses included an aggregate of
$133.2 million
in lower of cost or market charges for excess and obsolete INCIVEK inventories. See
Note F
to our financial statements.
|
|
(2)
|
Net loss (income) attributable to noncontrolling interest (Alios) relates to our collaboration with Alios. See
Note B
to our financial statements.
|
|
(3)
|
As of December 31, 2012, long-term debt obligations consisted of $400.0 million in aggregate principal amount of convertible senior subordinated notes (due 2015). See Note K to our financial statements.
|
|
(4)
|
In 2011, we entered into two leases for our future corporate headquarters. We are deemed for accounting purposes to be the owner of the buildings during the construction period. See
Note H
to our financial statements.
|
|
ITEM 7.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
We have completed enrollment in a Phase 3 clinical trial evaluating ivacaftor in patients six years of age and older with CF with gating mutations other than the G551D mutation.
|
|
•
|
We are continuing enrollment in a Phase 3 clinical trial evaluating ivacaftor in patients six years of age and older with CF with the R117H mutation in the
CFTR
gene on at least one allele.
|
|
•
|
We have begun dosing patients in a Phase 3 clinical trial in which we are evaluating a pediatric formulation of ivacaftor as a treatment for children two to five years of age with gating mutations in the
CFTR
gene, including the G551D mutation.
|
|
•
|
We are enrolling patients in a Phase 2 clinical trial in which we are evaluating ivacaftor in patients with CF who have clinical evidence of residual CFTR function.
|
|
2012/2011
Comparison |
2011/2010
Comparison |
||||||||||||||||||||||||
|
2012
|
2011
|
2010
|
Increase (Decrease)
|
Increase (Decrease)
|
Increase (Decrease)
|
Increase/ (Decrease)
|
|||||||||||||||||||
|
(in thousands)
|
(in thousands, except percentages)
|
||||||||||||||||||||||||
|
Revenues
|
$
|
1,527,042
|
|
$
|
1,410,626
|
|
$
|
143,370
|
|
$
|
116,416
|
|
8
|
%
|
$
|
1,267,256
|
|
884
|
%
|
||||||
|
Operating costs and expenses
|
1,524,710
|
|
1,296,806
|
|
839,447
|
|
227,904
|
|
18
|
%
|
457,359
|
|
54
|
%
|
|||||||||||
|
Other loss, net
|
(53,467
|
)
|
(72,641
|
)
|
(58,549
|
)
|
(19,174
|
)
|
(26
|
)%
|
14,092
|
|
24
|
%
|
|||||||||||
|
Net loss (income) attributable to noncontrolling interest (Alios)
|
(55,897
|
)
|
(11,605
|
)
|
—
|
|
44,292
|
|
382
|
%
|
11,605
|
|
n/a
|
|
|||||||||||
|
Net income (loss) attributable to Vertex
|
$
|
(107,032
|
)
|
$
|
29,574
|
|
$
|
(754,626
|
)
|
n/a
|
|
n/a
|
|
n/a
|
|
n/a
|
|
||||||||
|
2012/2011
Comparison |
2011/2010
Comparison |
||||||||||||||||||||||||
|
2012
|
2011
|
2010
|
Increase/(Decrease)
|
Increase/(Decrease)
|
Increase/(Decrease)
|
Increase/(Decrease)
|
|||||||||||||||||||
|
(in thousands)
|
(in thousands, except percentages)
|
||||||||||||||||||||||||
|
Product revenues, net
|
$
|
1,333,458
|
|
$
|
950,889
|
|
$
|
—
|
|
$
|
382,569
|
|
40
|
%
|
$
|
950,889
|
|
n/a
|
|
||||||
|
Royalty revenues
|
141,498
|
|
50,015
|
|
30,244
|
|
91,483
|
|
183
|
%
|
19,771
|
|
65
|
%
|
|||||||||||
|
Collaborative revenues
|
52,086
|
|
409,722
|
|
113,126
|
|
(357,636
|
)
|
(87
|
)%
|
296,596
|
|
262
|
%
|
|||||||||||
|
Total revenues
|
$
|
1,527,042
|
|
$
|
1,410,626
|
|
$
|
143,370
|
|
$
|
116,416
|
|
8
|
%
|
$
|
1,267,256
|
|
884
|
%
|
||||||
|
2012
|
2011
|
2010
|
|||||||||
|
(in thousands)
|
|||||||||||
|
INCIVEK
|
$
|
1,161,813
|
|
$
|
950,889
|
|
$
|
—
|
|
||
|
KALYDECO
|
171,645
|
|
—
|
|
—
|
|
|||||
|
Total product revenues, net
|
$
|
1,333,458
|
|
$
|
950,889
|
|
$
|
—
|
|
||
|
2012
|
2011
|
2010
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Collaborative revenues:
|
|||||||||||
|
Janssen
|
$
|
16,178
|
|
$
|
274,393
|
|
$
|
30,750
|
|
||
|
Mitsubishi Tanabe
|
18,879
|
|
121,675
|
|
81,868
|
|
|||||
|
CFFT
|
16,960
|
|
13,654
|
|
—
|
|
|||||
|
Other
|
69
|
|
—
|
|
508
|
|
|||||
|
Total collaborative revenues
|
$
|
52,086
|
|
$
|
409,722
|
|
$
|
113,126
|
|
||
|
2012/2011
Comparison |
2011/2010
Comparison |
||||||||||||||||||||||||
|
2012
|
2011
|
2010
|
Increase/
(Decrease) |
Increase/
(Decrease) |
Increase/
(Decrease) |
Increase/
(Decrease) |
|||||||||||||||||||
|
(in thousands)
|
(in thousands, except percentages)
|
||||||||||||||||||||||||
|
Cost of product revenues
|
$
|
236,742
|
|
$
|
63,625
|
|
$
|
—
|
|
$
|
173,117
|
|
272
|
%
|
$
|
63,625
|
|
n/a
|
|
||||||
|
Royalty expenses
|
43,143
|
|
16,880
|
|
12,730
|
|
26,263
|
|
156
|
%
|
4,150
|
|
33
|
%
|
|||||||||||
|
Research and development expenses
|
806,185
|
|
707,706
|
|
637,416
|
|
98,479
|
|
14
|
%
|
70,290
|
|
11
|
%
|
|||||||||||
|
Sales, general and administrative expenses
|
436,796
|
|
400,721
|
|
187,800
|
|
36,075
|
|
9
|
%
|
212,921
|
|
113
|
%
|
|||||||||||
|
Restructuring expense
|
1,844
|
|
2,074
|
|
1,501
|
|
(230
|
)
|
(11
|
)%
|
573
|
|
38
|
%
|
|||||||||||
|
Intangible asset impairment charge
|
—
|
|
105,800
|
|
—
|
|
(105,800
|
)
|
(100
|
)%
|
105,800
|
|
n/a
|
|
|||||||||||
|
Total costs and expenses
|
$
|
1,524,710
|
|
$
|
1,296,806
|
|
$
|
839,447
|
|
$
|
227,904
|
|
18
|
%
|
$
|
457,359
|
|
54
|
%
|
||||||
|
2012/2011
Comparison |
2011/2010
Comparison |
||||||||||||||||||||||||
|
2012
|
2011
|
2010
|
Increase
|
Increase
|
Increase
|
Increase
|
|||||||||||||||||||
|
(in thousands)
|
(in thousands, except percentages)
|
||||||||||||||||||||||||
|
Research expenses
|
$
|
235,588
|
|
$
|
216,903
|
|
$
|
189,273
|
|
$
|
18,685
|
|
9
|
%
|
$
|
27,630
|
|
15
|
%
|
||||||
|
Development expenses
|
570,597
|
|
490,803
|
|
448,143
|
|
79,794
|
|
16
|
%
|
42,660
|
|
10
|
%
|
|||||||||||
|
Total research and development expenses
|
$
|
806,185
|
|
$
|
707,706
|
|
$
|
637,416
|
|
$
|
98,479
|
|
14
|
%
|
$
|
70,290
|
|
11
|
%
|
||||||
|
2012/2011
Comparison |
2011/2010
Comparison |
||||||||||||||||||||||||
|
2012
|
2011
|
2010
|
Increase/
(Decrease) |
Increase/
(Decrease) |
Increase/
(Decrease) |
Increase/
(Decrease) |
|||||||||||||||||||
|
(in thousands)
|
(in thousands, except percentages)
|
||||||||||||||||||||||||
|
Research Expenses:
|
|||||||||||||||||||||||||
|
Salary and benefits
|
$
|
78,488
|
|
$
|
76,355
|
|
$
|
67,508
|
|
$
|
2,133
|
|
3
|
%
|
$
|
8,847
|
|
13
|
%
|
||||||
|
Stock-based compensation expense
|
25,147
|
|
25,305
|
|
23,496
|
|
(158
|
)
|
(1
|
)%
|
1,809
|
|
8
|
%
|
|||||||||||
|
Laboratory supplies and other direct expenses
|
40,005
|
|
35,641
|
|
29,145
|
|
4,364
|
|
12
|
%
|
6,496
|
|
22
|
%
|
|||||||||||
|
Contractual services
|
21,471
|
|
13,213
|
|
9,881
|
|
8,258
|
|
62
|
%
|
3,332
|
|
34
|
%
|
|||||||||||
|
Infrastructure costs
|
70,477
|
|
66,389
|
|
59,243
|
|
4,088
|
|
6
|
%
|
7,146
|
|
12
|
%
|
|||||||||||
|
Total research expenses
|
$
|
235,588
|
|
$
|
216,903
|
|
$
|
189,273
|
|
$
|
18,685
|
|
9
|
%
|
$
|
27,630
|
|
15
|
%
|
||||||
|
2012/2011
Comparison |
2011/2010
Comparison |
||||||||||||||||||||||||
|
2012
|
2011
|
2010
|
Increase/
(Decrease) |
Increase/
(Decrease) |
Increase/
(Decrease) |
Increase/
(Decrease) |
|||||||||||||||||||
|
(in thousands)
|
(in thousands, except percentages)
|
||||||||||||||||||||||||
|
Development Expenses:
|
|||||||||||||||||||||||||
|
Salary and benefits
|
$
|
147,574
|
|
$
|
126,441
|
|
$
|
108,617
|
|
$
|
21,133
|
|
17
|
%
|
$
|
17,824
|
|
16
|
%
|
||||||
|
Stock-based compensation expense
|
46,386
|
|
50,269
|
|
41,702
|
|
(3,883
|
)
|
(8
|
)%
|
8,567
|
|
21
|
%
|
|||||||||||
|
Laboratory supplies and other direct expenses
|
36,585
|
|
33,588
|
|
33,231
|
|
2,997
|
|
9
|
%
|
357
|
|
1
|
%
|
|||||||||||
|
Contractual services
|
217,406
|
|
149,033
|
|
113,031
|
|
68,373
|
|
46
|
%
|
36,002
|
|
32
|
%
|
|||||||||||
|
Drug supply costs
|
14,044
|
|
34,133
|
|
65,902
|
|
(20,089
|
)
|
(59
|
)%
|
(31,769
|
)
|
(48
|
)%
|
|||||||||||
|
Infrastructure costs
|
108,602
|
|
97,339
|
|
85,660
|
|
11,263
|
|
12
|
%
|
11,679
|
|
14
|
%
|
|||||||||||
|
Total development expenses
|
$
|
570,597
|
|
$
|
490,803
|
|
$
|
448,143
|
|
$
|
79,794
|
|
16
|
%
|
$
|
42,660
|
|
10
|
%
|
||||||
|
2012/2011
Comparison |
2011/2010
Comparison |
||||||||||||||||||||||||
|
2012
|
2011
|
2010
|
Increase
|
Increase
|
Increase
|
Increase
|
|||||||||||||||||||
|
(in thousands)
|
(in thousands, except percentages)
|
||||||||||||||||||||||||
|
Sales, general and administrative expenses
|
$
|
436,796
|
|
$
|
400,721
|
|
$
|
187,800
|
|
$
|
36,075
|
|
9
|
%
|
$
|
212,921
|
|
113
|
%
|
||||||
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Loss (income) before provision for (benefit from) income taxes
|
$
|
20,044
|
|
$
|
9,536
|
|
|
Decrease (increase) in fair value of contingent milestone and royalty payments
|
(114,970
|
)
|
(69,950
|
)
|
||
|
Provision for (benefit from) income taxes
|
39,029
|
|
48,809
|
|
||
|
Net loss (income) attributable to noncontrolling interest (Alios)
|
$
|
(55,897
|
)
|
$
|
(11,605
|
)
|
|
Payments Due by Period
|
|||||||||||||||||||
|
2013
|
2014-2015
|
2016-2017
|
2018 and later
|
Total
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Commitments and Obligations Recorded on the Consolidated Balance Sheet at December 31, 2012:
|
|||||||||||||||||||
|
Convertible senior subordinated notes (due October 2015) principal payment
|
$
|
—
|
|
$
|
400,000
|
|
$
|
—
|
|
$
|
—
|
|
$
|
400,000
|
|
||||
|
Convertible senior subordinated notes (due October 2015) interest payment
|
3,350
|
|
—
|
|
—
|
|
—
|
|
3,350
|
|
|||||||||
|
Capital lease obligations
|
13,707
|
|
15,170
|
|
—
|
|
—
|
|
28,877
|
|
|||||||||
|
Construction financing lease obligation
|
—
|
|
912
|
|
1,151
|
|
138,189
|
|
140,252
|
|
|||||||||
|
Research, development and drug supply costs
|
5,771
|
|
—
|
|
—
|
|
—
|
|
5,771
|
|
|||||||||
|
Additional Commitments and Obligations at December 31, 2012:
|
|||||||||||||||||||
|
Convertible senior subordinated notes (due October 2015) interest payments
|
10,050
|
|
26,800
|
|
—
|
|
—
|
|
36,850
|
|
|||||||||
|
Facility operating leases, excluding Fan Pier Leases
|
61,503
|
|
99,960
|
|
55,496
|
|
36,723
|
|
253,682
|
|
|||||||||
|
Fan Pier Leases
|
83,304
|
|
133,500
|
|
133,261
|
|
676,215
|
|
1,026,280
|
|
|||||||||
|
Research, development and drug supply costs
|
4,247
|
|
—
|
|
—
|
|
—
|
|
4,247
|
|
|||||||||
|
Other
|
13,681
|
|
2,497
|
|
164
|
|
—
|
|
16,342
|
|
|||||||||
|
Total contractual commitments and obligations
|
$
|
195,613
|
|
$
|
678,839
|
|
$
|
190,072
|
|
$
|
851,127
|
|
$
|
1,915,651
|
|
||||
|
•
|
revenue recognition;
|
|
•
|
consolidation of variable interest entity;
|
|
•
|
intangible assets;
|
|
•
|
accruals;
|
|
•
|
commercial supplies and inventories; and
|
|
•
|
income taxes.
