|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
|
¨
|
Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
|
Nevada
|
20-5093315
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
Large accelerated filer
¨
|
|
Accelerated filer
¨
|
|
Non-accelerated filer
¨
|
|
Smaller reporting company
x
|
|
|
|
(Do not check if a smaller
reporting company)
|
||||
|
|
Item No.
|
Page No.
|
||||||
|
|
||||||||
|
|
|
|
2
|
|||||
|
|
|
46
|
||||||
|
|
|
79
|
||||||
|
|
|
79
|
||||||
|
|
|
79
|
||||||
|
|
|
79
|
||||||
|
|
||||||||
|
|
|
80
|
||||||
|
|
|
81
|
||||||
|
|
|
81
|
||||||
|
|
|
93
|
||||||
|
|
|
94
|
||||||
|
|
|
143
|
||||||
|
|
|
143
|
||||||
|
|
|
143
|
||||||
|
|
||||||||
|
|
|
144
|
||||||
|
|
|
149
|
||||||
|
|
|
156
|
||||||
|
|
|
162
|
||||||
|
|
|
163
|
||||||
|
|
||||||||
|
|
|
165
|
||||||
| EXHIBIT INDEX | 165 | |||||||
|
169
|
||||||||
|
•
|
the availability of capital to satisfy our working capital requirements;
|
|
•
|
the accuracy of our estimates regarding expenses, future revenues and capital requirements;
|
|
•
|
our plans to develop and commercialize our lead product candidate, initially as a treatment for Major Depressive Disorder;
|
|
•
|
our ability to initiate and complete our clinical trials and to advance our product candidates into additional clinical trials, including pivotal clinical trials, and successfully complete such clinical trials;
|
|
•
|
regulatory developments in the United States and foreign countries;
|
|
•
|
the performance of the U.S. National Institute of Mental Health, our third-party contract manufacturer(s) and contract research organization(s);
|
|
•
|
our ability to obtain and maintain intellectual property protection for our proprietary assets;
|
|
•
|
the size of the potential markets for our product candidates and our ability to serve those markets;
|
|
•
|
the rate and degree of market acceptance of our product candidates for any indication once approved;
|
|
•
|
the success of competing products that are or become available for the indications that we are pursuing;
|
|
•
|
the loss of key scientific or management personnel, internally from one of our third-party collaborators; and
|
|
•
|
other risks and uncertainties, including those listed under Part I, Item 1A. Risk Factors.
|
|
Business
|
|
•
|
Develop and commercialize our lead product candidate, AV-101, for Major Depressive Disorder (MDD).
We are pursuing MDD as our lead indication for AV-101. We are preparing to launch our initial MDD Phase 2 clinical study in collaboration with the NIH in the second half of 2015. We intend to develop AV-101 internally, through Phase 3 clinical studies and submission of our NDA. If approved by the FDA, we plan to commercialize AV-101 for this indication in the U.S. either by (A) establishing or contracting for a specialty U.S. sales force focused primarily on psychiatrists and long-term care physicians who are high prescribers of currently-approved antidepressants or (B) collaborating with a pharmaceutical company with an extensive presence in U.S. CNS markets. Outside the U.S., we may choose to commercialize AV-101 in selected markets by establishing one or more strategic alliances.
|
|
•
|
Leverage the commercial potential AV-101 by expanding to additional CNS-related disorders.
We intend to pursue the development and commercialization of AV-101 in MDD and additional CNS-related indications that are underserved by currently available medicines and represent large unmet medical needs. Based on AV-101 preclinical studies, and by leveraging our AV-101 IND and successful Phase 1 clinical studies, we now have the opportunity to explore Phase 2 development of AV-101 as a potential treatment for chronic neuropathic pain, epilepsy and neurodegenerative diseases such as Parkinson’s disease and Huntington’s disease.
|
|
•
|
Grow our internal development pipeline by pursuing drug rescue opportunities using our stem cell technology.
