|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
FOR THE QUARTERLY PERIOD ENDED MARCH 31, 2019
|
|
|
or
|
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
FOR THE TRANSITION PERIOD FROM
TO
|
|
|
Massachusetts
|
04-3039129
|
|
(State or other jurisdiction of
incorporation or organization) |
(I.R.S. Employer
Identification No.) |
|
50 Northern Avenue, Boston, Massachusetts
|
02210
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Large accelerated filer
x
|
Accelerated filer
o
|
Non-accelerated filer
o
|
Smaller reporting company
o
|
|
Emerging growth company
o
|
|
||
|
Common Stock, par value $0.01 per share
|
256,121,360
|
|
Class
|
Outstanding at April 24, 2019
|
|
Page
|
||
|
Condensed Consolidated Statements of Operations - Three Months Ended March 31, 2019 and 20
18
|
||
|
Condensed Consolidated Statements of Comprehensive Income - Three Months Ended March 31, 2019 and 20
18
|
||
|
Condensed Consolidated Balance Sheets - March 31, 2019 and
December 31, 2018
|
||
|
Condensed Consolidated Statements of Shareholders' Equity and Noncontrolling Interest - Three Months Ended
March 31, 2019 and 2018
|
||
|
Condensed Consolidated Statements of Cash Flows - Three Months Ended
March 31, 2019 and 2018
|
||
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Revenues:
|
|||||||
|
Product revenues, net
|
$
|
857,253
|
|
$
|
637,729
|
|
|
|
Collaborative and royalty revenues
|
1,182
|
|
3,070
|
|
|||
|
Total revenues
|
858,435
|
|
640,799
|
|
|||
|
Costs and expenses:
|
|||||||
|
Cost of sales
|
95,092
|
|
71,613
|
|
|||
|
Research and development expenses
|
339,490
|
|
310,553
|
|
|||
|
Sales, general and administrative expenses
|
147,045
|
|
129,808
|
|
|||
|
Restructuring income
|
—
|
|
(76
|
)
|
|||
|
Total costs and expenses
|
581,627
|
|
511,898
|
|
|||
|
Income from operations
|
276,808
|
|
128,901
|
|
|||
|
Interest income
|
15,615
|
|
5,789
|
|
|||
|
Interest expense
|
(14,868
|
)
|
(16,886
|
)
|
|||
|
Other income, net
|
42,610
|
|
96,838
|
|
|||
|
Income before provision for (benefit from) income taxes
|
320,165
|
|
214,642
|
|
|||
|
Provision for (benefit from) income taxes
|
51,534
|
|
(12,659
|
)
|
|||
|
Net income
|
268,631
|
|
227,301
|
|
|||
|
Income attributable to noncontrolling interest
|
—
|
|
(17,038
|
)
|
|||
|
Net income attributable to Vertex
|
$
|
268,631
|
|
$
|
210,263
|
|
|
|
Amounts per share attributable to Vertex common shareholders:
|
|||||||
|
Net income:
|
|||||||
|
Basic
|
$
|
1.05
|
|
$
|
0.83
|
|
|
|
Diluted
|
$
|
1.03
|
|
$
|
0.81
|
|
|
|
Shares used in per share calculations:
|
|||||||
|
Basic
|
255,695
|
|
253,231
|
|
|||
|
Diluted
|
260,175
|
|
258,526
|
|
|||
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Net income
|
$
|
268,631
|
|
$
|
227,301
|
|
|
|
Changes in other comprehensive income (loss):
|
|||||||
|
Unrealized holding gains (losses) on marketable securities, net
|
596
|
|
(460
|
)
|
|||
|
Unrealized losses on foreign currency forward contracts, net of tax of $1.5 million and $0.3 million, respectively
|
(222
|
)
|
(862
|
)
|
|||
|
Foreign currency translation adjustment
|
4,967
|
|
(2,729
|
)
|
|||
|
Total changes in other comprehensive income (loss)
|
5,341
|
|
(4,051
|
)
|
|||
|
Comprehensive income
|
273,972
|
|
223,250
|
|
|||
|
Comprehensive income attributable to noncontrolling interest
|
—
|
|
(17,038
|
)
|
|||
|
Comprehensive income attributable to Vertex
|
$
|
273,972
|
|
$
|
206,212
|
|
|
|
March 31,
|
December 31,
|
||||||
|
2019
|
2018
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
2,893,885
|
|
$
|
2,650,134
|
|
|
|
Marketable securities
|
584,150
|
|
518,108
|
|
|||
|
Accounts receivable, net
|
438,297
|
|
409,688
|
|
|||
|
Inventories
|
136,698
|
|
124,360
|
|
|||
|
Prepaid expenses and other current assets
|
130,009
|
|
140,819
|
|
|||
|
Total current assets
|
4,183,039
|
|
3,843,109
|
|
|||
|
Property and equipment, net
|
742,559
|
|
812,005
|
|
|||
|
Goodwill
|
50,384
|
|
50,384
|
|
|||
|
Deferred tax assets
|
1,467,518
|
|
1,499,672
|
|
|||
|
Operating lease assets
|
60,573
|
|
—
|
|
|||
|
Other assets
|
39,041
|
|
40,728
|
|
|||
|
Total assets
|
$
|
6,543,114
|
|
$
|
6,245,898
|
|
|
|
Liabilities and Shareholders’ Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
82,262
|
|
$
|
110,987
|
|
|
|
Accrued expenses
|
532,745
|
|
604,495
|
|
|||
|
Early access sales accrual
|
382,703
|
|
354,404
|
|
|||
|
Other current liabilities
|
108,758
|
|
50,406
|
|
|||
|
Total current liabilities
|
1,106,468
|
|
1,120,292
|
|
|||
|
Long-term finance lease liabilities
|
560,381
|
|
581,550
|
|
|||
|
Long-term operating lease liabilities
|
63,484
|
|
—
|
|
|||
|
Long-term advance from collaborator
|
83,471
|
|
82,573
|
|
|||
|
Other long-term liabilities
|
5,997
|
|
26,280
|
|
|||
|
Total liabilities
|
1,819,801
|
|
1,810,695
|
|
|||
|
Commitments and contingencies
|
—
|
|
—
|
|
|||
|
Shareholders’ equity:
|
|||||||
|
Preferred stock, $0.01 par value; 1,000 shares authorized; none issued and outstanding
|
—
|
|
—
|
|
|||
|
Common stock, $0.01 par value; 500,000 shares authorized, 256,351 and 255,172 shares issued and outstanding, respectively
|
2,561
|
|
2,546
|
|
|||
|
Additional paid-in capital
|
7,475,909
|
|
7,421,476
|
|
|||
|
Accumulated other comprehensive income
|
6,000
|
|
659
|
|
|||
|
Accumulated deficit
|
(2,761,157
|
)
|
(2,989,478
|
)
|
|||
|
Total shareholders’ equity
|
4,723,313
|
|
4,435,203
|
|
|||
|
Total liabilities and shareholders’ equity
|
$
|
6,543,114
|
|
$
|
6,245,898
|
|
|
|
Common Stock
|
Additional
Paid-in Capital |
Accumulated
Other Comprehensive (Loss) Income |
Accumulated Deficit
|
Total Vertex
Shareholders’ Equity |
Noncontrolling
Interest |
Total
Shareholders’ Equity |
||||||||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||
|
Balance at December 31, 2017
|
253,253
|
|
$
|
2,512
|
|
$
|
7,157,362
|
|
$
|
(11,572
|
)
|
$
|
(5,119,723
|
)
|
$
|
2,028,579
|
|
$
|
13,727
|
|
$
|
2,042,306
|
|
|||||||
|
Cumulative effect adjustment for adoption of new accounting guidance
|
—
|
|
—
|
|
—
|
|
(24,120
|
)
|
33,349
|
|
9,229
|
|
—
|
|
9,229
|
|
||||||||||||||
|
Other comprehensive loss, net of tax
|
—
|
|
—
|
|
—
|
|
(4,051
|
)
|
—
|
|
(4,051
|
)
|
—
|
|
(4,051
|
)
|