|
|
•
|
there is persuasive evidence that an arrangement exists between us and our customer;
|
|
•
|
collectability is reasonably assured; and
|
|
•
|
the price is fixed or determinable.
|
|
•
|
In each period, we record net loss (income) attributable to the Alios noncontrolling interest. This net loss (income) reflects Alios’ net loss (income) for the period as adjusted for gains and losses in the fair value of the contingent milestone and royalty payments payable by us to Alios. Determining the fair value of the contingent milestone and royalty payments payable by us to Alios requires us to make significant estimates regarding the probability and potential timing of achieving each of the milestones pursuant to the agreement, future potential net sales of the HCV nucleotide analogues licensed from Alios and appropriate discount and tax rates. We base our estimate of the probability of achieving the relevant milestones on industry data for similar assets and our own experience. The discount rates used in the valuation model represent a measure of credit risk associated with settling the liability. Significant judgment is used in determining the appropriateness of these assumptions at each reporting period. Changes in these assumptions could have a material effect on the fair value of milestone and royalty payment obligation. We expect that the net loss (income) attributed to noncontrolling interest (Alios) will continue to be affected by changes in the fair value of the contingent milestone and royalty payments. For example, in 2012 we received positive results from a Phase 1 clinical trial of ALS-2200 and the fair value of the contingent milestone and royalty payments increased by
$115.0 million
due to increases in the likelihood of achieving milestones and obtaining regulatory approvals, together with decreases in the time period over which we are discounting potential milestone and royalty payments. Increases in the fair value of the contingent milestone payments and royalties payable by us to Alios result in a decrease in net income attributable to Vertex (or an increase in net loss attributable to Vertex) on a dollar-for-dollar basis. Changes in the fair value of these contingent milestone and royalty payments and the effects of these changes on net income (loss) attributable to Vertex were material in 2012 and 2011 and may be material in future periods.
|
|
•
|
Since the effective date of the collaboration agreement we have consolidated all of Alios’ expenses and revenues into our consolidated statements of operations, eliminating all intercompany balances and transactions. In future periods, if Alios increases its headcount and/or expands its activities related to its other programs, its operating expenses could increase substantially. To the extent that Alios pursues other programs, we expect that expenses of Alios related to those activities would be reflected in our research and development expenses and our sales, general and administrative expenses as a result of the financial statement consolidation. We would not be entitled to any benefits from those activities. If we cease to have the power to direct the activities that most significantly affect the economic performance of Alios because of the expansion of Alios' activities related to its other programs or for any other reason cease to be Alios' primary beneficiary, we would deconsolidate Alios.
|
|
•
|
We reflect all of Alios’ cash and cash equivalents as restricted cash and cash equivalents (Alios) when we consolidate Alios’ balance sheets. We do not have any rights to Alios’ cash or cash equivalents; these resources are not available to fund research and development programs pursuant to the collaboration agreement and these amounts do not provide us with any additional liquidity. As a result of payments we have made to Alios under the collaboration agreement, Alios had significant liquid assets as of December 31, 2012. Alios has control over the restricted cash and cash equivalents (Alios), including the ability to distribute the restricted cash and cash equivalents to Alios’ equityholders, and as a result this asset, although carried on our consolidated balance sheet, is not included in the discussion of our liquidity and should be disregarded when evaluating our financial condition.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
ITEM 9.
|
CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
•
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company;
|
|
•
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
|
Page Number in
this Form 10-K |
|
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Statements of Operations for the years ended December 31, 2012, 2011 and 2010
|
F-2
|
|
Consolidated Statements of Comprehensive Income (Loss) for the years ended December 31, 2012, 2011 and 2010
|
F-3
|
|
Consolidated Balance Sheets as of December 31, 2012 and 2011
|
F-4
|
|
Consolidated Statements of Shareholders’ Equity and Noncontrolling Interest for the years ended December 31, 2012, 2011 and 2010
|
F-5
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2012, 2011 and 2010
|
F-6
|
|
Notes to Consolidated Financial Statements
|
F-7
|
|
Exhibit Number
|
Exhibit Description
|
Filed with
this report |
Incorporated by
Reference herein from—Form or Schedule |
Filing Date/
Period Covered |
SEC File/
Reg. Number |
|
3.1
|
Restated Articles of Organization of Vertex Pharmaceuticals Incorporated, as amended.
|
10-Q
(Exhibit 3.1)
|
August 11, 2008
|
000-19319
|
|
|
3.2
|
By-laws of Vertex Pharmaceuticals Incorporated, as amended and restated as of February 5, 2013.
|
8-K
(Exhibit 3.1)
|
February 11, 2013
|
000-19319
|
|
|
4.1
|
Specimen stock certificate.
|
S-1
(Exhibit 4.1)
|
July 18, 1991
|
33-40966
|
|
|
4.2
|
Subordinated Indenture, dated as of September 28, 2010, by and between Vertex Pharmaceuticals Incorporated and U.S. Bank National Association, as trustee.
|
8-K
(Exhibit 4.1)
|
September 29, 2010
|
000-19319
|
|
|
4.3
|
First Supplemental Indenture, dated as of September 28, 2010, by and between Vertex Pharmaceuticals Incorporated and U.S. Bank National Association, as trustee.
|
8-K
(Exhibit 4.2)
|
September 29, 2010
|
000-19319
|
|
|
4.4
|
Form of 3.35% Convertible Senior Subordinated Note due 2015.
|
8-K
(Exhibit 4.3)
|
September 29, 2010
|
000-19319
|
|
|
Collaboration Agreements
|
|||||
|
10.1
|
License, Development, Manufacturing and Commercialization Agreement, dated June 30, 2006, by and between Vertex Pharmaceuticals Incorporated and Janssen Pharmaceutica, N.V.†
|
10-K
(Exhibit 10.1)
|
February 22, 2012
|
000-19319
|
|
|
10.2
|
License, Development and Commercialization Agreement, dated as of June 11, 2004, between Vertex Pharmaceuticals Incorporated and Mitsubishi Pharma Corporation.†
|
10-Q
(Exhibit 10.1)
|
November 9, 2009
|
000-19319
|
|
|
10.3
|
Second Amendment to License, Development and Commercialization Agreement, dated July 30, 2009, between Mitsubishi Tanabe Pharma Corporation and Vertex Pharmaceuticals Incorporated.†
|
10-Q
(Exhibit 10.2)
|
November 9, 2009
|
000-19319
|
|
|
10.4
|
Research Agreement and License Agreement, both dated December 16, 1993, between Vertex and Burroughs Wellcome Co.†
|
10-K
(Exhibit 10.16)
|
Year Ended
December 31, 1993
|
000-19319
|
|
|
10.5
|
Research, Development and Commercialization Agreement, dated as of May 24, 2004, between Vertex Pharmaceuticals Incorporated and Cystic Fibrosis Foundation Therapeutics Incorporated.†
|
10-Q/A
(Exhibit 10.2)
|
August 19, 2011
|
000-19319
|
|
|
10.6
|
Amendment No. 1 to Research, Development and Commercialization Agreement, dated as of January 6, 2006, between Vertex Pharmaceuticals Incorporated and Cystic Fibrosis Foundation Therapeutics Incorporated.†
|
10-K
(Exhibit 10.9)
|
March 16, 2006
|
000-19319
|
|
|
Exhibit Number
|
Exhibit Description
|
Filed with
this report |
Incorporated by
Reference herein from—Form or Schedule |
Filing Date/
Period Covered |
SEC File/
Reg. Number |
|
10.7
|
Amendment No. 2 to Research, Development and Commercialization Agreement, dated as of March 17, 2006, between Vertex Pharmaceuticals Incorporated and Cystic Fibrosis Foundation Therapeutics Incorporated.
|
10-Q/A
(Exhibit 10.6)
|
August 19, 2011
|
000-19319
|
|
|
10.8
|
Amendment No. 5 to Research, Development and Commercialization Agreement, effective as of April 1, 2011, between Vertex Pharmaceuticals Incorporated and Cystic Fibrosis Foundation Therapeutics Incorporated.†
|
10-Q
(Exhibit 10.3)
|
August 9, 2011
|
000-19319
|
|
|
10.9
|
Research and Development Agreement between the Company and Eli Lilly and Company effective June 11, 1997†
|
10-Q
(Exhibit 10.1)
|
August 14, 1997
|
000-19319
|
|
|
10.10
|
License and Collaboration Agreement, dated June 13, 2011, by and between Alios BioPharma, Inc. and Vertex Pharmaceuticals Incorporated and Vertex Pharmaceuticals (Switzerland) LLC.†
|
10-Q
(Exhibit 10.1)
|
August 9, 2011
|
000-19319
|
|
|
Financial Transaction
|
|||||
|
10.11
|
Purchase Agreement, dated May 30, 2008, by and between Vertex Pharmaceuticals Incorporated and Fosamprenavir Royalty, L.P.
|
10-Q
(Exhibit 10.2)
|
August 11, 2008
|
000-19319
|
|
|
Leases
|
|||||
|
10.12
|
Lease, dated May 5, 2011, between Fifty Northern Avenue LLC and Vertex Pharmaceuticals Incorporated.†
|
10-Q
(Exhibit 10.4)
|
August 9, 2011
|
000-19319
|
|
|
10.13
|
Lease, dated May 5, 2011, between Eleven Fan Pier Boulevard LLC and Vertex Pharmaceuticals Incorporated.†
|
10-Q
(Exhibit 10.5)
|
August 9, 2011
|
000-19319
|
|
|
10.14
|
Lease, dated as of March 3, 1995, between Fort Washington Realty Trust and Vertex.