W
e
are using our stem cell technology to screen and develop proprietary new chemical entities (NCEs) through drug rescue programs intended to produce proprietary NCEs for our internal drug development pipeline. We will focus on NCEs with established therapeutic and commercial potential. Our ability to build on that valuable head start with our biological and electrophysiological insights regarding cardiac and liver safety effects of NCEs obtained using
CardioSafe
3D and, eventually,
LiverSafe
3D, we believe will help us produce and then optimize patentable drug rescue NCEs for our internal pipeline without incurring many of the substantial costs and risks typically inherent in new drug discovery and nonclinical drug development.
|
|
•
|
Pursue other product candidates, including product candidates for treatment of CNS-related disorders.
While our resources are currently focused on developing AV-101 and producing drug rescue NCEs, we may pursue additional product candidates in the future. These may be directed at CNS-related disorders and may be developed independently or in partnerships. We believe that a diversified portfolio will mitigate risks inherent in drug development and increase the likelihood of our success.
|

|
1.
|
AV-101 is preferentially converted to 7-Cl-KYNA in brain areas related to neuronal injury. This is because astrocytes, which are responsible for the enzymatic transamination of 4-Cl-KYN prodrug to active 7-Cl-KYNA, are focally activated at sites of neuronal injury. Due to AV-101’s highly focused site of conversion, local concentrations of newly formed 7-Cl-KYNA are greatest at the site of therapeutic need. In addition to delivering the drug where it is needed, this reduces the chance of systemic and dangerous side effects with long-term use of the drug; and
|
|
2.
|
An active metabolite of AV-101, 4-Cl-3-hydroxyanthranilic acid, inhibits the synthesis of quinolinic acid, an endogenous NMDAR agonist that causes convulsions and excitotoxic neuronal damage.
|

Figure-1. Mean plasma concentrations of L-4-Cl-KYN
after oral administration of a single dose of AV-101.
|

Figure 2. Mean plasma concentrations of 7-Cl-KYNA
after oral administration of a single dose of AV-101.
|
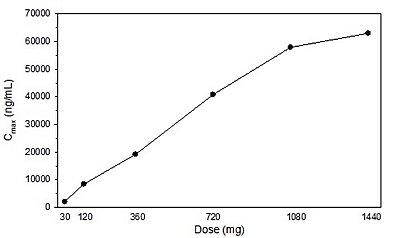
Figure 3. Mean C
max
values of L-4-Cl-KYN by dose of AV-101.
|
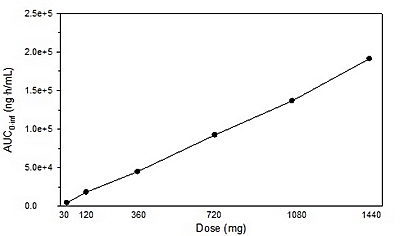
Figure 4. Mean AUC
0-∞
values of L-4-Cl-KYN by dose of AV-101.
|

Figure 5. Mean C
max
values of 7-Cl-KYNA by dose of AV-101.
|

Figure 6. Mean AUC
0-∞
values of 7-Cl-KYNA by dose of AV-101.
|
|
Placebo
(n = 18)
|
Cohorts (mg)
|
|||||||
|
MedDRA SOC and Preferred Term
|
30
(n = 3)
|
120
(n = 3)
|
360
(n = 3)
|
720
(n = 3)
|
1,080
(n = 3)
|
1,440
(n = 3)
|
Overall
(n = 36)
|
|
|
Infections and Infestations
|
0
(0%)
|
1
(33.3%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
1
(2.8%)
|
|
Gastroenteritis
|
0
(0%)
|
1
(33.3%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
1
(2.8%)
|
|
Nervous System Disorders
|
1
(5.6%)
|
1
(33.3%)
|
1
(33.3%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
3
(8.3%)
|
|
Headache
|
1
(5.6%)
|
1
(33.3%)
|
1
(33.3%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
3
(8.3%)
|
|
Psychiatric Disorder
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
2
(66.7%)
|
2
(5.6%)
|
|
Euphoric mood
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
2
(66.7%)
|
2
(5.6%)
|
|
Skin and Subcutaneous Tissue Disorder
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
|
Dermatitis contact
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
0
(0%)
|
|
·
|
Blinded safety and tolerability data were reviewed and assessed as being satisfactory by the investigator and medical monitor.