||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
210,263
|
|
210,263
|
|
17,038
|
|
227,301
|
|
||||||||||||||
|
Repurchase of common stock
|
(67
|
)
|
(1
|
)
|
(11,250
|
)
|
—
|
|
—
|
|
(11,251
|
)
|
—
|
|
(11,251
|
)
|
||||||||||||||
|
Issuance of common stock under benefit plans
|
1,682
|
|
30
|
|
89,656
|
|
—
|
|
—
|
|
89,686
|
|
—
|
|
89,686
|
|
||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
78,601
|
|
—
|
|
—
|
|
78,601
|
|
—
|
|
78,601
|
|
||||||||||||||
|
Other VIE activity
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,000
|
)
|
(1,000
|
)
|
||||||||||||||
|
Balance at March 31, 2018
|
254,868
|
|
$
|
2,541
|
|
$
|
7,314,369
|
|
$
|
(39,743
|
)
|
$
|
(4,876,111
|
)
|
$
|
2,401,056
|
|
$
|
29,765
|
|
$
|
2,430,821
|
|
|||||||
|
Balance at December 31, 2018
|
255,172
|
|
$
|
2,546
|
|
$
|
7,421,476
|
|
$
|
659
|
|
$
|
(2,989,478
|
)
|
$
|
4,435,203
|
|
$
|
—
|
|
$
|
4,435,203
|
|
|||||||
|
Cumulative effect adjustment for adoption of new accounting guidance
|
—
|
|
—
|
|
—
|
|
—
|
|
(40,310
|
)
|
(40,310
|
)
|
—
|
|
(40,310
|
)
|
||||||||||||||
|
Other comprehensive income, net of tax
|
—
|
|
—
|
|
—
|
|
5,341
|
|
—
|
|
5,341
|
|
—
|
|
5,341
|
|
||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
268,631
|
|
268,631
|
|
—
|
|
268,631
|
|
||||||||||||||
|
Repurchases of common stock
|
(564
|
)
|
(6
|
)
|
(103,833
|
)
|
—
|
|
—
|
|
(103,839
|
)
|
—
|
|
(103,839
|
)
|
||||||||||||||
|
Issuance of common stock under benefit plans
|
1,743
|
|
21
|
|
64,023
|
|
—
|
|
—
|
|
64,044
|
|
—
|
|
64,044
|
|
||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
94,243
|
|
—
|
|
—
|
|
94,243
|
|
—
|
|
94,243
|
|
||||||||||||||
|
Balance at March 31, 2019
|
256,351
|
|
$
|
2,561
|
|
$
|
7,475,909
|
|
$
|
6,000
|
|
$
|
(2,761,157
|
)
|
$
|
4,723,313
|
|
$
|
—
|
|
$
|
4,723,313
|
|
|||||||
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Cash flows from operating activities:
|
|||||||
|
Net income
|
$
|
268,631
|
|
$
|
227,301
|
|
|
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|||||||
|
Stock-based compensation expense
|
93,791
|
|
78,136
|
|
|||
|
Depreciation expense
|
27,140
|
|
16,343
|
|
|||
|
Write-downs of inventories to net realizable value
|
1,270
|
|
3,619
|
|
|||
|
Deferred income taxes
|
43,425
|
|
3,587
|
|
|||
|
Unrealized gain on equity securities
|
(43,551
|
)
|
(95,458
|
)
|
|||
|
Other non-cash items, net
|
(3,701
|
)
|
5,827
|
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Accounts receivable, net
|
(30,136
|
)
|
(13,473
|
)
|
|||
|
Inventories
|
(13,139
|
)
|
(8,208
|
)
|
|||
|
Prepaid expenses and other assets
|
7,941
|
|
25,482
|
|
|||
|
Accounts payable
|
(24,145
|
)
|
2,154
|
|
|||
|
Accrued expenses and other liabilities
|
(38,425
|
)
|
(31,469
|
)
|
|||
|
Early access sales accrual
|
35,683
|
|
38,816
|
|
|||
|
Net cash provided by operating activities
|
324,784
|
|
252,657
|
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Purchases of available-for-sale debt securities
|
(128,215
|
)
|
(38,653
|
)
|
|||
|
Maturities of available-for-sale debt securities
|
107,118
|
|
94,365
|
|
|||
|
Expenditures for property and equipment
|
(18,041
|
)
|
(29,279
|
)
|
|||
|
Investment in equity securities
|
—
|
|
(21,500
|
)
|
|||
|
Net cash (used in) provided by investing activities
|
(39,138
|
)
|
4,933
|
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Issuances of common stock under benefit plans
|
63,620
|
|
88,403
|
|
|||
|
Repurchase of common stock
|
(99,839
|
)
|
(10,000
|
)
|
|||
|
Advance from collaborator
|
5,000
|
|
2,500
|
|
|||
|
Payments on capital lease and construction financing lease obligations
|
—
|
|
(9,331
|
)
|
|||
|
Payments on finance leases
|
(9,385
|
)
|
—
|
|
|||
|
Proceeds related to construction financing lease obligation
|
—
|
|
9,566
|
|
|||
|
Repayments of advanced funding
|
(1,385
|
)
|
(1,182
|
)
|
|||
|
Other financing activities
|
—
|
|
(1,000
|
)
|
|||
|
Net cash (used in) provided by financing activities
|
(41,989
|
)
|
78,956
|
|
|||
|
Effect of changes in exchange rates on cash
|
(378
|
)
|
1,656
|
|
|||
|
Net increase in cash and cash equivalents
|
243,279
|
|
338,202
|
|
|||
|
Cash, cash equivalents and restricted cash—beginning of period
|
2,658,253
|
|
1,667,526
|
|
|||
|
Cash, cash equivalents and restricted cash—end of period
|
$
|
2,901,532
|
|
$
|
2,005,728
|
|
|
|
Supplemental disclosure of cash flow information:
|
|||||||
|
Cash paid for interest
|
$
|
13,148
|
|
$
|
16,825
|
|
|
|
Cash paid for income taxes
|
$
|
1,835
|
|
$
|
1,897
|
|
|
|
Capitalization of costs related to construction financing lease obligation
|
$
|
—
|
|
$
|
3,716
|
|
|
|
Issuances of common stock from employee benefit plans receivable
|
$
|
510
|
|
$
|
2,124
|
|
|
|
Accrued share repurchase liability
|
$
|
4,000
|
|
$
|
—
|
|
|
|
Balance as of
|
Balance as of
|
||||||||||
|
December 31, 2018 ^
|
Adjustments
|
January 1, 2019
|
|||||||||
|
Assets
|
(in thousands)
|
||||||||||
|
Prepaid expenses and other current assets
|
$
|
140,819
|
|
$
|
(2,930
|
)
|
$
|
137,889
|
|
||
|
Property and equipment, net
|
812,005
|
|
(53,920
|
)
|
758,085
|
|
|||||
|
Deferred tax assets
|
1,499,672
|
|
11,236
|
|
1,510,908
|
|
|||||
|
Operating lease assets
|
—
|
|
61,674
|
|
61,674
|
|
|||||
|
Total assets
|
$
|
6,245,898
|
|
$
|
16,060
|
|
$
|
6,261,958
|
|
||
|
Liabilities and Shareholders’ Equity
|
|||||||||||
|
Capital lease obligations, current portion
|
$
|
9,817
|
|
$
|
(9,817
|
)
|
$
|
—
|
|
||
|
Other current liabilities
|
40,589
|
|
34,304
|
|
74,893
|
|
|||||
|
Capital lease obligations, excluding current portion
|
19,658
|
|
(19,658
|
)
|
—
|
|
|||||
|
Construction financing lease obligation, excluding current portion
|
561,892
|
|
(561,892
|
)
|
—
|
|
|||||
|
Long-term finance lease liabilities
|
—
|
|
569,487
|
|
569,487
|
|
|||||
|
Long-term operating lease liabilities
|
—
|
|
64,849
|
|
64,849
|
|
|||||
|
Other long-term liabilities
|
26,280
|
|
(20,903
|
)
|
5,377
|
|
|||||
|
Accumulated deficit
|
(2,989,478
|
)
|
(40,310
|
)
|
(3,029,788
|
)
|
|||||
|
Total liabilities and shareholders’ equity
|
$
|
6,245,898
|
|
$
|
16,060
|
|
$
|
6,261,958
|
|
||
|
^ As reported in the Company’s 2018 Annual Report on Form 10-K.