|
10-K
(Exhibit 10.15)
|
Year Ended
December 31, 1994
|
000-19319
|
|
|
10.15
|
First Amendment to Lease, dated as of December 29, 1995, between Fort Washington Realty Trust and Vertex Pharmaceuticals Incorporated.
|
10-K
(Exhibit 10.15)
|
Year Ended
December 31, 1995
|
000-19319
|
|
|
10.16
|
Second Amendment to Lease, dated as of June 13, 1997, between Fort Washington Realty Trust and Vertex Pharmaceuticals Incorporated.
|
10-K
(Exhibit 10.20)
|
March 26, 1998
|
000-19319
|
|
|
10.17
|
Third, Fourth and Fifth Amendments to Lease between Fort Washington Realty Trust and Vertex Pharmaceuticals Incorporated.†
|
10-K
(Exhibit 10.14)
|
March 26, 2001
|
000-19319
|
|
|
10.18
|
Lease, dated as of September 17, 1999, between Trustees of Fort Washington Realty Trust and Vertex Pharmaceuticals Incorporated.†
|
10-Q
(Exhibit 10.27)
|
November 15, 1999
|
000-19319
|
|
|
10.19
|
Amendment to Lease, dated January 12, 2009, by and between BMR-200 Sidney Street LLC (as successor in interest to Fort Washington Realty Trust), and Vertex Pharmaceuticals Incorporated.
|
10-Q
(Exhibit 10.4)
|
May 11, 2009
|
000-19319
|
|
|
10.20
|
Lease, dated as of January 18, 2001, between Kendall Square, LLC and Vertex Pharmaceuticals Incorporated.†
|
10-K
(Exhibit 10.16)
|
March 26, 2001
|
000-19319
|
|
|
10.21
|
Agreement for Lease, dated as of November 4, 1998, between Milton Park Limited, Vertex Pharmaceuticals Incorporated and Vertex Pharmaceuticals (Europe) Limited.
|
10-K
(Exhibit 10.21)
|
March 30, 1999
|
000-19319
|
|
|
10.22
|
Lease between MEPC Milton Park No.1 Limited and MEPC Milton Park No. 2 Limited, Vertex Pharmaceuticals (Europe) Limited and Vertex Pharmaceuticals Incorporated, dated June 10, 2009.
|
10-Q
(Exhibit 10.1)
|
August 10, 2009
|
000-19319
|
|
|
Equity Plans
|
|||||
|
10.23
|
1996 Stock and Option Plan, as amended and restated as of March 14, 2005.*
|
10-K
(Exhibit 10.3)
|
March 16, 2005
|
000-19319
|
|
|
10.24
|
Form of Stock Option Grant under 1996 Stock and Option Plan.*
|
8-K
(Exhibit 10.1)
|
February 9, 2005
|
000-19319
|
|
|
10.25
|
Amended and Restated 2006 Stock and Option Plan.*
|
10-Q
(Exhibit 10.3)
|
August 8, 2012
|
000-19319
|
|
|
10.26
|
Form of Stock Option Grant under 2006 Stock and Option Plan.*
|
8-K
(Exhibit 10.2)
|
May 15, 2006
|
000-19319
|
|
|
10.27
|
Form of Restricted Stock Award under 2006 Stock and Option Plan.*
|
8-K
(Exhibit 10.3)
|
May 15, 2006
|
000-19319
|
|
|
10.28
|
Form of Restricted Stock Award (Performance Accelerated Restricted Stock) under 2006 Stock and Option Plan.*
|
8-K
(Exhibit 10.4)
|
May 15, 2006
|
000-19319
|
|
|
10.29
|
Form of Stock Option Grant-Performance Accelerated 2009 Stock-Options.*
|
10-K
(Exhibit 10.33)
|
February 19, 2010
|
000-19319
|
|
|
10.30
|
Vertex Pharmaceuticals Incorporated Employee Stock Purchase Plan, as amended and restated.*
|
10-Q
(Exhibit 10.4)
|
August 8, 2012
|
000-19319
|
|
|
Exhibit Number
|
Exhibit Description
|
Filed with
this report |
Incorporated by
Reference herein from—Form or Schedule |
Filing Date/
Period Covered |
SEC File/
Reg. Number |
|
Agreements with Executive Officers and Directors
|
|||||
|
10.31
|
Agreement between Jeffrey M. Leiden and Vertex, dated December 14, 2011.*
|
10-K
(Exhibit 10.34)
|
February 22, 2012
|
000-19319
|
|
|
10.32
|
Employee Non-disclosure, Non-competition and Inventions Agreement between Jeffrey M. Leiden and Vertex, dated December 14, 2011.*
|
10-K
(Exhibit 10.35)
|
February 22, 2012
|
000-19319
|
|
|
10.33
|
Transition Agreement between Matthew W. Emmens and Vertex, dated December 14, 2011.*
|
10-K
(Exhibit 10.38)
|
February 22, 2012
|
000-19319
|
|
|
10.34
|
Employment Agreement, dated as of August 27, 2012, between Vertex Pharmaceuticals Incorporated and Stuart Arbuckle.*
|
10-Q
(Exhibit 10.1)
|
November 6, 2012
|
000-19319
|
|
|
10.35
|
Change of Control Agreement, dated as of August 27, 2012, between Vertex Pharmaceuticals Incorporated and Stuart Arbuckle.*
|
10-Q
(Exhibit 10.2)
|
November 6, 2012
|
000-19319
|
|
|
10.36
|
Employment Agreement, dated as of June 11, 2012, between Vertex Pharmaceuticals Incorporated and Kenneth L. Horton.*
|
10-Q
(Exhibit 10.1)
|
August 8, 2012
|
000-19319
|
|
|
10.37
|
Change of Control Agreement, dated as of June 11, 2012, between Vertex Pharmaceuticals Incorporated and Kenneth L. Horton.*
|
10-Q
(Exhibit 10.2)
|
August 8, 2012
|
000-19319
|
|
|
10.38
|
Second Amended and Restated Employment Agreement, dated November 15, 2012, between Peter Mueller and Vertex.*
|
X
|
|
|
|
|
10.39
|
Second Amended and Restated Change of Control Agreement, dated November 15, 2012, between Vertex and Peter Mueller.*
|
X
|
|
|
|
|
10.40
|
Amended and Restated Employment Agreement, dated as of November 8, 2004, between Vertex and Ian F. Smith.*
|
10-Q
(Exhibit 10.13)
|
November 9, 2004
|
000-19319
|
|
|
10.41
|
Amendment No. 1 to Amended and Restated Employment Agreement between Ian F. Smith and Vertex, dated December 29, 2008.*
|
10-K
(Exhibit 10.66)
|
February 17, 2009
|
000-19319
|
|
|
10.42
|
Employment Agreement, dated as of January 26, 2012 between Vertex and David T. Howton.*
|
10-K
(Exhibit 10.50)
|
February 22, 2012
|
000-19319
|
|
|
10.43
|
Change of Control Agreement, dated as of January 26, 2012 between Vertex and David T. Howton.*
|
10-K
(Exhibit 10.51)
|
February 22, 2012
|
000-19319
|
|
|
10.44
|
Separation and General Release Agreement, dated as of November 29, 2012, between Vertex and David T. Howton.*
|
X
|
|||
|
10.45
|
Form of Employee Non-Disclosure and Inventions Agreement.*
|
S-1
(Exhibit 10.4)
|
May 30, 1991
|
33-40966
|
|
|
10.46
|
Vertex Employee Compensation Plan.*
|
X
|
|||
|
10.47
|
Vertex Pharmaceuticals Non-Employee Board Compensation.*
|
10-K
(Exhibit 10.57)
|
February 22, 2012
|
000-19319
|
|
|
Subsidiaries
|
|||||
|
21.1
|
Subsidiaries of Vertex Pharmaceuticals Incorporated.
|
X
|
|||
|
Consent
|
|||||
|
23.1
|
Consent of Independent Registered Public Accounting Firm Ernst & Young LLP.
|
X
|
|||
|
Certifications
|
|||||
|
31.1
|
Certification of the Chief Executive Officer under Section 302 of the Sarbanes-Oxley Act of 2002.
|
X
|
|||
|
31.2
|
Certification of the Chief Financial Officer under Section 302 of the Sarbanes-Oxley Act of 2002.
|
X
|
|||
|
32.1
|
Certification of the Chief Executive Officer and the Chief Financial Officer under Section 906 of the Sarbanes-Oxley Act of 2002.
|
X
|
|||
|
101.INS
|
XBRL Instance
|
X
|
|||
|
101.SCH
|
XBRL Taxonomy Extension Schema
|
X
|
|||
|
101.CAL
|
XBRL Taxonomy Extension Calculation
|
X
|
|||
|
101.LAB
|
XBRL Taxonomy Extension Labels
|
X
|
|||
|
101.PRE
|
XBRL Taxonomy Extension Presentation
|
X
|
|||
|
101.DEF
|
XBRL Taxonomy Extension Definition
|
X
|
|||
|
†
|
Confidential portions of this document have been filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment.