|
|
·
|
PK assessments were reviewed by the blinded Cato Research PK specialist to determine if the PK stopping criteria were reached.
|
| · Dose Cohorts | ||||||||
|
·
·
|
360 mgAV-101
N = 12)
[n (%)]
|
·
·
|
1,080 mg AV-101
N = 13)
[n (%)]
|
·
·
|
1,440 mg AV-101
(N = 12)
[n (%)]
|
·
·
|
Pooled Placebo
(N = 13)
|
|
|
·
Number of AEs
|
· |
16
|
· |
14
|
·
|
10
|
·
|
17
|
|
·
Number of subjects with any AE
|
· |
9 (75.0%)
|
· |
8 (61.5%)
|
· |
7 (58.3%)
|
· |
10 (76.9%)
|
|
·
Number of SAEs
|
· |
0 (0%)
|
· |
0 (0%)
|
· |
0 (0%)
|
· |
0 (0%)
|
|
·
Number of AEs resulting in death
|
· |
0 (0%)
|
· |
0 (0%)
|
·
|
0 (0%)
|
· |
0 (0%)
|
|
Number of AEs leading to discontinuation of study drug
|
· |
0 (0%)
|
· |
0 (0%)
|
·
|
0 (0%)
|
· |
1 (7.7%)
|
|
Placebo
% of Adverse Events/N
|
AV-101
% of Adverse Events/N
|
|
|
·
Phase 1a
|
||
|
·
Nonserious Adverse Events
|
22% (4/18)
|
28% (5/18)
|
|
·
Feelings of Well-being (coded as euphoric mood)
|
0% (0/18)
|
11% (2/18)
|
|
·
Phase 1b
|
||
|
·
Nonserious Adverse Events
|
77% (10/13)
|
65% (24/37)
|
|
·
Feelings of Well-being (coded as euphoric mood)
|
0% (0/13)
|
8% (3/37)
|
|
·
Phase 1a and 1b
|
||
|
·
Feelings of Well-being (coded as euphoric mood)
|
0% (0/31)
|
9% (5/55)
|
N = number of subjects
|
●
|
individuals with specific inheritable diseases and conditions that predispose the individual to respond differently to drugs; or
|
|
●
|
individuals with specific variations in genes that directly affect drug levels in the body or alter the manner or efficiency of their metabolism, breakdown and/or elimination of drugs.
|
|
●
|
specific growth and differentiation factors used in the tissue culture medium, applied in specific combinations, at critical concentrations, and at critical times unique to each desired human cell type;
|
|
●
|
the experimentally controlled regulation of developmental genes, which is critical for determining what differentiation path a human cell will take; and
|
|
●
|
biological markers characteristic of precursor cells, which are committed to becoming specific human cells and tissues, and which can be used to identify, enrich and purify the desired mature human cell type.
|
|
|
1. cell viability;
|
|
|
2. apoptosis;
|
|
|
3. mitochondrial membrane depolarization;
|
|
|
4. oxidative stress; and
|
|
|
5. energy metabolism disruption.
|
|
1. ion channel blockers: amiodarone, nifedipine;
|
|
|
2. hERG trafficking blockers: pentamidine, amoxapine;
|
|
|
3. α-1 adrenoreceptors: doxazosin;
|
|
|
4. protein and DNA synthesis inhibitors: emetine;
|
|
|
5. DNA intercalating agents: doxorubicin;
|
|
|
6. antibiotics: ampicillin, cefazolin;
|
|
|
7. NSAID: aspirin; and
|
|
|
8. kinase inhibitors: staurosporine.