|
|||||||||||
|
B.
|
Revenue Recognition
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
SYMDEKO/SYMKEVI
|
$
|
320,275
|
|
$
|
34,124
|
|
|
|
ORKAMBI
|
293,007
|
|
354,066
|
|
|||
|
KALYDECO
|
243,971
|
|
249,539
|
|
|||
|
Total product revenues, net
|
$
|
857,253
|
|
$
|
637,729
|
|
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
United States
|
$
|
641,104
|
|
$
|
482,667
|
|
|
|
Outside of the United States
|
|||||||
|
Europe
|
167,751
|
|
131,895
|
|
|||
|
Other
|
49,580
|
|
26,237
|
|
|||
|
Total revenues outside of the United States
|
217,331
|
|
158,132
|
|
|||
|
Total revenues
|
$
|
858,435
|
|
$
|
640,799
|
|
|
|
C.
|
Collaborative Arrangements and Acquisitions
|
|
D.
|
Earnings Per Share
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands, except per share amounts)
|
|||||||
|
Basic net income attributable to Vertex per common share calculation:
|
|||||||
|
Net income attributable to Vertex common shareholders
|
$
|
268,631
|
|
$
|
210,263
|
|
|
|
Less: Undistributed earnings allocated to participating securities
|
—
|
|
(99
|
)
|
|||
|
Net income attributable to Vertex common shareholders—basic
|
$
|
268,631
|
|
$
|
210,164
|
|
|
|
Basic weighted-average common shares outstanding
|
255,695
|
|
253,231
|
|
|||
|
Basic net income attributable to Vertex per common share
|
$
|
1.05
|
|
$
|
0.83
|
|
|
|
Diluted net income attributable to Vertex per common share calculation:
|
|||||||
|
Net income attributable to Vertex common shareholders
|
$
|
268,631
|
|
$
|
210,263
|
|
|
|
Less: Undistributed earnings allocated to participating securities
|
—
|
|
(97
|
)
|
|||
|
Net income attributable to Vertex common shareholders—diluted
|
$
|
268,631
|
|
$
|
210,166
|
|
|
|
Weighted-average shares used to compute basic net income per common share
|
255,695
|
|
253,231
|
|
|||
|
Effect of potentially dilutive securities:
|
|||||||
|
Stock options
|
2,585
|
|
3,248
|
|
|||
|
Restricted stock and restricted stock units (including PSUs)
|
1,870
|
|
2,013
|
|
|||
|
Employee stock purchase program
|
25
|
|
34
|
|
|||
|
Weighted-average shares used to compute diluted net income per common share
|
260,175
|
|
258,526
|
|
|||
|
Diluted net income attributable to Vertex per common share
|
$
|
1.03
|
|
$
|
0.81
|
|
|
|
Three Months Ended March 31,
|
|||||
|
2019
|
2018
|
||||
|
(in thousands)
|
|||||
|
Stock options
|
2,837
|
|
1,633
|
|
|
|
Unvested restricted stock and restricted stock units (including PSUs)
|
6
|
|
4
|
|
|
|
E.
|
Fair Value Measurements
|
|
Level 1:
|
Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis.
|
|
Level 2:
|
Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active.
|
|
Level 3:
|
Unobservable inputs based on the Company’s assessment of the assumptions that market participants would use in pricing the asset or liability.