|
|
Vertex Pharmaceuticals Incorporated
|
||
|
March 1, 2013
|
By:
|
/s/ Jeffrey M. Leiden
|
|
Jeffrey M. Leiden
Chief Executive Officer
|
||
|
Name
|
Title
|
Date
|
|||||||||||
|
/s/ Jeffrey M. Leiden
|
Chair of the Board, President and Chief Executive Officer (Principal Executive Officer)
|
||||||||||||
|
Jeffrey M. Leiden
|
March 1, 2013
|
||||||||||||
|
/s/ Ian F. Smith
|
Executive Vice President and Chief Financial Officer (Principal Financial Officer)
|
||||||||||||
|
Ian F. Smith
|
March 1, 2013
|
||||||||||||
|
/s/ Paul M. Silva
|
Senior Vice President and Corporate Controller (Principal Accounting Officer)
|
||||||||||||
|
Paul M. Silva
|
March 1, 2013
|
||||||||||||
|
/s/ David Altshuler
|
Director
|
||||||||||||
|
David Altshuler
|
March 1, 2013
|
||||||||||||
|
/s/ Joshua S. Boger
|
Director
|
||||||||||||
|
Joshua S. Boger
|
March 1, 2013
|
||||||||||||
|
/s/ Matthew W. Emmens
|
Director
|
||||||||||||
|
Matthew W. Emmens
|
March 1, 2013
|
||||||||||||
|
/s/ Terrence C. Kearney
|
Director
|
||||||||||||
|
Terrence C. Kearney
|
March 1, 2013
|
||||||||||||
|
/s/ Yuchun Lee
|
Director
|
||||||||||||
|
Yuchun Lee
|
March 1, 2013
|
||||||||||||
|
/s/ Margaret G. McGlynn
|
Director
|
||||||||||||
|
Margaret G. McGlynn
|
March 1, 2013
|
||||||||||||
|
/s/ Wayne J. Riley
|
Director
|
||||||||||||
|
Wayne J. Riley
|
March 1, 2013
|
||||||||||||
|
/s/ Bruce I. Sachs
|
Director
|
||||||||||||
|
Bruce I. Sachs
|
March 1, 2013
|
||||||||||||
|
/s/ Elaine S. Ullian
|
Director
|
||||||||||||
|
Elaine S. Ullian
|
March 1, 2013
|
||||||||||||
|
/s/ Ernst & Young LLP
|
|
|
VERTEX PHARMACEUTICALS INCORPORATED
(in thousands, except per share amounts)
|
|||||||||||
|
Year Ended December 31,
|
|||||||||||
|
2012
|
2011
|
2010
|
|||||||||
|
Revenues:
|
|||||||||||
|
Product revenues, net
|
$
|
1,333,458
|
|
$
|
950,889
|
|
$
|
—
|
|
||
|
Royalty revenues
|
141,498
|
|
50,015
|
|
30,244
|
|
|||||
|
Collaborative revenues
|
52,086
|
|
409,722
|
|
113,126
|
|
|||||
|
Total revenues
|
1,527,042
|
|
1,410,626
|
|
143,370
|
|
|||||
|
Costs and expenses:
|
|||||||||||
|
Cost of product revenues (Note F)
|
236,742
|
|
63,625
|
|
—
|
|
|||||
|
Royalty expenses
|
43,143
|
|
16,880
|
|
12,730
|
|
|||||
|
Research and development expenses
|
806,185
|
|
707,706
|
|
637,416
|
|
|||||
|
Sales, general and administrative expenses
|
436,796
|
|
400,721
|
|
187,800
|
|
|||||
|
Restructuring expense
|
1,844
|
|
2,074
|
|
1,501
|
|
|||||
|
Intangible asset impairment charge
|
—
|
|
105,800
|
|
—
|
|
|||||
|
Total costs and expenses
|
1,524,710
|
|
1,296,806
|
|
839,447
|
|
|||||
|
Income (loss) from operations
|
2,332
|
|
113,820
|
|
(696,077
|
)
|
|||||
|
Interest income
|
1,940
|
|
1,878
|
|
1,955
|
|
|||||
|
Interest expense
|
(16,653
|
)
|
(38,452
|
)
|
(19,275
|
)
|
|||||
|
Change in fair value of derivative instruments
|
—
|
|
(16,801
|
)
|
(41,229
|
)
|
|||||
|
Income (loss) before provision for (benefit from) income taxes
|
(12,381
|
)
|
60,445
|
|
(754,626
|
)
|
|||||
|
Provision for (benefit from) income taxes
|
38,754
|
|
19,266
|
|
—
|
|
|||||
|
Net income (loss)
|
(51,135
|
)
|
41,179
|
|
(754,626
|
)
|
|||||
|
Net loss (income) attributable to noncontrolling interest (Alios)
|
(55,897
|
)
|
(11,605
|
)
|
—
|
|
|||||
|
Net income (loss) attributable to Vertex
|
$
|
(107,032
|
)
|
$
|
29,574
|
|
$
|
(754,626
|
)
|
||
|
Net income (loss) per share attributable to Vertex common shareholders:
|
|||||||||||
|
Basic
|
$
|
(0.50
|
)
|
$
|
0.14
|
|
$
|
(3.77
|
)
|
||
|
Diluted
|
$
|
(0.50
|
)
|
$
|
0.14
|
|
$
|
(3.77
|
)
|
||
|
Shares used in per share calculations:
|
|||||||||||
|
Basic
|
211,946
|
|
204,891
|
|
200,402
|
|
|||||
|
Diluted
|
211,946
|
|
208,807
|
|
200,402
|
|
|||||
|
VERTEX PHARMACEUTICALS INCORPORATED
Consolidated Statements of Comprehensive Income (Loss)
(in thousands)
|
|||||||||||
|
Year ended December 31,
|
|||||||||||
|
2012
|
2011
|
2010
|
|||||||||
|
Net income (loss)
|
$
|
(51,135
|
)
|
$
|
41,179
|
|
$
|
(754,626
|
)
|
||
|
Changes in other comprehensive income (loss):
|
|||||||||||
|
Unrealized holding gains (losses) on marketable securities, net of tax
|
305
|
|
(119
|
)
|
46
|
|
|||||
|
Foreign currency translation adjustment
|
198
|
|
133
|
|
(473
|
)
|
|||||
|
Total changes in other comprehensive income (loss)
|
503
|
|
14
|
|
(427
|
)
|
|||||
|
Comprehensive income (loss)
|
(50,632
|
)
|
41,193
|
|
(755,053
|
)
|
|||||
|
Comprehensive loss (income) attributable to noncontrolling interest (Alios)
|
(55,897
|
)
|
(11,605
|
)
|
—
|
|
|||||
|
Comprehensive income (loss) attributable to Vertex
|
$
|
(106,529
|
)
|
$
|
29,588
|
|
$
|
(755,053
|
)
|
||
|
VERTEX PHARMACEUTICALS INCORPORATED
(in thousands, except share and per share amounts)
|
|||||||
|
December 31,
|
|||||||
|
2012
|
2011
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
489,407
|
|
$
|
475,320
|
|
|
|
Marketable securities, available for sale
|
831,808
|
|
493,602
|
|
|||
|
Restricted cash and cash equivalents (Alios)
|
69,983
|
|
51,878
|
|
|||
|
Accounts receivable, net
|
143,250
|
|
183,135
|
|
|||
|
Inventories
|
30,464
|
|
112,430
|
|
|||
|
Prepaid expenses and other current assets
|
24,673
|
|
14,889
|
|
|||
|
Total current assets
|
1,589,585
|
|
1,331,254
|
|
|||
|
Restricted cash
|
31,934
|
|
34,090
|
|
|||
|
Property and equipment, net
|
433,609
|
|
133,176
|
|
|||
|
Intangible assets
|
663,500
|
|
663,500
|
|
|||
|
Goodwill
|
30,992
|
|
30,992
|
|
|||
|
Other assets
|
9,668
|
|
11,268
|
|
|||
|
Total assets
|
$
|
2,759,288
|
|
$
|
2,204,280
|
|
|
|
Liabilities and Shareholders’ Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
101,292
|
|
$
|
74,642
|
|
|
|
Accrued expenses
|
264,884
|
|
243,187
|
|
|||
|
Deferred revenues, current portion
|
27,566
|
|
45,037
|
|
|||
|
Accrued restructuring expense, current portion
|
4,758
|
|
4,932
|
|
|||
|
Capital lease obligations, current portion
|
13,707
|
|
—
|
|
|||
|
Income taxes payable (Alios)
|
715
|
|
12,075
|
|
|||
|
Other liabilities, current portion
|
19,702
|
|
12,475
|
|
|||
|
Total current liabilities
|
432,624
|
|
392,348
|
|
|||
|
Deferred revenues, excluding current portion
|
96,242
|
|
118,095
|
|
|||
|
Accrued restructuring expense, excluding current portion
|
18,570
|
|
21,381
|
|
|||
|
Capital lease obligations, excluding current portion
|
15,170
|
|
—
|
|
|||
|
Convertible senior subordinated notes (due 2015)
|
400,000
|
|
400,000
|
|
|||
|
Deferred tax liability
|
280,367
|
|
243,707
|
|
|||
|
Construction financing lease obligation
|
268,031
|
|
55,950
|
|
|||
|
Other liabilities, excluding current portion
|
13,902
|
|
7,287
|
|
|||
|
Total liabilities
|
1,524,906
|
|
1,238,768
|
|
|||
|
Commitments and contingencies (Note S and Note U)
|
|
|
|
|
|||
|
Redeemable noncontrolling interest (Alios)
|
38,530
|
|
37,036
|
|
|||
|
Shareholders’ equity:
|
|||||||
|
Preferred stock, $0.01 par value; 1,000,000 shares authorized; none issued and outstanding at December 31, 2012 and 2011
|
—
|
|
—
|
|
|||
|
Common stock, $0.01 par value; 300,000,000 shares authorized at December 31, 2012 and 2011; 217,286,868 and 209,303,995 shares issued and outstanding at December 31, 2012 and 2011, respectively
|
2,149
|
|
2,072
|
|
|||
|
Additional paid-in capital
|
4,519,448
|
|
4,200,659
|
|
|||
|
Accumulated other comprehensive loss
|
(550
|
)
|
(1,053
|
)
|
|||
|
Accumulated deficit
|
(3,521,867
|
)
|
(3,414,835
|
)
|
|||
|
Total Vertex shareholders’ equity
|
999,180
|
|
786,843
|
|
|||
|
Noncontrolling interest (Alios)
|
196,672
|
|
141,633
|
|
|||
|
Total shareholders’ equity
|
1,195,852
|
|
928,476
|
|
|||
|
Total liabilities and shareholders’ equity
|
$
|
2,759,288
|
|
$
|
2,204,280
|
|
|
|
(1)
|
Amounts include the assets and liabilities of Vertex’s variable interest entity (“VIE”), Alios BioPharma, Inc. (“Alios”). Vertex’s interests and obligations with respect to the VIE’s assets and liabilities are limited to those accorded to Vertex in its agreement with Alios. See
Note B, "Collaborative Arrangements,"
to these consolidated financial statements for amounts.
|
|
Vertex Pharmaceuticals Incorporated
Consolidated Statements of Shareholders’ Equity and Noncontrolling Interest
(in thousands)
|
||||||||||||||||||||||||||||||||||
|
Common Stock
|
Additional
Paid-in Capital |
Accumulated
Other Comprehensive Income (Loss) |
Accumulated Deficit
|
Total Vertex
Shareholders’ Equity |
Noncontrolling
Interest (Alios) |
Total
Shareholders’ Equity |
Redeemable
Noncontrolling Interest (Alios) |
|||||||||||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||
|
Balance, December 31, 2009
|
199,955
|
|
$
|
1,982
|
|
$
|
3,784,787
|
|
$
|
(640
|
)
|
$
|
(2,689,783
|
)
|
$
|
1,096,346
|
|
$
|
—
|
|
$
|
1,096,346
|
|
$
|
—
|
|
||||||||
|
Unrealized holding gains (losses) on marketable securities, net of tax
|
46
|
|
46
|
|
46
|
|
||||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
(473
|
)
|
(473
|
)
|
(473
|
)
|
||||||||||||||||||||||||||||
|
Net income (loss)
|
(754,626
|
)
|
(754,626
|
)
|
(754,626
|
)
|
||||||||||||||||||||||||||||
|
Issuances of common stock:
|
||||||||||||||||||||||||||||||||||
|
Convertible senior subordinated notes (due 2013) conversion
|
1,386
|
|
14
|
|
31,551
|
|
31,565
|
|
31,565
|
|
||||||||||||||||||||||||
|
Benefit plans
|
2,182
|
|
20
|
|
39,971
|
|
39,991
|
|
39,991
|
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
91,124
|
|
91,124
|
|
91,124
|
|
||||||||||||||||||||||||||||
|
Balance, December 31, 2010
|
203,523
|
|
$
|
2,016
|
|
$
|
3,947,433
|
|
$
|
(1,067
|
)
|
$
|
(3,444,409
|
)
|
$
|
503,973
|
|
$
|
—
|
|
$
|
503,973
|
|
$
|
—
|
|
||||||||
|
Unrealized holding gains (losses) on marketable securities, net of tax
|
(119
|
)
|
(119
|
)
|
(119
|
)
|
||||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
133
|
|
133
|
|
133
|
|
||||||||||||||||||||||||||||
|
Net income (loss)
|
29,574
|
|
29,574
|
|
11,605
|
|
41,179
|
|
||||||||||||||||||||||||||
|
Issuances of common stock:
|
||||||||||||||||||||||||||||||||||
|
Benefit plans
|
5,781
|
|
56
|
|
133,362
|
|
133,418
|
|
(25
|
)
|
133,393
|
|
||||||||||||||||||||||