|
|
1.
|
One FDA-approved drug (aspirin) without cardiac liability to serve as a negative control;
|
|
2.
|
Five FDA-approved drugs (astemizole, sotalol, cisapride, terfenadine and sertindole) that were withdrawn from the market due to heart toxicity concerns;
|
|
3.
|
Five FDA-approved drugs (fexofenadine, nifedipine, verapamil, lidocaine and propranolol) that have certain measurable non-toxic cardiac effects consistent with clinical experience with such compounds. Note: fexofenadine is a non-cardiotoxic drug variant of terfenadine; and
|
|
4.
|
One research compound (E-4031) failed in Phase I human clinical study before being discontinued due to inducing heart arrhythmias.
|
|
Detects cardiac effects mediated by:
|
hERG assay
|
CardioSafe
3D™
|
|
hERG potassium ion channels
|
ü
|
ü
|
|
Other potassium ion channels
|
ü
|
|
|
Calcium ion channels
|
ü
|
|
|
Sodium ion channels
|
ü
|
|
|
Interactions between ion channels
|
ü
|
|
|
Channel regulatory proteins
|
ü
|
|
|
Cell viability
|
ü
|
|
|
Apoptosis
|
ü
|
|
|
Mitochondria
|
ü
|
|
|
Energy
|
ü
|
|
|
Oxidative Stress
|
ü
|
|
1.
|
Inhibitors of growth factor receptors: sunitinib, axitinib, imatinib, dasatinib, sorafenib, erlotinib, lapatinib, tyrphostin and AG1478;
|
|
|
2.
|
Inhibitors of the mTOR pathway: everolimus, temsirolimus;
|
|
|
3.
|
Inhibitors of cell cycle regulators: tozasertib, barasertib, alvocidib;
|
|
|
4.
|
Inhibitors of the PI3K pathway : perifosine, LY294002, XL765;
|
|
|
5.
|
Inhibitors of the MEK pathway: PD325901, AZD6264; and
|
|
|
6.
|
Inhibitors of the JAK and other pathways: lestaurtinib.
|
|
Characteristics of
in vitro
hepatocyte assays:
|
Primary hepatocytes
|
VSTA-heps™
|
|
Human cells
|
ü
|
ü
|
|
Liver enzyme activity
|
ü
|
ü
|
|
Within batch reproducibility
|
ü
|
ü
|
|
Batch-to-batch reproducibility
|
ü
|
|
|
Long term culture
|
ü
|
|
|
Maintenance of function in culture
|
ü
|
|
|
Parental cells can be expanded into large batches
|
ü
|
|
|
Uniform genetic background between batches
|
ü
|
|
|
Uniform donor health status between batches
|
ü
|
|
|
Gene “reporters” can be genetically inserted
|
ü
|
|
●
|
academic research institutions, such as the University Health Network (
UHN
) for hPSC technology research and development;
|
|
●
|
contract medicinal chemistry companies, such as Synterys, Inc., to design, produce and analyze drug rescue NCEs; and
|
|
●
|
contract clinical development and regulatory organizations (
CROs
), such as Cato Research, Ltd., for regulatory expertise and clinical development support.
|
|
●
|
a combination of growth factors (molecules that stimulate the growth of cells);
|
|
●
|
the experimentally controlled regulation of developmental genes, which is critical for determining what differentiation path a human cell will take; and
|
|
●
|
precise selection of immature cell populations for further growth and development.
|
|
●
|
the use of certain growth factors to generate mesoderm (that is, the precursors capable of developing into cells of the heart, blood system, connective tissues, and vascular system) from hESCs;
|
|
●
|
the use of certain growth factors to generate endoderm (that is, the precursors capable of developing into cells of the liver, pancreas, lungs, gut, intestines, thymus, thyroid gland, bladder, and parts of the auditory system) from hESCs; and
|
|
●
|
applications of cells derived from mesoderm and endoderm precursors, especially those relating to drug discovery and testing for applications in the field of
in vitro
drug discovery and development applications.