|
|
Fair Value Measurements as of March 31, 2019
|
|||||||||||||||
|
Fair Value Hierarchy
|
|||||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Financial instruments carried at fair value (asset positions):
|
|||||||||||||||
|
Cash equivalents:
|
|||||||||||||||
|
Money market funds
|
$
|
1,354,656
|
|
$
|
1,354,656
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
U.S. Treasury securities
|
5,996
|
|
5,996
|
|
—
|
|
—
|
|
|||||||
|
Government-sponsored enterprise securities
|
11,786
|
|
11,786
|
|
—
|
|
—
|
|
|||||||
|
Corporate debt securities
|
4,519
|
|
—
|
|
4,519
|
|
—
|
|
|||||||
|
Commercial paper
|
35,955
|
|
—
|
|
35,955
|
|
—
|
|
|||||||
|
Marketable securities:
|
|||||||||||||||
|
Corporate equity securities
|
210,874
|
|
192,207
|
|
18,667
|
|
—
|
|
|||||||
|
Government-sponsored enterprise securities
|
15,181
|
|
15,181
|
|
—
|
|
—
|
|
|||||||
|
Corporate debt securities
|
231,572
|
|
—
|
|
231,572
|
|
—
|
|
|||||||
|
Commercial paper
|
126,523
|
|
—
|
|
126,523
|
|
—
|
|
|||||||
|
Prepaid expenses and other current assets:
|
|||||||||||||||
|
Foreign currency forward contracts
|
19,210
|
|
—
|
|
19,210
|
|
—
|
|
|||||||
|
Other assets:
|
|||||||||||||||
|
Foreign currency forward contracts
|
973
|
|
—
|
|
973
|
|
—
|
|
|||||||
|
Total financial assets
|
$
|
2,017,245
|
|
|
$
|
1,579,826
|
|
$
|
437,419
|
|
$
|
—
|
|
||
|
Financial instruments carried at fair value (liability positions):
|
|||||||||||||||
|
Other current liabilities:
|
|||||||||||||||
|
Foreign currency forward contracts
|
$
|
(310
|
)
|
$
|
—
|
|
$
|
(310
|
)
|
$
|
—
|
|
|||
|
Other long-term liabilities:
|
|||||||||||||||
|
Foreign currency forward contracts
|
(68
|
)
|
—
|
|
(68
|
)
|
—
|
|
|||||||
|
Total financial liabilities
|
$
|
(378
|
)
|
$
|
—
|
|
$
|
(378
|
)
|
$
|
—
|
|
|||
|
Fair Value Measurements as of December 31, 2018
|
|||||||||||||||
|
Fair Value Hierarchy
|
|||||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Financial instruments carried at fair value (asset positions):
|
|||||||||||||||
|
Cash equivalents:
|
|||||||||||||||
|
Money market funds
|
$
|
1,226,603
|
|
$
|
1,226,603
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
U.S. Treasury securities
|
5,966
|
|
5,966
|
|
—
|
|
—
|
|
|||||||
|
Government-sponsored enterprise securities
|
7,123
|
|
7,123
|
|
—
|
|
—
|
|
|||||||
|
Commercial paper
|
58,268
|
|
—
|
|
58,268
|
|
—
|
|
|||||||
|
Marketable securities:
|
|||||||||||||||
|
Corporate equity securities
|
167,323
|
|
153,733
|
|
13,590
|
|
—
|
|
|||||||
|
U.S. Treasury securities
|
6,026
|
|
6,026
|
|
—
|
|
—
|
|
|||||||
|
Government-sponsored enterprise securities
|
10,704
|
|
10,704
|
|
—
|
|
—
|
|
|||||||
|
Corporate debt securities
|
233,665
|
|
—
|
|
233,665
|
|
—
|
|
|||||||
|
Commercial paper
|
100,390
|
|
—
|
|
100,390
|
|
—
|
|
|||||||
|
Prepaid expenses and other current assets:
|
|||||||||||||||
|
Foreign currency forward contracts
|
19,023
|
|
—
|
|
19,023
|
|
—
|
|
|||||||
|
Other assets:
|
|||||||||||||||
|
Foreign currency forward contracts
|
1,514
|
|
—
|
|
1,514
|
|
—
|
|
|||||||
|
Total financial assets
|
$
|
1,836,605
|
|
$
|
1,410,155
|
|
$
|
426,450
|
|
$
|
—
|
|
|||
|
Financial instruments carried at fair value (liability positions):
|
|||||||||||||||
|
Other current liabilities:
|
|||||||||||||||
|
Foreign currency forward contracts
|
$
|
(340
|
)
|
$
|
—
|
|
$
|
(340
|
)
|
$
|
—
|
|
|||
|
Other long-term liabilities:
|
|||||||||||||||
|
Foreign currency forward contracts
|
(108
|
)
|
—
|
|
(108
|
)
|
—
|
|
|||||||
|
Total financial liabilities
|
$
|
(448
|
)
|
$
|
—
|
|
$
|
(448
|
)
|
$
|
—
|
|
|||
|
F.
|
Marketable Securities and Equity Investments
|
|
Amortized Cost
|
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Fair Value
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
As of March 31, 2019
|
|||||||||||||||
|
Cash equivalents:
|
|||||||||||||||
|
Money market funds
|
$
|
1,354,656
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,354,656
|
|
|||
|
U.S. Treasury securities
|
5,996
|
|
—
|
|
—
|
|
5,996
|
|
|||||||
|
Government-sponsored enterprise securities
|
11,787
|
|
—
|
|
(1
|
)
|
11,786
|
|
|||||||
|
Corporate debt securities
|
4,519
|
|
—
|
|
—
|
|
4,519
|
|
|||||||
|
Commercial paper
|
35,959
|
|
—
|
|
(4
|
)
|
35,955
|
|
|||||||
|
Total cash equivalents
|
1,412,917
|
|
—
|
|
(5
|
)
|
1,412,912
|
|
|||||||
|
Marketable securities:
|
|||||||||||||||
|
Government-sponsored enterprise securities
|
15,180
|
|
2
|
|
(1
|
)
|
15,181
|
|
|||||||
|
Corporate debt securities
|
231,516
|
|
88
|
|
(32
|
)
|
231,572
|
|
|||||||
|
Commercial paper
|
126,515
|
|
34
|
|
(26
|
)
|
126,523
|
|
|||||||
|
Total marketable debt securities
|
373,211
|
|
124
|
|
(59
|
)
|
373,276
|
|
|||||||
|
Corporate equity securities
|
133,157
|
|
79,093
|
|
(1,376
|
)
|
210,874
|
|
|||||||
|
Total marketable securities
|
$
|
506,368
|
|
$
|
79,217
|
|
$
|
(1,435
|
)
|
$
|
584,150
|
|
|||
|
As of December 31, 2018
|
|||||||||||||||
|
Cash equivalents:
|
|||||||||||||||
|
Money market funds
|
$
|
1,226,603
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,226,603
|
|
|||
|
U.S. Treasury securities
|
5,967
|
|
—
|
|
(1
|
)
|
5,966
|
|
|||||||
|
Government-sponsored enterprise securities
|
7,124
|
|
—
|
|
(1
|
)
|
7,123
|
|
|||||||
|
Commercial paper
|
58,271
|
|
—
|
|
(3
|
)
|
58,268
|
|
|||||||
|
Total cash equivalents
|
1,297,965
|
|
—
|
|
(5
|
)
|
1,297,960
|
|
|||||||
|
Marketable securities:
|
|||||||||||||||
|
U.S. Treasury securities
|
6,026
|
|
—
|
|
—
|
|
6,026
|
|
|||||||
|
Government-sponsored enterprise securities
|
10,704
|
|
—
|
|
—
|
|
10,704
|
|
|||||||
|
Corporate debt securities
|
234,088
|
|
|
27
|
|
(450
|
)
|
233,665
|
|
||||||
|
Commercial paper
|
100,498
|
|
—
|
|
(108
|
)
|
100,390
|
|
|||||||
|
Total marketable debt securities
|
351,316
|
|
27
|
|
(558
|
)
|
350,785
|
|
|||||||
|
Corporate equity securities
|
133,157
|
|
40,619
|
|
(6,453
|
)
|
167,323
|
|
|||||||
|
Total marketable securities
|
$
|
484,473
|
|
$
|
40,646
|
|
$
|
(7,011
|
)
|
$
|
518,108
|
|
|||
|
As of March 31, 2019
|
As of December 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Cash and cash equivalents
|
$
|
1,412,912
|
|
$
|
1,297,960
|
|
|
|
Marketable securities
|
373,276
|
|
350,785
|
|
|||
|
Total
|
$
|
1,786,188
|
|
$
|
1,648,745
|
|
|
|
As of March 31, 2019
|
As of December 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Matures within one year
|
$
|
1,775,571
|
|
$
|
1,647,500
|
|
|
|
Matures after one year through five years
|
10,617
|
|
1,245
|
|
|||
|
Total
|
$
|
1,786,188
|
|
$
|
1,648,745
|
|
|
|
G.