|
Stock-based compensation expense
|
118,964
|
|
118,964
|
|
304
|
|
119,268
|
|
||||||||||||||||||||||||||
|
Tax benefit from equity compensation
|
900
|
|
900
|
|
|
|
900
|
|
||||||||||||||||||||||||||
|
Alios noncontrolling interest upon consolidation
|
130,486
|
|
130,486
|
|
36,299
|
|
||||||||||||||||||||||||||||
|
Change in liquidation value of noncontrolling interest
|
(737
|
)
|
(737
|
)
|
737
|
|
||||||||||||||||||||||||||||
|
Balance, December 31, 2011
|
209,304
|
|
$
|
2,072
|
|
$
|
4,200,659
|
|
$
|
(1,053
|
)
|
$
|
(3,414,835
|
)
|
$
|
786,843
|
|
$
|
141,633
|
|
$
|
928,476
|
|
$
|
37,036
|
|
||||||||
|
Unrealized holding gains (losses) on marketable securities, net of tax
|
305
|
|
305
|
|
305
|
|
||||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
198
|
|
198
|
|
198
|
|
||||||||||||||||||||||||||||
|
Net income (loss)
|
(107,032
|
)
|
(107,032
|
)
|
55,897
|
|
(51,135
|
)
|
||||||||||||||||||||||||||
|
Issuances of common stock:
|
||||||||||||||||||||||||||||||||||
|
Benefit plans
|
7,983
|
|
77
|
|
201,760
|
|
201,837
|
|
155
|
|
201,992
|
|
||||||||||||||||||||||
|
Stock-based compensation expense
|
115,058
|
|
115,058
|
|
481
|
|
115,539
|
|
||||||||||||||||||||||||||
|
Tax benefit from equity compensation
|
1,971
|
|
1,971
|
|
|
|
1,971
|
|
||||||||||||||||||||||||||
|
Change in liquidation value of redeemable noncontrolling interest
|
(1,494
|
)
|
(1,494
|
)
|
1,494
|
|
||||||||||||||||||||||||||||
|
Balance, December 31, 2012
|
217,287
|
|
$
|
2,149
|
|
$
|
4,519,448
|
|
$
|
(550
|
)
|
$
|
(3,521,867
|
)
|
$
|
999,180
|
|
$
|
196,672
|
|
$
|
1,195,852
|
|
$
|
38,530
|
|
||||||||
|
VERTEX PHARMACEUTICALS INCORPORATED
(in thousands)
|
|||||||||||
|
Year Ended December 31,
|
|||||||||||
|
2012
|
2011
|
2010
|
|||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income (loss)
|
$
|
(51,135
|
)
|
$
|
41,179
|
|
$
|
(754,626
|
)
|
||
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities:
|
|||||||||||
|
Depreciation and amortization expense
|
38,191
|
|
35,041
|
|
30,459
|
|
|||||
|
Stock-based compensation expense
|
114,285
|
|
118,226
|
|
91,124
|
|
|||||
|
Other non-cash based compensation expense
|
10,261
|
|
8,525
|
|
6,552
|
|
|||||
|
Intangible asset impairment charge
|
—
|
|
105,800
|
|
—
|
|
|||||
|
Secured notes (due 2012) discount amortization expense
|
—
|
|
18,409
|
|
13,589
|
|
|||||
|
Change in fair value of derivative instruments
|
—
|
|
16,801
|
|
41,229
|
|
|||||
|
Deferred income taxes
|
36,660
|
|
(7,501
|
)
|
—
|
|
|||||
|
Loss on disposal of property and equipment
|
390
|
|
55
|
|
39
|
|
|||||
|
Write-downs of inventories to net realizable value
|
133,189
|
|
—
|
|
—
|
|
|||||
|
Other non-cash items, net
|
(212
|
)
|
264
|
|
(31
|
)
|
|||||
|
Changes in operating assets and liabilities, excluding the effects of the acquisition of a variable interest entity (Alios):
|
|||||||||||
|
Accounts receivable, net
|
39,912
|
|
(170,606
|
)
|
(2,923
|
)
|
|||||
|
Inventories
|
(29,925
|
)
|
(111,388
|
)
|
—
|
|
|||||
|
Prepaid expenses and other current assets
|
(12,259
|
)
|
(1,717
|
)
|
(600
|
)
|
|||||
|
Accounts payable
|
14,892
|
|
37,468
|
|
(1,182
|
)
|
|||||
|
Accrued expenses and other liabilities
|
29,232
|
|
116,822
|
|
11,213
|
|
|||||
|
Excess tax benefit from share-based payment arrangements
|
(1,971
|
)
|
(900
|
)
|
—
|
|
|||||
|
Accrued restructuring expense
|
(2,985
|
)
|
(3,282
|
)
|
(4,422
|
)
|
|||||
|
Income taxes payable (Alios)
|
(11,360
|
)
|
12,075
|
|
—
|
|
|||||
|
Deferred revenues
|
(39,324
|
)
|
(71,536
|
)
|
(65,863
|
)
|
|||||
|
Net cash provided by (used in) operating activities
|
267,841
|
|
143,735
|
|
(635,442
|
)
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Purchases of marketable securities
|
(1,705,829
|
)
|
(721,545
|
)
|
(1,234,719
|
)
|
|||||
|
Sales and maturities of marketable securities
|
1,367,927
|
|
1,016,040
|
|
1,284,806
|
|
|||||
|
Payment for acquisition of a variable interest entity (Alios)
|
—
|
|
(60,000
|
)
|
—
|
|
|||||
|
Expenditures for property and equipment
|
(71,140
|
)
|
(34,595
|
)
|
(38,054
|
)
|
|||||
|
Decrease (increase) in restricted cash and cash equivalents
|
2,156
|
|
—
|
|
(3,777
|
)
|
|||||
|
Decrease (increase) in restricted cash and cash equivalents (Alios)
|
(18,105
|
)
|
12,695
|
|
—
|
|
|||||
|
Decrease (increase) in other assets
|
(826
|
)
|
(183
|
)
|
(955
|
)
|
|||||
|
Net cash provided by (used in) investing activities
|
(425,817
|
)
|
212,412
|
|
7,301
|
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Excess tax benefit from share-based payment arrangements
|
1,971
|
|
900
|
|
—
|
|
|||||
|
Issuances of common stock from employee benefit plans
|
191,721
|
|
124,862
|
|
33,434
|
|
|||||
|
Issuance of convertible senior subordinated notes (due 2015)
|
—
|
|
—
|
|
391,645
|
|
|||||
|
Payments to redeem secured notes (due 2012)
|
—
|
|
(155,000
|
)
|
—
|
|
|||||
|
Settlement of milestone derivatives
|
—
|
|
(95,000
|
)
|
—
|
|
|||||
|
Payments on capital lease obligations
|
(2,615
|
)
|
—
|
|
—
|
|
|||||
|
Payments on construction financing lease obligation
|
(18,873
|
)
|
—
|
|
—
|
|
|||||
|
Debt conversion costs
|
—
|
|
—
|
|
(22
|
)
|
|||||
|
Net cash provided by (used in) financing activities
|
172,204
|
|
(124,238
|
)
|
425,057
|
|
|||||
|
Effect of changes in exchange rates on cash
|
(141
|
)
|
214
|
|
(377
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
14,087
|
|
232,123
|
|
(203,461
|
)
|
|||||
|
Cash and cash equivalents—beginning of period
|
475,320
|
|
243,197
|
|
446,658
|
|
|||||
|
Cash and cash equivalents—end of period
|
$
|
489,407
|
|
$
|
475,320
|
|
$
|
243,197
|
|
||
|
Supplemental disclosure of cash flow information:
|
|||||||||||
|
Cash paid for interest
|
$
|
13,400
|
|
$
|
13,512
|
|
$
|
761
|
|
||
|
Cash paid for income taxes
|
$
|
9,318
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Conversion of convertible senior subordinated notes (due 2013) for common stock
|
$
|
—
|
|
$
|
—
|
|
$
|
32,071
|
|
||
|
Accrued interest offset to additional paid-in capital on conversion of convertible senior subordinated notes (due 2013)
|
$
|
—
|
|
$
|
—
|
|
$
|
140
|
|
||
|
Unamortized debt issuance costs of converted convertible senior subordinated notes (due 2013) offset to additional paid-in capital
|
$
|
—
|
|
$
|
—
|
|
$
|
624
|
|
||
|
Capitalization of construction in-process related to construction financing lease obligation
|
$
|
235,594
|
|
$
|
54,655
|
|
$
|
—
|
|
||
|
Assets acquired under capital lease obligations
|
$
|
30,101
|
|
$
|
—
|
|
$
|
—
|
|
||
|
A.
|
Nature of Business and Accounting Policies
|
|
Trade
Allowances |
Rebates,
Chargebacks and Discounts |
Product
Returns |
Other
Incentives |
Total
|
|||||||||||||
|
(in thousands)
|
|||||||||||||||||
|
2012
|
|||||||||||||||||
|
Beginning Balance
|
$
|
11,162
|
|
$
|
52,659
|
|
$
|
340
|
|
$
|
5,202
|
|
$
|
69,363
|
|
||
|
Provision related to current period sales
|
55,913
|
|
216,942
|
|
2,067
|
|
19,103
|
|
294,025
|
|
|||||||
|
Adjustments related to prior period sales
|
29
|
|
3,883
|
|
1,498
|
|
72
|
|
5,482
|
|
|||||||
|
Credits/payments made
|
(61,688
|
)
|
(209,924
|
)
|
(1,053
|
)
|
(20,812
|
)
|
(293,477
|
)
|
|||||||
|
Ending Balance
|
$
|
5,416
|
|
$
|
63,560
|
|
$
|
2,852
|
|
$
|
3,565
|
|
$
|
75,393
|
|
||
|
2011
|
|||||||||||||||||
|
Beginning Balance
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Provision related to current period sales
|
38,228
|
|
75,145
|
|
553
|
|
9,692
|
|
123,618
|
|
|||||||
|
Credits/payments made
|
(27,066
|
)
|
(22,486
|
)
|
(213
|
)
|
(4,490
|
)
|
(54,255
|
)
|
|||||||
|
Ending Balance
|
$
|
11,162
|
|
$
|
52,659
|
|
$
|
340
|
|
$
|
5,202
|
|
$
|
69,363
|
|
||
|
B.
|
Collaborative Arrangements
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Royalty revenues
|
$
|
117,592
|
|
$
|
20,289
|
|
$
|
—
|
|
|
Collaborative revenues:
|
|||||||||
|
Amortized portion of up-front payment
|
$
|
12,428
|
|
$
|
12,428
|
|
$
|
12,428
|
|
|
Milestone revenues
|
—
|
|
250,000
|
|
—
|
|
|||
|
Net reimbursement (payment) for telaprevir development costs
|
(3,507
|
)
|
(8,418
|
)
|
9,245
|
|
|||
|
Reimbursement for manufacturing services
|
7,257
|
|
20,383
|
|
9,077
|
|
|||
|
Total collaborative revenues attributable to the Janssen collaboration
|
$
|
16,178
|
|
$
|
274,393
|
|
$
|
30,750
|
|
|
Total revenues attributable to the Janssen collaboration
|
$
|
133,770
|
|
$
|
294,682
|
|
$
|
30,750
|
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Amortized portion of up-front payments
|
$
|
12,744
|
|
$
|
38,232
|
|
$
|
38,232
|
|
|
Milestone revenues
|
485
|
|
68,515
|
|
—
|
|
|||
|
Payments for manufacturing services
|
5,650
|
|
14,928
|
|
43,636
|
|
|||
|
Total collaborative revenues attributable to the Mitsubishi Tanabe collaboration
|
$
|
18,879
|
|
$
|
121,675
|
|
$
|
81,868
|
|
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Loss (income) before provision for (benefit from) income taxes
|
$
|
20,044
|
|
$
|
9,536
|
|
|
Decrease (increase) in fair value of contingent milestone and royalty payments
|
(114,970
|
)
|
(69,950
|
)
|
||
|
Provision for (benefit from) income taxes
|
39,029
|
|
48,809
|
|
||
|
Net loss (income) attributable to noncontrolling interest (Alios)
|
$
|
(55,897
|
)
|
$
|
(11,605
|
)
|
|
As of December 31,
|
||||||
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Restricted cash and cash equivalents (Alios)
|
$
|
69,983
|
|
$
|
51,878
|
|
|
Prepaid expenses and other current assets
|
672
|
|
2,299
|
|
||
|
Property and equipment, net
|
1,728
|
|
1,925
|
|
||
|
Intangible assets
|
250,600
|
|
250,600
|
|
||
|
Goodwill
|
4,890
|
|
4,890
|
|
||
|
Other assets
|
861
|
|
133
|
|
||
|
Accounts payable
|
1,054
|
|
4,132
|
|
||
|
Accrued expenses
|
6,099
|
|
4,304
|
|
||
|
Income taxes payable (Alios)
|
715
|
|
12,075
|
|
||
|
Deferred tax liability
|
152,781
|
|
116,121
|
|
||
|
Other liabilities, excluding current portion
|
910
|
|
1,030
|
|
||
|
Redeemable noncontrolling interest (Alios)
|
38,530
|
|
37,036
|
|
||
|
Noncontrolling interest (Alios)
|
196,672
|
|
141,633
|
|
||
|
C.