|
|
Territory
|
Patent No.
|
General Subject Matter
|
Expiration
|
||||
|
US
|
7,763,466
|
Method to produce endoderm cells
|
May 2025
|
||||
|
US
|
7,955,849
|
Method of enriching population of mesoderm cells
|
May 2023
|
||||
|
US
|
8,143,009
|
Toxicity typing using liver stem cells
|
June 2023
|
||||
|
·
|
Completion of extensive non-clinical, sometimes referred to as non-clinical laboratory tests, non-clinical animal studies and formulation studies in accordance with applicable regulations, including the FDA’s current Good Laboratory Practice, or GLP, regulations;
|
|
·
|
Submission to the FDA of an IND application, which must become effective before human clinical trials may begin;
|
|
·
|
Approval by an independent institutional review board, or IRB, or ethics committee at each clinical trial site before each trial may be initiated;
|
|
·
|
Performance of adequate and well-controlled human clinical trials in accordance with applicable IND and other clinical trial-related regulations, sometimes referred to as good clinical practices, or GCPs, to establish the safety and efficacy of the proposed drug for each proposed indication;
|
|
·
|
Submission to the FDA of an NDA, for a new drug;
|
|
·
|
A determination by the FDA within 60 days of its receipt of an NDA to file the NDA for review;
|
|
·
|
Satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities where the drug is produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity;
|
|
·
|
Potential FDA audit of the non-clinical and/or clinical trial sites that generated the data in support of the NDA; and
|
|
·
|
FDA review and approval of the NDA, including consideration of the views of any FDA advisory committee, prior to any commercial marketing or sale of the drug in the United States.
|
|
·
|
Phase 1 clinical trials generally involve a small number of healthy volunteers who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, side effect tolerability and safety of the drug.
|
|
·
|
Phase 2 clinical trials typically involve studies in disease-affected patients to determine the dose required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, as well as identification of possible adverse effects and safety risks and preliminary evaluation of efficacy.
|
|
·
|
Phase 3 clinical trials generally involve large numbers of patients at multiple sites (from several hundred to several thousand subjects) and are designed to provide the data necessary to demonstrate the effectiveness of the product for its intended use, its safety in use, and to establish the overall benefit/risk relationship of the product and provide an adequate basis for product approval. Phase 3 clinical trials may include comparisons with placebo and/or other comparator treatments. The duration of treatment is often extended to mimic the actual use of a product during marketing.
|
|
•
|
distribution restricted to certain facilities or physicians with special training or experience; or
|
|
•
|
distribution conditioned on the performance of specified medical procedures.
|
|
·
|
we may not be able to demonstrate that AV-101 is safe and effective in treating MDD, to the satisfaction of the FDA;
|
|
·
|
the results of our non-clinical studies and clinical trials may not meet the level of statistical or clinical significance required by the FDA for marketing approval;
|
|
·
|
the FDA may disagree with the number, design, size, conduct or implementation of our non-clinical studies and clinical trials;
|
|
·
|
the FDA may require that we conduct additional non-clinical studies and clinical trials;
|
|
·
|
the FDA or the applicable foreign regulatory agency may not approve the formulation, labeling or specifications of any of our product candidates;
|
|
·
|
the contract research organizations, or CROs, that we retain to conduct our non-clinical studies and clinical trials may take actions outside of our control that materially adversely impact our non-clinical studies and clinical trials;
|
|
·
|
the FDA may find the data from non-clinical studies and clinical trials insufficient to demonstrate that our product candidates’ clinical and other benefits outweigh their safety risks;
|
|
·
|
the FDA may disagree with our interpretation of data from our non-clinical studies and clinical trials;
|
|
·
|
the FDA may not accept data generated at our non-clinical studies and clinical trial sites;
|
|
·
|
if our NDA, if and when submitted, is reviewed by an advisory committee, the FDA may have difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, additional non-clinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions;
|
|
·
|
the FDA may require development of a Risk Evaluation and Mitigation Strategy, or REMS, as a condition of approval or post-approval;
|
|
·
|
the FDA or the applicable foreign regulatory agency may determine that the manufacturing processes or facilities of third-party contract manufacturers with which we contract do not conform to applicable requirements, including current Good Manufacturing Practices, or cGMPs; or
|
|
·
|
the FDA or applicable foreign regulatory agency may change its approval policies or adopt new regulations.