|
Accumulated Other Comprehensive Income (Loss)
|
|
Unrealized Holding Gains (Losses), Net of Tax
|
|||||||||||||||
|
Foreign Currency Translation Adjustment
|
On Available-For-Sale Debt Securities
|
On Foreign Currency Forward Contracts
|
Total
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Balance at December 31, 2018
|
$
|
(11,227
|
)
|
$
|
(536
|
)
|
$
|
12,422
|
|
$
|
659
|
|
|||
|
Other comprehensive income before reclassifications
|
4,967
|
|
596
|
|
5,126
|
|
10,689
|
|
|||||||
|
Amounts reclassified from accumulated other comprehensive income
|
—
|
|
—
|
|
(5,348
|
)
|
(5,348
|
)
|
|||||||
|
Net current period other comprehensive income (loss)
|
4,967
|
|
596
|
|
(222
|
)
|
5,341
|
|
|||||||
|
Balance at March 31, 2019
|
$
|
(6,260
|
)
|
$
|
60
|
|
$
|
12,200
|
|
$
|
6,000
|
|
|||
|
Unrealized Holding Gains (Losses), Net of Tax
|
|||||||||||||||||||
|
Foreign Currency Translation Adjustment
|
On Available-For-Sale Debt Securities
|
On Equity Securities
|
On Foreign Currency Forward Contracts
|
Total
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Balance at December 31, 2017
|
$
|
(21,031
|
)
|
$
|
(594
|
)
|
$
|
25,069
|
|
$
|
(15,016
|
)
|
$
|
(11,572
|
)
|
||||
|
Other comprehensive loss before reclassifications
|
(2,729
|
)
|
(460
|
)
|
—
|
|
(7,639
|
)
|
(10,828
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
—
|
|
—
|
|
—
|
|
6,777
|
|
6,777
|
|
|||||||||
|
Net current period other comprehensive loss
|
(2,729
|
)
|
(460
|
)
|
—
|
|
(862
|
)
|
(4,051
|
)
|
|||||||||
|
Amounts reclassified to accumulated deficit pursuant to adoption of new accounting standard
|
949
|
|
—
|
|
(25,069
|
)
|
—
|
|
(24,120
|
)
|
|||||||||
|
Balance at March 31, 2018
|
$
|
(22,811
|
)
|
$
|
(1,054
|
)
|
$
|
—
|
|
$
|
(15,878
|
)
|
$
|
(39,743
|
)
|
||||
|
H.
|
Hedging
|
|
As of March 31, 2019
|
As of December 31, 2018
|
||||||
|
Foreign Currency
|
(in thousands)
|
||||||
|
Euro
|
$
|
373,264
|
|
$
|
335,179
|
|
|
|
British pound sterling
|
76,685
|
|
73,460
|
|
|||
|
Australian dollar
|
70,889
|
|
52,820
|
|
|||
|
Canadian dollar
|
40,089
|
|
43,759
|
|
|||
|
Total foreign currency forward contracts
|
$
|
560,927
|
|
$
|
505,218
|
|
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Designated as hedging instruments - Reclassified from AOCI
|
|||||||
|
Product revenues, net
|
$
|
6,839
|
|
$
|
(6,485
|
)
|
|
|
Not designated as hedging instruments
|
|||||||
|
Other income, net
|
$
|
3,151
|
|
$
|
1,539
|
|
|
|
As of March 31, 2019
|
||||||||||
|
Assets
|
Liabilities
|
|||||||||
|
Classification
|
Fair Value
|
Classification
|
Fair Value
|
|||||||
|
(in thousands)
|
||||||||||
|
Prepaid expenses and other current assets
|
$
|
19,210
|
|
Other current liabilities
|
$
|
(310
|
)
|
|||
|
Other assets
|
973
|
|
Other long-term liabilities
|
(68
|
)
|
|||||
|
Total assets
|
$
|
20,183
|
|
Total liabilities
|
$
|
(378
|
)
|
|||
|
As of December 31, 2018
|
||||||||||
|
Assets
|
Liabilities
|
|||||||||
|
Classification
|
Fair Value
|
Classification
|
Fair Value
|
|||||||
|
(in thousands)
|
||||||||||
|
Prepaid expenses and other current assets
|
$
|
19,023
|
|
Other current liabilities
|
$
|
(340
|
)
|
|||
|
Other assets
|
1,514
|
|
Other long-term liabilities
|
(108
|
)
|
|||||
|
Total assets
|
$
|
20,537
|
|
Total liabilities
|
$
|
(448
|
)
|
|||
|
As of March 31, 2019
|
|||||||||||||||||||
|
Gross Amounts Recognized
|
Gross Amounts Offset
|
Gross Amounts Presented
|
Gross Amounts Not Offset
|
Legal Offset
|
|||||||||||||||
|
Foreign currency forward contracts
|
(in thousands)
|
||||||||||||||||||
|
Total assets
|
$
|
20,183
|
|
$
|
—
|
|
$
|
20,183
|
|
$
|
(378
|
)
|
$
|
19,805
|
|
||||
|
Total liabilities
|
$
|
(378
|
)
|
$
|
—
|
|
$
|
(378
|
)
|
$
|
378
|
|
$
|
—
|
|
||||
|
As of December 31, 2018
|
|||||||||||||||||||
|
Gross Amounts Recognized
|
Gross Amounts Offset
|
Gross Amounts Presented
|
Gross Amounts Not Offset
|
Legal Offset
|
|||||||||||||||
|
Foreign currency forward contracts
|
(in thousands)
|
||||||||||||||||||
|
Total assets
|
$
|
20,537
|
|
$
|
—
|
|
$
|
20,537
|
|
$
|
(448
|
)
|
$
|
20,089
|
|
||||
|
Total liabilities
|
$
|
(448
|
)
|
$
|
—
|
|
$
|
(448
|
)
|
$
|
448
|
|
$
|
—
|
|
||||
|
As of March 31, 2019
|
As of December 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Raw materials
|
$
|
11,649
|
|
$
|
9,677
|
|
|
|
Work-in-process
|
80,529
|
|
87,944
|
|
|||
|
Finished goods
|
44,520
|
|
26,739
|
|
|||
|
Total
|
$
|
136,698
|
|
$
|
124,360
|
|
|
|
Three Months Ended March 31, 2019
|
|||
|
(in thousands)
|
|||
|
Operating lease cost
|
$
|
2,639
|
|
|
Finance lease cost
|
|||
|
Amortization of leased assets
|
12,365
|
|
|
|
Interest on lease liabilities
|
13,449
|
|
|
|
Variable lease cost
|
6,762
|
|
|
|
Sublease income
|
(1,484
|
)
|
|
|
Net lease cost
|
$
|
33,731
|
|
|
As of March 31, 2019
|
As of December 31, 2018 ^
|
||||||
|
(in thousands)
|
|||||||
|
Finance leases
|
|||||||
|
Property and equipment, net
|
$
|
473,129
|
|
$
|
640,952
|
|
|
|
Total finance lease assets
|
$
|
473,129
|
|
$
|
640,952
|
|
|
|
Capital lease obligations, current portion
|
$
|
—
|
|
$
|
9,817
|
|
|
|
Other current liabilities
|
35,725
|
|
5,271
|
|
|||
|
Capital lease obligations, excluding current portion
|
—
|
|
19,658
|
|
|||
|
Construction financing lease obligation, excluding current portion
|
—
|
|
561,892
|
|
|||
|
Long-term finance lease liabilities
|
560,381
|
|
—
|
|
|||
|
Total finance lease liabilities
|
$
|
596,106
|
|
$
|
596,638
|
|
|
|
Operating leases
|
|||||||
|
Operating lease assets
|
$
|
60,573
|
|
$
|
—
|
|
|
|
Total operating lease assets
|
$
|
60,573
|
|
$
|
—
|
|
|
|
Other current liabilities
|
$
|
7,520
|
|
$
|
—
|
|
|
|
Long-term operating lease liabilities
|
63,484
|
|
—
|
|
|||
|
Total operating lease liabilities
|
$
|
71,004
|
|
$
|
—
|
|
|
|
^ As reported in the Company’s 2018 Annual Report on Form 10-K.