|
Net Income (Loss) Per Share Attributable to Vertex Common Shareholders
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands, except per share amounts)
|
|||||||||
|
Basic net income (loss) attributable to Vertex per common share calculation:
|
|||||||||
|
Net income (loss) attributable to Vertex common shareholders
|
$
|
(107,032
|
)
|
$
|
29,574
|
|
$
|
(754,626
|
)
|
|
Less: Undistributed earnings allocated to participating securities
|
—
|
|
(291
|
)
|
—
|
|
|||
|
Net income (loss) attributable to Vertex common shareholders—basic
|
$
|
(107,032
|
)
|
$
|
29,283
|
|
$
|
(754,626
|
)
|
|
Basic weighted-average common shares outstanding
|
211,946
|
|
204,891
|
|
200,402
|
|
|||
|
Basic net income (loss) attributable to Vertex per common share
|
$
|
(0.50
|
)
|
$
|
0.14
|
|
$
|
(3.77
|
)
|
|
Diluted net income (loss) attributable to Vertex per common share calculation:
|
|||||||||
|
Net income (loss) attributable to Vertex common shareholders
|
$
|
(107,032
|
)
|
$
|
29,574
|
|
$
|
(754,626
|
)
|
|
Less: Undistributed earnings allocated to participating securities
|
—
|
|
(285
|
)
|
—
|
|
|||
|
Net income (loss) attributable to Vertex common shareholders—diluted
|
$
|
(107,032
|
)
|
$
|
29,289
|
|
$
|
(754,626
|
)
|
|
Weighted-average shares used to compute basic net income (loss) per common share
|
211,946
|
|
204,891
|
|
200,402
|
|
|||
|
Effect of potentially dilutive securities:
|
|||||||||
|
Stock options
|
—
|
|
3,863
|
|
—
|
|
|||
|
Other
|
—
|
|
53
|
|
—
|
|
|||
|
Weighted-average shares used to compute diluted net income (loss) per common share
|
211,946
|
|
208,807
|
|
200,402
|
|
|||
|
Diluted net income (loss) attributable to Vertex per common share
|
$
|
(0.50
|
)
|
$
|
0.14
|
|
$
|
(3.77
|
)
|
|
2012
|
2011
|
2010
|
||||
|
(in thousands)
|
||||||
|
Stock options
|
19,726
|
|
9,626
|
|
21,293
|
|
|
Convertible senior subordinated notes
|
8,192
|
|
8,192
|
|
8,192
|
|
|
Unvested restricted stock and restricted stock units
|
2,350
|
|
8
|
|
1,950
|
|
|
D.
|
Fair Value Measurements
|
|
Level 1:
|
Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis.
|
|
Level 2:
|
Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active.
|
|
Level 3:
|
Unobservable inputs based on the Company’s assessment of the assumptions that market participants would use in pricing the asset or liability.
|
|
Fair Value Measurements as
of December 31, 2012 |
||||||||||||
|
Fair Value Hierarchy
|
||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|||||||||
|
(in thousands)
|
||||||||||||
|
Financial assets carried at fair value:
|
||||||||||||
|
Cash equivalents:
|
||||||||||||
|
Money market funds
|
$
|
268,463
|
|
$
|
268,463
|
|
$
|
—
|
|
$
|
—
|
|
|
Marketable securities:
|
||||||||||||
|
U.S. Treasury securities
|
111,350
|
|
111,350
|
|
—
|
|
—
|
|
||||
|
Government-sponsored enterprise securities
|
440,225
|
|
440,225
|
|
—
|
|
—
|
|
||||
|
Commercial paper
|
225,449
|
|
—
|
|
225,449
|
|
—
|
|
||||
|
Corporate debt securities
|
54,784
|
|
—
|
|
54,784
|
|
—
|
|
||||
|
Restricted cash
|
31,934
|
|
31,934
|
|
—
|
|
—
|
|
||||
|
Total
|
$
|
1,132,205
|
|
$
|
851,972
|
|
$
|
280,233
|
|
$
|
—
|
|
|
E.
|
Marketable Securities
|
|
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Fair Value
|
|||||||||
|
(in thousands)
|
||||||||||||
|
December 31, 2012
|
||||||||||||
|
Cash and cash equivalents:
|
||||||||||||
|
Cash and money market funds
|
$
|
489,407
|
|
$
|
—
|
|
$
|
—
|
|
$
|
489,407
|
|
|
Total cash and cash equivalents
|
$
|
489,407
|
|
$
|
—
|
|
$
|
—
|
|
$
|
489,407
|
|
|
Marketable securities:
|
||||||||||||
|
U.S. Treasury securities (due within 1 year)
|
$
|
111,350
|
|
$
|
2
|
|
$
|
(2
|
)
|
$
|
111,350
|
|
|
Government-sponsored enterprise securities (due within 1 year)
|
440,181
|
|
49
|
|
(5
|
)
|
440,225
|
|
||||
|
Commercial paper (due within 1 year)
|
225,294
|
|
155
|
|
—
|
|
225,449
|
|
||||
|
Corporate debt securities (due within 1 year)
|
15,429
|
|
1
|
|
(1
|
)
|
15,429
|
|
||||
|
Corporate debt securities (due after 1 year through 5 years)
|
39,358
|
|
10
|
|
(13
|
)
|
39,355
|
|
||||
|
Total marketable securities
|
$
|
831,612
|
|
$
|
217
|
|
$
|
(21
|
)
|
$
|
831,808
|
|
|
Total cash, cash equivalents and marketable securities
|
$
|
1,321,019
|
|
$
|
217
|
|
$
|
(21
|
)
|
$
|
1,321,215
|
|
|
December 31, 2011
|
||||||||||||
|
Cash and cash equivalents:
|
||||||||||||
|
Cash and money market funds
|
$
|
362,035
|
|
$
|
—
|
|
$
|
—
|
|
$
|
362,035
|
|
|
Government-sponsored enterprise securities
|
113,302
|
|
—
|
|
(17
|
)
|
113,285
|
|
||||
|
Total cash and cash equivalents
|
$
|
475,337
|
|
$
|
—
|
|
$
|
(17
|
)
|
$
|
475,320
|
|
|
Marketable securities:
|
||||||||||||
|
U.S. Treasury securities (due within 1 year)
|
$
|
22,105
|
|
$
|
2
|
|
$
|
—
|
|
$
|
22,107
|
|
|
Government-sponsored enterprise securities (due within 1 year)
|
471,589
|
|
8
|
|
(102
|
)
|
471,495
|
|
||||
|
Total marketable securities
|
$
|
493,694
|
|
$
|
10
|
|
$
|
(102
|
)
|
$
|
493,602
|
|
|
Total cash, cash equivalents and marketable securities
|
$
|
969,031
|
|
$
|
10
|
|
$
|
(119
|
)
|
$
|
968,922
|
|
|
F.
|
Inventories
|
|
As of December 31,
|
||||||
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
INCIVEK
|
$
|
22,792
|
|
$
|
112,430
|
|
|
KALYDECO
|
7,672
|
|
—
|
|
||
|
Total
|
$
|
30,464
|
|
$
|
112,430
|
|
|
As of December 31,
|
||||||
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Raw materials
|
$
|
3,754
|
|
$
|
32,213
|
|
|
Work-in-process
|
11,317
|
|
47,010
|
|
||
|
Finished goods
|
15,393
|
|
33,207
|
|
||
|
Total
|
$
|
30,464
|
|
$
|
112,430
|
|
|
G.
|
Property and Equipment
|
|
As of December 31,
|
||||||
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Furniture and equipment
|
$
|
173,766
|
|
$
|
151,961
|
|
|
Leasehold improvements
|
123,770
|
|
107,169
|
|
||
|
Software
|
101,276
|
|
56,923
|
|
||
|
Computers
|
40,779
|
|
33,116
|
|
||
|
Construction-in-process
|
290,703
|
|
55,070
|
|
||
|
Total property and equipment, gross
|
730,294
|
|
404,239
|
|
||
|
Less: accumulated depreciation
|
(296,685
|
)
|
(271,063
|
)
|
||
|
Total property and equipment, net
|
$
|
433,609
|
|
$
|
133,176
|
|
|
H.
|
Fan Pier Leases
|
|
I.
|
Intangible Assets and Goodwill
|
|
J.
|
Accrued Expenses
|
|
As of December 31,
|
||||||
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Research, development and commercial contract costs
|
$
|
63,960
|
|
$
|
66,426
|
|
|
Payroll and benefits
|
62,140
|
|
57,453
|
|
||
|
Product revenue allowances
|
69,936
|
|
58,201
|
|
||
|
Royalty payable
|
29,007
|
|
28,603
|
|
||
|
Unrecognized tax benefits
|
4,106
|
|
4,360
|
|
||
|
Interest
|
3,395
|
|
3,363
|
|
||
|
Professional fees
|
11,226
|
|
12,785
|
|
||
|
Other
|
21,114
|
|
11,996
|
|
||
|
Total
|
$
|
264,884
|
|
$
|
243,187
|
|
|
K.
|
Convertible Senior Subordinated Notes
|
|
L.
|
Common Stock, Preferred Stock and Equity Plans
|
|
As of December 31, 2012
|
|||||||
|
Title of Plan
|
Group Eligible
|
Type of Award
Granted |
Awards
Outstanding |
Additional Awards
Authorized for Grant |
|||
|
2006 Stock and Option Plan
|
Employees, Non-employee Directors and Consultants
|
NSO, ISO,
RS and RSU |
20,551,584
|
|
5,751,677
|
|
|
|
1996 Stock and Option Plan
|
Employees, Non-employee Directors, Advisors and Consultants
|
NSO, ISO and RS
|
1,524,010
|
|
—
|
|
|
|
Total
|
22,075,594
|
|
5,751,677
|
|
|||
|
Stock Options
|
Weighted-average
Exercise Price |
Weighted-average
Remaining Contractual Life |
Aggregate Intrinsic
Value |
||||||
|
(in thousands)
|
(per share)
|
(in years)
|
(in thousands)
|
||||||
|
Outstanding at December 31, 2011
|
20,923
|
|
$
|
34.23
|
|
||||
|
Granted
|
6,043
|
|
43.80
|
|
|||||
|
Exercised
|
(5,856
|
)
|
29.51
|
|
|||||
|
Forfeited
|
(1,310
|
)
|
41.01
|
|
|||||
|
Expired
|
(74
|
)
|
39.47
|
|
|||||
|
Outstanding at December 31, 2012
|
19,726
|
|
$
|
38.09
|
|
7.15
|
$
|
107,016
|
|
|
Exercisable at December 31, 2012
|
10,849
|
|
$
|
34.54
|
|
5.93
|
$
|
84,297
|
|
|
Total exercisable or expected to vest at December 31, 2012
|
18,782
|
|
$
|
37.83
|
|
7.06
|
$
|
104,931
|
|
|
Options Outstanding
|
Options Exercisable
|
||||||||||
|
Range of Exercise Prices
|
Number
Outstanding |
Weighted-average
Remaining Contractual Life |
Weighted-average
Exercise Price |
Number
Exercisable |
Weighted-average
Exercise Price |
||||||
|
(in thousands)
|
(in years)
|
(per share)
|
(in thousands)
|
(per share)
|
|||||||
|
$ 9.07–$20.00
|
1,000
|
|
3.12
|
$
|
15.38
|
|
1,000
|
|
$
|
15.38
|
|
|
$20.01–$30.00
|
1,415
|
|
6.35
|
$
|
29.30
|
|
1,039
|
|
$
|
29.11
|
|
|
$30.01–$40.00
|
12,590
|
|
6.86
|
$
|
36.09
|
|
7,770
|
|
$
|
35.29
|
|
|
$40.01–$50.00
|
2,331
|
|
9.32
|
$
|
48.04
|
|
216
|
|
$
|
46.92
|
|
|
$50.01–$60.00
|
2,328
|
|
8.68
|
$
|
53.41
|
|
816
|
|
$
|
54.18
|
|
|
$60.01–$64.30
|
62
|
|
9.34
|
$
|
63.17
|
|
8
|
|
$
|
63.14
|
|
|
Restricted
Stock |
Weighted-average
Grant-date Fair Value |
||||
|
(in thousands)
|
(per share)
|
||||
|
Unvested at December 31, 2011
|
2,100
|
|
$
|
37.13
|
|
|
Granted
|
1,428
|
|
45.46
|
|
|
|
Vested
|
(998
|
)
|
35.11
|
|
|
|
Cancelled
|
(260
|
)
|
40.09
|
|
|
|
Unvested at December 31, 2012
|
2,270
|
|
$
|
42.92
|
|
|
Year Ended December 31, 2012
|
|||
|
(in thousands,
except per share amount) |
|||
|
Number of shares
|
702
|
|
|
|
Average price paid per share
|
|
$26.71
|
|
|
M.