|
|
·
|
the FDA may deny permission to proceed with our planned clinical trials or any other clinical trials we may initiate, or may place a clinical trial on hold;
|
|
·
|
delays in filing or receiving approvals of additional INDs that may be required;
|
|
·
|
negative results from our ongoing non-clinical studies;
|
|
·
|
delays in reaching or failing to reach agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
·
|
inadequate quantity or quality of a product candidate or other materials necessary to conduct clinical trials, for example delays in the manufacturing of sufficient supply of finished drug product;
|
|
·
|
difficulties obtaining Institutional Review Board, or IRB, approval to conduct a clinical trial at a prospective site or sites;
|
|
·
|
challenges in recruiting and enrolling patients to participate in clinical trials, including the proximity of patients to trial sites;
|
|
·
|
eligibility criteria for the clinical trial, the nature of the clinical trial protocol, the availability of approved effective treatments for the relevant disease and competition from other clinical trial programs for similar indications;
|
|
·
|
severe or unexpected drug-related side effects experienced by patients in a clinical trial;
|
|
·
|
delays in validating any endpoints utilized in a clinical trial;
|
|
·
|
the FDA may disagree with our clinical trial design and our interpretation of data from clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our clinical trials;
|
|
·
|
reports from non-clinical or clinical testing of other CNS therapies that raise safety or efficacy concerns; and
|
|
·
|
difficulties retaining patients who have enrolled in a clinical trial but may be prone to withdraw due to rigors of the clinical trials, lack of efficacy, side effects, personal issues or loss of interest.
|
|
·
|
failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols;
|
|
·
|
inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities that reveals deficiencies or violations that require us to undertake corrective action, including the imposition of a clinical hold;
|
|
·
|
unforeseen safety issues, including any that could be identified in our ongoing non-clinical carcinogenicity studies, adverse side effects or lack of effectiveness;
|
|
·
|
changes in government regulations or administrative actions;
|
|
·
|
problems with clinical supply materials; and
|
|
·
|
lack of adequate funding to continue clinical trials.
|
|
·
|
have staffing difficulties;
|
|
·
|
fail to comply with contractual obligations;
|
|
·
|
experience regulatory compliance issues;
|
|
·
|
undergo changes in priorities or become financially distressed; or
|
|
·
|
form relationships with other entities, some of which may be our competitors.
|
|
·
|
the efficacy of our product candidates as demonstrated in clinical trials, and, if required by any applicable regulatory authority in connection with the approval for the applicable indications, to provide patients with incremental health benefits, as compared with other available CNS therapies;
|
|
·
|
limitations or warnings contained in the labeling approved for our product candidates by the FDA or other applicable regulatory authorities;
|
|
·
|
the clinical indications for which our product candidates are approved;
|
|
·
|
availability of alternative treatments already approved or expected to be commercially launched in the near future;
|
|
·
|
the potential and perceived advantages of our product candidates over current treatment options or alternative treatments, including future alternative treatments;
|
|
·
|
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
|
|
·
|
the strength of marketing and distribution support and timing of market introduction of competitive products;
|
|
·
|
publicity concerning our products or competing products and treatments;
|
|
·
|
pricing and cost effectiveness;
|
|
·
|
the effectiveness of our sales and marketing strategies;
|
|
·
|
our ability to increase awareness of our product candidates through marketing efforts;
|
|
·
|
our ability to obtain sufficient third-party coverage or reimbursement; or
|
|
·
|
the willingness of patients to pay out-of-pocket in the absence of third-party coverage.