|
|||||||
|
Year
|
Finance Leases
|
Operating Leases
|
Total
|
|||||||||
|
(in thousands)
|
||||||||||||
|
Remainder of 2019
|
$
|
61,339
|
|
$
|
7,331
|
|
$
|
68,670
|
|
|||
|
2020
|
88,998
|
|
10,366
|
|
99,364
|
|
||||||
|
2021
|
87,365
|
|
8,658
|
|
96,023
|
|
||||||
|
2022
|
85,016
|
|
8,233
|
|
93,249
|
|
||||||
|
2023
|
84,092
|
|
8,151
|
|
92,243
|
|
||||||
|
Thereafter
|
512,804
|
|
46,212
|
|
559,016
|
|
||||||
|
Total lease payments
|
919,614
|
|
88,951
|
|
1,008,565
|
|
||||||
|
Less: amount representing interest
|
(323,508
|
)
|
(17,947
|
)
|
(341,455
|
)
|
||||||
|
Present value of lease liabilities
|
$
|
596,106
|
|
$
|
71,004
|
|
$
|
667,110
|
|
|||
|
Three Months Ended March 31, 2019
|
||
|
Weighted-average remaining lease term (in years)
|
||
|
Finance leases
|
10.45
|
|
|
Operating leases
|
10.91
|
|
|
Weighted-average discount rate
|
||
|
Finance leases
|
9.11
|
%
|
|
Operating leases
|
4.05
|
%
|
|
Year
|
Fan Pier
Leases |
San Diego
Lease
|
Other
Leases |
Total Lease
Commitments |
||||||||||||
|
(in thousands)
|
||||||||||||||||
|
2019
|
$
|
66,540
|
|
$
|
5,324
|
|
$
|
13,207
|
|
$
|
85,071
|
|
||||
|
2020
|
72,589
|
|
9,127
|
|
14,270
|
|
95,986
|
|
||||||||
|
2021
|
72,589
|
|
9,127
|
|
12,529
|
|
94,245
|
|
||||||||
|
2022
|
72,589
|
|
9,127
|
|
12,045
|
|
93,761
|
|
||||||||
|
2023
|
72,589
|
|
9,530
|
|
11,952
|
|
94,071
|
|
||||||||
|
Thereafter
|
389,855
|
|
119,864
|
|
65,472
|
|
575,191
|
|
||||||||
|
Total minimum lease payments
|
$
|
746,751
|
|
$
|
162,099
|
|
$
|
129,475
|
|
$
|
1,038,325
|
|
||||
|
Year
|
(in thousands)
|
|||
|
2019
|
$
|
10,770
|
|
|
|
2020
|
7,282
|
|
||
|
2021
|
5,649
|
|
||
|
2022
|
3,300
|
|
||
|
2023
|
1,974
|
|
||
|
Thereafter
|
3,085
|
|
||
|
Total payments
|
32,060
|
|
||
|
Less: amount representing interest
|
(2,585
|
)
|
||
|
Present value of payments
|
$
|
29,475
|
|
|
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Stock-based compensation expense by type of award:
|
|||||||
|
Restricted stock and restricted stock units (including PSUs)
|
$
|
63,510
|
|
$
|
50,418
|
|
|
|
Stock options
|
28,156
|
|
26,055
|
|
|||
|
ESPP share issuances
|
2,577
|
|
2,128
|
|
|||
|
Stock-based compensation expense related to inventories
|
(452
|
)
|
(465
|
)
|
|||
|
Total stock-based compensation included in costs and expenses
|
$
|
93,791
|
|
$
|
78,136
|
|
|
|
Stock-based compensation expense by line item:
|
|
|
|||||
|
Cost of sales
|
$
|
1,338
|
|
$
|
813
|
|
|
|
Research and development expenses
|
59,715
|
|
48,488
|
|
|||
|
Sales, general and administrative expenses
|
32,738
|
|
28,835
|
|
|||
|
Total stock-based compensation included in costs and expenses
|
93,791
|
|
78,136
|
|
|||
|
Income tax effect
|
(39,524
|
)
|
(21,859
|
)
|
|||
|
Total stock-based compensation included in costs and expenses, net of tax
|
$
|
54,267
|
|
$
|
56,277
|
|
|
|
As of March 31, 2019
|
|||||
|
Unrecognized Expense
|
Weighted-average
Recognition Period |
||||
|
(in thousands)
|
(in years)
|
||||
|
Type of award:
|
|||||
|
Restricted stock and restricted stock units (including PSUs)
|
$
|
475,787
|
|
2.55
|
|
|
Stock options
|
$
|
193,321
|
|
2.84
|
|
|
ESPP share issuances
|
$
|
2,555
|
|
0.43
|
|
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||
|
Range of Exercise Prices
|
Number
Outstanding |
Weighted-average
Remaining Contractual Life |
Weighted-average
Exercise Price |
Number
Exercisable |
Weighted-average
Exercise Price |
|||||||||||
|
(in thousands)
|
(in years)
|
(per share)
|
(in thousands)
|
(per share)
|
||||||||||||
|
$29.07–$40.00
|
252
|
|
1.67
|
$
|
35.87
|
|
252
|
|
$
|
35.87
|
|
|||||
|
$40.01–$60.00
|
437
|
|
3.20
|
$
|
50.23
|
|
437
|
|
$
|
50.23
|
|
|||||
|
$60.01–$80.00
|
525
|
|
5.02
|
$
|
74.83
|
|
516
|
|
$
|
74.82
|
|
|||||
|
$80.01–$100.00
|
2,481
|
|
6.95
|
$
|
89.20
|
|
1,315
|
|
$
|
89.81
|
|
|||||
|
$100.01–$120.00
|
622
|
|
5.88
|
$
|
109.32
|
|
614
|
|
$
|
109.24
|
|
|||||
|
$120.01–$140.00
|
773
|
|
6.36
|
$
|
130.20
|
|
653
|
|
$
|
130.24
|
|
|||||
|
$140.01–$160.00
|
1,287
|
|
8.82
|
$
|
155.51
|
|
333
|
|
$
|
155.35
|
|
|||||
|
$160.01–$180.00
|
502
|
|
8.30
|
$
|
162.94
|
|
177
|
|
$
|
162.95
|
|
|||||
|
$180.01–$187.53
|
1,823
|
|
9.65
|
$
|
185.29
|
|
115
|
|
$
|
182.72
|
|
|||||
|
Total
|
8,702
|
|
7.28
|
$
|
124.11
|
|
4,412
|
|
$
|
100.04
|
|
|||||
|
Three Months Ended March 31,
|
|||||||||||||||
|
2019
|
2018
|
||||||||||||||
|
Beginning of period
|
End of period
|
Beginning of period
|
End of period
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
2,650,134
|
|
$
|
2,893,885
|
|
$
|
1,665,412
|
|
$
|
1,995,893
|
|
|||
|
Prepaid expenses and other current assets
|
4,910
|
|
6,250
|
|
2,114
|
|
9,835
|
|
|||||||
|
Other assets
|
3,209
|
|
1,397
|
|
—
|
|
—
|
|
|||||||
|
Cash, cash equivalents and restricted cash per statement of cash flows
|
$
|
2,658,253
|
|
$
|
2,901,532
|
|
$
|
1,667,526
|
|
$
|
2,005,728
|
|
|||
|
Three Months Ended March 31, 2019
|
|||
|
(in thousands)
|
|||
|
Cash paid for amounts included in the measurement of lease liabilities:
|
|||
|
Operating cash flows from operating leases
|
$
|
2,531
|
|
|
Operating cash flows from finance leases
|
$
|
11,910
|
|
|
Financing cash flows from finance leases
|
$
|
9,385
|
|
|
Right-of-use assets obtained in exchange for lease obligations
|
|||
|
Operating leases
|
$
|
—
|
|
|
Finance leases
|
$
|
—
|
|
|
•
|
Announced positive data from two Phase 3 clinical trials evaluating the triple combination of VX-445, tezacaftor and ivacaftor in F508del/Min patients and F508del homozygous patients 12 years of age or older.