|
Stock-based Compensation Expense
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Stock-based compensation expense by line item:
|
|||||||||
|
Research and development expenses
|
$
|
71,533
|
|
$
|
75,574
|
|
$
|
65,198
|
|
|
Sales, general and administrative expenses
|
42,752
|
|
42,652
|
|
25,926
|
|
|||
|
Total stock-based compensation expense included in costs and expenses
|
$
|
114,285
|
|
$
|
118,226
|
|
$
|
91,124
|
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Stock-based compensation expense by type of award:
|
|||||||||
|
Stock options
|
$
|
79,047
|
|
$
|
83,098
|
|
$
|
64,005
|
|
|
Restricted stock and restricted stock units
|
29,194
|
|
30,708
|
|
22,960
|
|
|||
|
ESPP share issuances
|
7,298
|
|
5,462
|
|
4,159
|
|
|||
|
Less stock-based compensation expense capitalized to inventories
|
(1,254
|
)
|
(1,042
|
)
|
—
|
|
|||
|
Total stock-based compensation expense included in costs and expenses
|
$
|
114,285
|
|
$
|
118,226
|
|
$
|
91,124
|
|
|
As of December 31, 2012
|
|||
|
Unrecognized Expense
Net of Estimated Forfeitures |
Weighted-average
Recognition Period |
||
|
(in thousands)
|
(in years)
|
||
|
Type of award:
|
|||
|
Stock options
|
$156,225
|
2.69
|
|
|
Restricted stock and restricted stock units
|
68,094
|
|
2.58
|
|
ESPP share issuances
|
5,661
|
|
0.65
|
|
2012
|
2011
|
2010
|
||||
|
Expected stock price volatility
|
47.93
|
%
|
49.53
|
%
|
52.17
|
%
|
|
Risk-free interest rate
|
0.95
|
%
|
2.09
|
%
|
2.44
|
%
|
|
Expected term of options (in years)
|
5.78
|
|
5.74
|
|
5.71
|
|
|
Expected annual dividends
|
—
|
|
—
|
|
—
|
|
|
•
|
Expected stock price volatility:
Options to purchase the Company’s stock with remaining terms of greater than one year are regularly traded in the market. Expected stock price volatility is calculated using the trailing one month average of daily implied volatilities prior to grant date.
|
|
•
|
Risk-free interest rate:
The Company bases the risk-free interest rate on the interest rate payable on U.S. Treasury securities in effect at the time of grant for a period that is commensurate with the assumed expected option term.
|
|
•
|
Expected term of options:
The expected term of options represents the period of time options are expected to be outstanding. The Company uses historical data to estimate employee exercise and post-vest termination behavior. The Company believes that all groups of employees exhibit similar exercise and post-vest termination behavior and therefore does not stratify employees into multiple groups in determining the expected term of options.
|
|
•
|
Expected annual dividends:
The estimate for annual dividends is
$0.00
because the Company has not historically paid, and does not intend for the foreseeable future to pay, a dividend.
|
|
2012
|
2011
|
2010
|
||||
|
Expected stock price volatility
|
46.90
|
%
|
51.32
|
%
|
43.92
|
%
|
|
Risk-free interest rate
|
0.16
|
%
|
0.08
|
%
|
0.24
|
%
|
|
Expected term (in years)
|
0.74
|
|
0.72
|
|
0.71
|
|
|
Expected annual dividends
|
—
|
|
—
|
|
—
|
|
|
N.
|
September 2009 Financial Transactions
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Expenses and Losses (Gains):
|
|||||||||
|
Interest expense related to 2012 Notes
|
$
|
—
|
|
$
|
21,687
|
|
$
|
15,068
|
|
|
Change in fair value of embedded derivative related to 2012 Notes
|
—
|
|
(400
|
)
|
1,637
|
|
|||
|
Change in fair value of free-standing derivatives related to the sale of milestone payments
|
—
|
|
17,201
|
|
39,592
|
|
|||
|
Total September 2009 financial transaction expenses
|
$
|
—
|
|
$
|
38,488
|
|
$
|
56,297
|
|
|
O.
|
Sale of HIV Protease Inhibitor Royalty Stream
|
|
P.
|
Income Taxes
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
United States
|
$
|
256,816
|
|
$
|
343,515
|
|
$
|
(719,859
|
)
|
|
Foreign
|
(269,197
|
)
|
(283,070
|
)
|
(34,767
|
)
|
|||
|
Income (loss) before provision for (benefit from) income taxes
|
$
|
(12,381
|
)
|
$
|
60,445
|
|
$
|
(754,626
|
)
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Current taxes:
|
|||||||||
|
United States
|
$
|
2,057
|
|
$
|
22,275
|
|
$
|
—
|
|
|
Foreign
|
(1,865
|
)
|
(561
|
)
|
—
|
|
|||
|
State
|
1,902
|
|
8,655
|
|
—
|
|
|||
|
Total current taxes
|
$
|
2,094
|
|
$
|
30,369
|
|
$
|
—
|
|
|
Deferred taxes:
|
|||||||||
|
United States
|
$
|
31,308
|
|
$
|
19,629
|
|
$
|
—
|
|
|
Foreign
|
—
|
|
(32,692
|
)
|
—
|
|
|||
|
State
|
5,352
|
|
1,960
|
|
—
|
|
|||
|
Total deferred taxes
|
$
|
36,660
|
|
$
|
(11,103
|
)
|
$
|
—
|
|
|
Provision for (benefit from) income taxes
|
$
|
38,754
|
|
$
|
19,266
|
|
$
|
—
|
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Income (loss) before provision for (benefit from) income taxes
|
$
|
(12,381
|
)
|
$
|
60,445
|
|
$
|
(754,626
|
)
|
|
Expected tax provision (benefit)
|
(4,333
|
)
|
21,156
|
|
(256,574
|
)
|
|||
|
State taxes, net of federal benefit
|
7,075
|
|
10,624
|
|
(46,108
|
)
|
|||
|
Foreign rate differential
|
62,425
|
|
43,629
|
|
632
|
|
|||
|
Tax credits
|
(1,980
|
)
|
(51,086
|
)
|
(23,292
|
)
|
|||
|
Unbenefited operating losses
|
(30,364
|
)
|
(6,286
|
)
|
322,551
|
|
|||
|
Non-deductible expenses
|
3,198
|
|
1,953
|
|
2,158
|
|
|||
|
Rate change
|
3,275
|
|
—
|
|
—
|
|
|||
|
Other
|
(542
|
)
|
(724
|
)
|
633
|
|
|||
|
Provision for (benefit from) income taxes
|
$
|
38,754
|
|
$
|
19,266
|
|
$
|
—
|
|
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Unrecognized tax benefits beginning of year
|
$
|
4,360
|
|
$
|
2,374
|
|
|
Gross change for current year positions
|
598
|
|
2,569
|
|
||
|
Increase for prior period positions
|
—
|
|
—
|
|
||
|
Decrease for prior period positions
|
—
|
|
—
|
|
||
|
Decrease due to settlements and payments
|
—
|
|
—
|
|
||
|
Decrease due to statute limitations
|
(852
|
)
|
(583
|
)
|
||
|
Unrecognized tax benefits end of year
|
$
|
4,106
|
|
$
|
4,360
|
|
|
As of December 31,
|
||||||
|
2012
|
2011
|
|||||
|
(in thousands)
|
||||||
|
Deferred tax assets:
|
||||||
|
Net operating loss
|
$
|
777,687
|
|
$
|
870,367
|
|
|
Tax credit carryforwards
|
147,074
|
|
167,759
|
|
||
|
Property and equipment
|
10,701
|
|
15,537
|
|
||
|
Intangibles
|
63,353
|
|
71,076
|
|
||
|
Deferred revenues
|
44,867
|
|
59,939
|
|
||
|
Stock-based compensation
|
83,979
|
|
90,563
|
|
||
|
Inventories
|
56,564
|
|
23,883
|
|
||
|
Accrued expenses
|
27,945
|
|
30,636
|
|
||
|
Unrealized loss
|
—
|
|
245
|
|
||
|
Gross deferred tax assets
|
1,212,170
|
|
1,330,005
|
|
||
|
Valuation allowance
|
(1,211,561
|
)
|
(1,329,775
|
)
|
||
|
Total deferred tax assets
|
609
|
|
230
|
|
||
|
Deferred tax liabilities:
|
||||||
|
Unrealized gain
|
(376
|
)
|
—
|
|
||
|
Contingent milestone and royalty payment obligation
|
(50,904
|
)
|
(14,241
|
)
|
||
|
Acquired intangibles
|
(229,696
|
)
|
(229,696
|
)
|
||
|
Net deferred tax liabilities
|
$
|
(280,367
|
)
|
$
|
(243,707
|
)
|
|
Q.
|
Restructuring Expense
|
|
Charge
in 2003 |
Cash
payments in 2003 |
Non-cash
write-off in 2003 |
Liability as of
December 31, 2003 |
|||||||||
|
(in thousands)
|
||||||||||||
|
Lease restructuring and other operating lease expense
|
$
|
84,726
|
|
$
|
(15,200
|
)
|
$
|
—
|
|
$
|
69,526
|
|
|
Employee severance, benefits and related costs
|
2,616
|
|
(2,616
|
)
|
—
|
|
—
|
|
||||
|
Leasehold improvements and asset impairments
|
4,482
|
|
—
|
|
(4,482
|
)
|
—
|
|
||||
|
Total
|
$
|
91,824
|
|
$
|
(17,816
|
)
|
$
|
(4,482
|
)
|
$
|
69,526
|
|
|
2012
|
2011
|
2010
|
2004-2012
|
|||||||||
|
(in thousands)
|
||||||||||||
|
Liability, beginning of the period
|
$
|
26,313
|
|
$
|
29,595
|
|
$
|
34,017
|
|
$
|
69,526
|
|
|
Cash payments
|
(14,853
|
)
|
(14,904
|
)
|
(14,759
|
)
|
(163,697
|
)
|
||||
|
Cash received from subleases
|
10,024
|
|
9,548
|
|
8,836
|
|
65,038
|
|
||||
|
Credit for portion of facility Vertex decided to occupy in 2005
|
—
|
|
—
|
|
—
|
|
(10,018
|
)
|
||||
|
Restructuring expense
|
1,844
|
|
2,074
|
|
1,501
|
|
62,479
|
|
||||
|
Liability, end of the period
|
$
|
23,328
|
|
$
|
26,313
|
|
$
|
29,595
|
|
$
|
23,328
|
|
|
R.
|
Employee Benefits
|
|
2012
|
2011
|
2010
|
|||||||
|
(in thousands)
|
|||||||||
|
Discretionary matching contributions during the year ended December 31,
|
$
|
10,261
|
|
$
|
8,619
|
|
$
|
6,552
|
|
|
Shares issued during the year ended December 31,
|
242
|
|
183
|
|
174
|
|
|||
|
Shares issuable as of the year ended December 31,
|
53
|
|
62
|
|
42
|
|
|||
|
S.