|
|
•
|
Obtained approval for SYMDEKO in Australia for certain patients 12 years of age or older.
|
|
•
|
Obtained approval for ORKAMBI in the European Union for children 2 to 5 years of age.
|
|
•
|
Initiated Phase 2 dose-ranging clinical trial to evaluate the potentiator VX-561 as a potential once-daily monotherapy.
|
|
•
|
Obtained approval for KALYDECO in the United States for infants 6 to <12 months of age.
|
|
•
|
Initiated Phase 2 clinical trial evaluating the potential once-daily triple combination of VX-121 (an additional next-generation corrector) with VX-561 and tezacaftor.
|
|
•
|
Obtained Fast Track Designation for VX-814, our first small molecule alpha-1 antitrypsin deficiency corrector.
|
|
•
|
Announced with CRISPR Therapeutics that the first patient has been treated with CTX001 in a Phase 1/2 clinical trial of patients with transfusion-dependent beta thalassemia.
|
|
•
|
Announced with CRISPR Therapeutics that first patient has been enrolled in a Phase 1/2 clinical trial of patients with sickle cell disease.
|
|
•
|
Obtained Fast Track designation for CTX001 for both transfusion-dependent beta thalassemia and sickle cell disease.
|
|
•
|
CRISPR Therapeutics AG and its affiliates, or CRISPR, pursuant to which we are collaborating on the discovery and development of potential new treatments aimed at the underlying genetic causes of human diseases using CRISPR-Cas9 gene-editing technology;
|
|
•
|
Arbor Biotechnologies, Inc., or Arbor, pursuant to which we are collaborating on the discovery of novel proteins, including DNA endonucleases, to advance the development of new gene-editing therapies; and
|
|
•
|
Moderna Therapeutics, Inc., or Moderna, pursuant to which we are seeking to identify and develop messenger ribonucleic acid, or mRNA therapeutics for the treatment of CF.
|
|
•
|
Janssen Pharmaceuticals, Inc., or Janssen which is evaluating pimodivir in Phase 3 clinical trials for the treatment of influenza; and
|
|
•
|
Merck KGaA, Darmstadt, Germany, which licensed oncology research and development programs from us in early 2017.
|
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Revenues
|
$
|
858,435
|
|
$
|
640,799
|
|
$
|
217,636
|
|
34
|
%
|
|||
|
Operating costs and expenses
|
581,627
|
|
511,898
|
|
69,729
|
|
14
|
%
|
||||||
|
Other non-operating income, net
|
43,357
|
|
68,703
|
|
(25,346
|
)
|
**
|
|
||||||
|
Provision for (benefit from) income taxes
|
51,534
|
|
(12,659
|
)
|
**
|
|
**
|
|
||||||
|
Net income attributable to Vertex
|
$
|
268,631
|
|
$
|
210,263
|
|
$
|
58,368
|
|
28
|
%
|
|||
|
Net income per diluted share attributable to Vertex common shareholders
|
$
|
1.03
|
|
$
|
0.81
|
|
||||||||
|
Diluted shares used in per share calculations
|
260,175
|
|
258,526
|
|
||||||||||
|
** Not meaningful
|
||||||||||||||
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
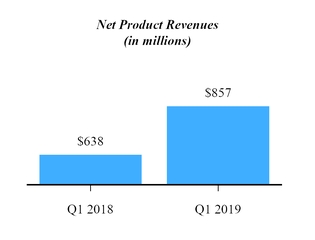
|
Product revenues, net
|
$
|
857,253
|
|
$
|
637,729
|
|
$
|
219,524
|
|
34
|
%
|
|||
|
Collaborative and royalty revenues
|
1,182
|
|
3,070
|
|
(1,888
|
)
|
(61
|
)%
|
||||||
|
Total revenues
|
$
|
858,435
|
|
$
|
640,799
|
|
$
|
217,636
|
|
34
|
%
|
|||
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
SYMDEKO/SYMKEVI
|
$
|
320,275
|
|
$
|
34,124
|
|
$
|
286,151
|
|
839
|
%
|
|||
|
ORKAMBI
|
293,007
|
|
354,066
|
|
(61,059
|
)
|
(17
|
)%
|
||||||
|
KALYDECO
|
243,971
|
|
249,539
|
|
(5,568
|
)
|
(2
|
)%
|
||||||
|
Total product revenues, net
|
$
|
857,253
|
|
$
|
637,729
|
|
$
|
219,524
|
|
34
|
%
|
|||
|
`
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Cost of sales
|
$
|
95,092
|
|
$
|
71,613
|
|
$
|
23,479
|
|
33
|
%
|
|||
|
Research and development expenses
|
339,490
|
|
310,553
|
|
28,937
|
|
9
|
%
|
||||||
|
Sales, general and administrative expenses
|
147,045
|
|
129,808
|
|
17,237
|
|
13
|
%
|
||||||
|
Restructuring income
|
—
|
|
(76
|
)
|
76
|
|
**
|
|
||||||
|
Total costs and expenses
|
$
|
581,627
|
|
$
|
511,898
|
|
$
|
69,729
|
|
14
|
%
|
|||
|
** Not Meaningful
|
||||||||||||||
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Research expenses
|
$
|
90,463
|
|
$
|
77,942
|
|
$
|
12,521
|
|
16
|
%
|
|||
|
Development expenses
|
249,027
|
|
232,611
|
|
16,416
|
|
7
|
%
|
||||||
|
Total research and development expenses
|
$
|
339,490
|
|
$
|
310,553
|
|
$
|
28,937
|
|
9
|
%
|
|||
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Research Expenses:
|
||||||||||||||
|
Salary and benefits
|
$
|
24,379
|
|
$
|
24,088
|
|
$
|
291
|
|
1
|
%
|
|||
|
Stock-based compensation expense
|
17,535
|
|
14,760
|
|
2,775
|
|
19
|
%
|
||||||
|
Outsourced services and other direct expenses
|
23,364
|
|
18,813
|
|
4,551
|
|
24
|
%
|
||||||
|
Collaboration and asset acquisition payments
|
—
|
|
308
|
|
(308
|
)
|
**
|
|
||||||
|
Infrastructure costs
|
25,185
|
|
19,973
|
|
5,212
|
|
26
|
%
|
||||||
|
Total research expenses
|
$
|
90,463
|
|
$
|
77,942
|
|
$
|
12,521
|
|
16
|
%
|
|||
|
** Not meaningful
|
||||||||||||||
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Development Expenses:
|
||||||||||||||
|
Salary and benefits
|
$
|
60,507
|
|
$
|
57,002
|
|
$
|
3,505
|
|
6
|
%
|
|||
|
Stock-based compensation expense
|
42,180
|
|
33,728
|
|
8,452
|
|
25
|
%
|
||||||
|
Outsourced services and other direct expenses
|
89,874
|
|
99,192
|
|
(9,318
|
)
|
(9
|
)%
|
||||||
|
Collaboration and asset acquisition payments
|
5,250
|
|
250
|
|
5,000
|
|
**
|
|
||||||
|
Drug supply costs
|
7,894
|
|
8,421
|
|
(527
|
)
|
(6
|
)%
|
||||||
|
Infrastructure costs
|
43,322
|
|
34,018
|
|
9,304
|
|
27
|
%
|
||||||
|
Total development expenses
|
$
|
249,027
|
|
$
|
232,611
|
|
$
|
16,416
|
|
7
|
%
|
|||
|
** Not meaningful
|
||||||||||||||
|
Three Months Ended March 31,
|
Increase/(Decrease)
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Sales, general and administrative expenses
|
$
|
147,045
|
|
$
|
129,808
|
|
$
|
17,237
|
|
13
|
%
|
|||
|
March 31,
|
December 31,
|
Increase/(Decrease)
|
||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
3,478,035
|
|
$
|
3,168,242
|
|
$
|
309,793
|
|
10
|
%
|
|||
|
Working Capital
|
||||||||||||||
|
Total current assets
|
4,183,039
|
|
3,843,109
|
|
339,930
|
|
9
|
%
|
||||||
|
Total current liabilities
|
(1,106,468
|
)
|
(1,120,292
|
)
|
(13,824
|
)
|
(1
|
)%
|
||||||
|
Total working capital
|
$
|
3,076,571
|
|
$
|
2,722,817
|
|
$
|
353,754
|
|
13
|
%
|
|||
|
•
|
significant expected operating expenses to conduct research and development activities and to operate our organization; and
|
|
•
|
substantial facility and capital lease obligations, including leases for two buildings in Boston, Massachusetts that continue through 2028 and a lease in San Diego, California that continues through 2034.
|
|
•
|
As of
March 31, 2019
, we have accrued approximately
$382.7 million
from ORKAMBI early access programs in France. We expect we will be required to repay a portion of the collected amounts to the French government based
|
|
•
|
We have entered into certain collaboration agreements with third parties that include the funding of certain research, development and commercialization efforts with the potential for future milestone and royalty payments by us upon the achievement of pre-established developmental and regulatory targets and/or commercial targets, and we may enter into additional business development transactions, including acquisitions, collaborations and equity investments, that require additional capital. For example, in 2018, we made
$100.4 million
of upfront and milestone payments related to collaborations and asset acquisitions.
|
|
•
|
To the extent we borrow amounts under the credit agreement we entered into in October 2016, we would be required to repay any outstanding principal amounts in 2021.
|
|
•
|
our expectations regarding the amount of, timing of and trends with respect to our revenues, costs and expenses and other gains and losses, including those related to net product revenues;
|
|
•
|
our expectations regarding clinical trials, development timelines and regulatory authority filings and submissions for ivacaftor, lumacaftor, tezacaftor, VX-659, VX-445, VX-150 and the timelines for regulatory filings for a triple combination regimen;
|
|
•
|
our ability to obtain reimbursement for our medicines in ex-U.S. markets and our ability to otherwise successfully market our medicines or any of our other drug candidates for which we obtain regulatory approval;
|
|
•
|
our expectations regarding the timing and structure of clinical trials of our drugs and drug candidates and the expected timing of our receipt of data from our ongoing and planned clinical trials;
|
|
•
|
the data that will be generated by ongoing and planned clinical trials and the ability to use that data to advance compounds, continue development or support regulatory filings;
|
|
•
|
our beliefs regarding the support provided by clinical trials and preclinical and nonclinical studies of our drug candidates for further investigation, clinical trials or potential use as a treatment;
|
|
•
|
our plan to continue investing in our research and development programs and our strategy to develop our drug candidates, alone or with third party-collaborators;
|
|
•
|
the establishment, development and maintenance of collaborative relationships;
|
|
•
|
potential business development activities;
|
|
•
|
potential fluctuations in foreign currency exchange rates;
|
|
•
|
our ability to use our research programs to identify and develop new drug candidates to address serious diseases and significant unmet medical needs; and
|
|
•
|
our liquidity and our expectations regarding the possibility of raising additional capital.
|
|
Period
|
Total Number
of Shares Purchased (1) |
Average Price
Paid per Share |
Total Number of Shares
Purchased as Part of Publicly Announced Plans or Programs (2) |
Approximate Dollar Value of Shares that May Yet be Purchased Under the Plans or Programs (2)
|
|
|
January 1, 2019 to January 31, 2019
|
70,691
|
$169.74
|
69,778
|
$138,000,783
|
|
|
February 1, 2019 to February 28, 2019
|
183,914
|
$184.86
|
183,366
|
$104,002,661
|
|
|
March 1, 2019 to March 31, 2019
|
285,909
|
$181.87
|
283,874
|
$52,004,541
|
|
|
Total
|
540,514
|
$181.30
|
537,018
|
$52,004,541
|
|
|
Exhibit Number
|
Exhibit Description
|
|
10.1
|
|
|
10.2
|
|
|
31.1
|
|
|
31.2
|
|
|
32.1
|
|
|
101.INS
|
XBRL Instance
|
|
101.SCH
|
XBRL Taxonomy Extension Schema
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation
|
|
101.LAB
|
XBRL Taxonomy Extension Labels
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation
|
|
101.DEF
|
XBRL Taxonomy Extension Definition
|
|
Vertex Pharmaceuticals Incorporated
|
||
|
May 1, 2019
|
By:
|
/s/ Charles Wagner
|
|
Charles Wagner
|
||
|
Executive Vice President, Chief Financial Officer
(principal financial officer and duly authorized officer) |
||