|
Commitments
|
|
Year
|
Fan Pier
Leases |
Kendall Square
Lease |
Kendall Sublease
Income |
Other
Operating Leases |
Total Lease
Commitments (Net of Sublease Income) |
||||||||||
|
(in thousands)
|
|||||||||||||||
|
2013
|
$
|
83,304
|
|
$
|
18,338
|
|
$
|
(8,495
|
)
|
$
|
43,165
|
|
$
|
136,312
|
|
|
2014
|
67,206
|
|
18,338
|
|
(8,495
|
)
|
35,375
|
|
112,424
|
|
|||||
|
2015
|
67,206
|
|
18,338
|
|
(3,976
|
)
|
27,909
|
|
109,477
|
|
|||||
|
2016
|
67,206
|
|
18,338
|
|
—
|
|
9,916
|
|
95,460
|
|
|||||
|
2017
|
67,206
|
|
18,338
|
|
—
|
|
8,904
|
|
94,448
|
|
|||||
|
Thereafter
|
814,404
|
|
6,113
|
|
—
|
|
30,610
|
|
851,127
|
|
|||||
|
Total minimum lease payments
|
$
|
1,166,532
|
|
$
|
97,803
|
|
$
|
(20,966
|
)
|
$
|
155,879
|
|
$
|
1,399,248
|
|
|
Year
|
(in thousands)
|
||
|
2013
|
$
|
14,502
|
|
|
2014
|
9,005
|
|
|
|
2015
|
6,537
|
|
|
|
Total payments
|
30,044
|
|
|
|
Less: amount representing interest
|
(1,167
|
)
|
|
|
Present value of payments
|
$
|
28,877
|
|
|
T.
|
Legal Proceedings
|
|
U.
|
Contingencies
|
|
V.
|
Guarantees
|
|
W.
|
Segment Information
|
|
2012
|
2011
|
2010
|
|||||||||
|
(in thousands)
|
|||||||||||
|
INCIVEK
|
$
|
1,161,813
|
|
$
|
950,889
|
|
$
|
—
|
|
||
|
KALYDECO
|
171,645
|
|
—
|
|
—
|
|
|||||
|
Total product revenues, net
|
$
|
1,333,458
|
|
$
|
950,889
|
|
$
|
—
|
|
||
|
2012
|
2011
|
2010
|
|||||||||
|
(in thousands)
|
|||||||||||
|
United States
|
$
|
1,373,516
|
|
$
|
1,389,568
|
|
$
|
143,370
|
|
||
|
Outside of the United States
|
|||||||||||
|
Europe
|
129,786
|
|
20,289
|
|
—
|
|
|||||
|
Other
|
23,740
|
|
769
|
|
—
|
|
|||||
|
Total revenues outside of the United States
|
153,526
|
|
21,058
|
|
—
|
|
|||||
|
Total revenues
|
$
|
1,527,042
|
|
$
|
1,410,626
|
|
$
|
143,370
|
|
||
|
Percent of Total Gross Revenues
|
Percent of Accounts Receivable
|
|||||||||||
|
Year Ended December 31,
|
As of December 31,
|
|||||||||||
|
2012
|
2011
|
2010
|
2012
|
2011
|
||||||||
|
AmerisourceBergen Drug Corporation
|
32
|
%
|
25
|
%
|
—
|
%
|
22
|
%
|
35
|
%
|
||
|
McKesson Corporation
|
29
|
%
|
24
|
%
|
—
|
%
|
26
|
%
|
30
|
%
|
||
|
Cardinal Health Incorporated
|
15
|
%
|
15
|
%
|
—
|
%
|
<10
|
%
|
20
|
%
|
||
|
Janssen
|
<10
|
%
|
19
|
%
|
21
|
%
|
26
|
%
|
10
|
%
|
||
|
Mitsubishi Tanabe
|
<10
|
%
|
<10
|
%
|
57
|
%
|
—
|
%
|
<10
|
%
|
||
|
As of December 31,
|
|||||||
|
2012
|
2011
|
||||||
|
(in thousands)
|
|||||||
|
United States
|
$
|
400,102
|
|
$
|
109,480
|
|
|
|
Outside of the United States
|
|||||||
|
United Kingdom
|
30,622
|
|
21,377
|
|
|||
|
Other
|
2,885
|
|
2,319
|
|
|||
|
Total property and equipment, net outside of the United States
|
33,507
|
|
23,696
|
|
|||
|
Total property and equipment, net
|
$
|
433,609
|
|
$
|
133,176
|
|
|
|
X.
|
Quarterly Financial Data (unaudited)
|
|
Three Months Ended
|
||||||||||||
|
March 31,
2012 |
June 30,
2012 (1) |
Sept. 30,
2012 |
Dec. 31,
2012 (2) |
|||||||||
|
(in thousands, except per share amounts)
|
||||||||||||
|
Revenues:
|
||||||||||||
|
Product revenues, net
|
$
|
375,375
|
|
$
|
373,273
|
|
$
|
303,501
|
|
$
|
281,309
|
|
|
Royalty revenues
|
38,981
|
|
33,480
|
|
25,586
|
|
43,451
|
|
||||
|
Collaborative revenues
|
24,381
|
|
11,552
|
|
6,919
|
|
9,234
|
|
||||
|
Total revenues
|
438,737
|
|
418,305
|
|
336,006
|
|
333,994
|
|
||||
|
Costs and expenses:
|
||||||||||||
|
Cost of product revenues
|
25,918
|
|
104,549
|
|
30,680
|
|
75,595
|
|
||||
|
Royalty expenses
|
13,293
|
|
9,874
|
|
7,856
|
|
12,120
|
|
||||
|
Research and development expenses
|
196,371
|
|
196,544
|
|
200,161
|
|
213,109
|
|
||||
|
Sales, general and administrative expenses
|
111,146
|
|
117,514
|
|
97,684
|
|
110,452
|
|
||||
|
Restructuring expense (credit)
|
360
|
|
594
|
|
696
|
|
194
|
|
||||
|
Intangible asset impairment charge
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
Total costs and expenses
|
347,088
|
|
429,075
|
|
337,077
|
|
411,470
|
|
||||
|
Income (loss) from operations
|
91,649
|
|
(10,770
|
)
|
(1,071
|
)
|
(77,476
|
)
|
||||
|
Interest income
|
364
|
|
560
|
|
519
|
|
497
|
|
||||
|
Interest expense
|
(4,105
|
)
|
(4,195
|
)
|
(4,560
|
)
|
(3,793
|
)
|
||||
|
Change in fair value of derivative instruments
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
Income (loss) before provision for (benefit from) income taxes
|
87,908
|
|
(14,405
|
)
|
(5,112
|
)
|
(80,772
|
)
|
||||
|
Provision for (benefit from) income taxes
|
32
|
|
20,063
|
|
21,355
|
|
(2,696
|
)
|
||||
|
Net income (loss)
|
87,876
|
|
(34,468
|
)
|
(26,467
|
)
|
(78,076
|
)
|
||||
|
Net loss (income) attributable to noncontrolling interest (Alios)
|
3,714
|
|
(30,463
|
)
|
(31,076
|
)
|
1,928
|
|
||||
|
Net income (loss) attributable to Vertex
|
$
|
91,590
|
|
$
|
(64,931
|
)
|
$
|
(57,543
|
)
|
$
|
(76,148
|
)
|
|
Net income (loss) per share attributable to Vertex common shareholders:
|
||||||||||||
|
Basic
|
$
|
0.44
|
|
$
|
(0.31
|
)
|
$
|
(0.27
|
)
|
$
|
(0.35
|
)
|
|
Diluted
|
$
|
0.43
|
|
$
|
(0.31
|
)
|
$
|
(0.27
|
)
|
$
|
(0.35
|
)
|
|
Shares used in per share calculations:
|
||||||||||||
|
Basic
|
208,018
|
|
211,344
|
|
213,767
|
|
214,607
|
|
||||
|
Diluted
|
219,264
|
|
211,344
|
|
213,767
|
|
214,607
|
|
||||
|
Three Months Ended
|
||||||||||||
|
March 31,
2011 |
June 30,
2011 |
Sept. 30,
2011 (3) |
Dec. 31,
2011 |
|||||||||
|
(in thousands, except per share amounts)
|
||||||||||||
|
Revenues:
|
||||||||||||
|
Product revenues, net
|
$
|
—
|
|
$
|
74,535
|
|
$
|
419,595
|
|
$
|
456,759
|
|
|
Royalty revenues
|
6,061
|
|
10,010
|
|
8,539
|
|
25,405
|
|
||||
|
Collaborative revenues
|
67,601
|
|
29,879
|
|
231,066
|
|
81,176
|
|
||||
|
Total revenues
|
73,662
|
|
114,424
|
|
659,200
|
|
563,340
|
|
||||
|
Costs and expenses:
|
||||||||||||
|
Cost of product revenues
|
—
|
|
5,404
|
|
35,285
|
|
22,936
|
|
||||
|
Royalty expenses
|
2,666
|
|
3,902
|
|
3,121
|
|
7,191
|
|
||||
|
Research and development expenses
|
158,612
|
|
173,604
|
|
189,052
|
|
186,438
|
|
||||
|
Sales, general and administrative expenses
|
71,523
|
|
96,663
|
|
110,654
|
|
121,881
|
|
||||
|
Restructuring expense (credit)
|
760
|
|
741
|
|
(419
|
)
|
992
|
|
||||
|
Intangible asset impairment charge
|
—
|
|
—
|
|
105,800
|
|
—
|
|
||||
|
Total costs and expenses
|
233,561
|
|
280,314
|
|
443,493
|
|
339,438
|
|
||||
|
Income (loss) from operations
|
(159,899
|
)
|
(165,890
|
)
|
215,707
|
|
223,902
|
|
||||
|
Interest income
|
1,402
|
|
202
|
|
77
|
|
197
|
|
||||
|
Interest expense
|
(12,001
|
)
|
(6,962
|
)
|
(7,059
|
)
|
(12,430
|
)
|
||||
|
Change in fair value of derivative instruments
|
(5,598
|
)
|
(2,220
|
)
|
(8,115
|
)
|
(868
|
)
|
||||
|
Income (loss) before provision for (benefit from) income taxes
|
(176,096
|
)
|
(174,870
|
)
|
200,610
|
|
210,801
|
|
||||
|
Provision for (benefit from) income taxes
|
—
|
|
24,448
|
|
(27,842
|
)
|
22,660
|
|
||||
|
Net income (loss)
|
(176,096
|
)
|
(199,318
|
)
|
228,452
|
|
188,141
|
|
||||
|
Net loss (income) attributable to noncontrolling interest (Alios)
|
—
|
|
25,249
|
|
(7,342
|
)
|
(29,512
|
)
|
||||
|
Net income (loss) attributable to Vertex
|
$
|
(176,096
|
)
|
$
|
(174,069
|
)
|
$
|
221,110
|
|
$
|
158,629
|
|
|
Net income (loss) per share attributable to Vertex common shareholders:
|
||||||||||||
|
Basic
|
$
|
(0.87
|
)
|
$
|
(0.85
|
)
|
$
|
1.06
|
|
$
|
0.76
|
|
|
Diluted
|
$
|
(0.87
|
)
|
$
|
(0.85
|
)
|
$
|
1.02
|
|
$
|
0.74
|
|
|
Shares used in per share calculations:
|
||||||||||||
|
Basic
|
202,329
|
|
204,413
|
|
206,002
|
|
206,758
|
|
||||
|
Diluted
|
202,329
|
|
204,413
|
|
219,349
|
|
217,602
|
|
||||
|
(1)
|
During the second quarter of 2012, the Company recorded within cost of product revenues a lower of cost or market charge of
$78.0 million
for excess and obsolete INCIVEK inventories. This charge affected net income (loss) attributable to Vertex per diluted share, net of tax, by
$(0.36)
for the second quarter of 2012, resulting in a net loss attributable to Vertex per diluted share in the second quarter of 2012. See
Note F, "Inventories,"
for further information.
|
|
(2)
|
During the fourth quarter of 2012, the Company recorded within cost of product revenues a lower of cost or market charge of
$55.2 million
for excess and obsolete INCIVEK inventories. This charge resulted in a
$0.25
increase in the net loss attributable to Vertex per diluted share for the fourth quarter of 2012. See
Note F, "Inventories,"
for further information.
|
|
(3)
|
During the third quarter of 2011, the Company recorded an impairment charge of
$105.8 million
. In connection with this impairment charge, the Company recorded a benefit from income taxes of
$32.7 million
resulting in a net decrease in net income attributable to Vertex related to this impairment charge of
$73.1 million
in the third quarter of 2011.
|