|
|
·
|
regulatory authorities may withdraw or limit their approval of such product candidates;
|
|
·
|
regulatory authorities may require the addition of labeling statements, such as a “boxed” warning or a contraindication;
|
|
·
|
we may be required to change the way such product candidates are distributed or administered, conduct additional clinical trials or change the labeling of the product candidates;
|
|
·
|
we may be subject to regulatory investigations and government enforcement actions;
|
|
·
|
we may decide to remove such product candidates from the marketplace;
|
|
·
|
we could be sued and held liable for injury caused to individuals exposed to or taking our product candidates; and
|
|
·
|
our reputation may suffer.
|
|
·
|
issue warning letters or untitled letters;
|
|
·
|
seek an injunction or impose civil or criminal penalties or monetary fines;
|
|
·
|
suspend or withdraw marketing approval;
|
|
·
|
suspend any ongoing clinical trials;
|
|
·
|
refuse to approve pending applications or supplements to applications submitted by us;
|
|
·
|
suspend or impose restrictions on operations, including costly new manufacturing requirements; or
|
|
·
|
seize or detain products, refuse to permit the import or export of products, or require that we initiate a product recall.
|
|
·
|
The federal anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid.
|
|
·
|
The federal False Claims Act imposes criminal and civil penalties, including those from civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government.
|
|
·
|
The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
|
|
·
|
The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services.
|
|
·
|
The federal transparency requirements, sometimes referred to as the “Sunshine Act,” under the Patient Protection and Affordable Care Act, require manufacturers of drugs, devices, biologics and medical supplies that are reimbursable under Medicare, Medicaid, or the Children’s Health Insurance Program to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests.
|
|
·
|
Analogous state laws and regulations, such as state anti-kickback and false claims laws and transparency laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance.
|
|
·
|
guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures and drug pricing.
|
|
•
|
our customers’ ability to obtain reimbursement for our product candidates in foreign markets;
|
|
•
|
our inability to directly control commercial activities because we are relying on third parties;
|
|
•
|
the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements;
|
|
•
|
different medical practices and customs in foreign countries affecting acceptance in the marketplace;
|
|
•
|
import or export licensing requirements;
|
|
•
|
longer accounts receivable collection times;
|
|
•
|
longer lead times for shipping;
|
|
•
|
language barriers for technical training;
|
|
•
|
reduced protection of intellectual property rights in some foreign countries;
|
|
•
|
the existence of additional potentially relevant third party intellectual property rights;
|
|
•
|
foreign currency exchange rate fluctuations; and
|
|
•
|
the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute.
|
|
●
|
produce product candidates;
|
|
●
|
develop and obtain required regulatory approvals for commercialization of products we produce;
|
|
●
|
maintain, leverage and expand our intellectual property portfolio;
|
|
●
|
establish and maintain sales, distribution and marketing capabilities, and/or enter into strategic partnering arrangements to access such capabilities;
|
|
●
|
gain market acceptance for our products; and
|
|
●
|
obtain adequate capital resources and manage our spending as costs and expenses increase due to research, production, development, regulatory approval and commercialization of product candidates.
|
|
●
|
our drug rescue research methodology may not be successful in identifying potential drug rescue NCEs;
|
|
●
|
competitors may develop alternatives that render our drug rescue NCEs obsolete;
|
|
●
|
a drug rescue NCE may, on further study, be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria;
|
|
●
|
a drug rescue NCE may not be capable of being produced in commercial quantities at an acceptable cost, or at all; or
|
|
●
|
a drug rescue NCE may not be accepted as safe and effective by regulatory authorities, patients, the medical community or third-party payors.
|
|
●
|
our ability to identify potential drug rescue candidates in the public domain, obtain sufficient quantities of them, and assess them using our bioassay systems;
|
|
●
|
if we seek to rescue drug rescue candidates that are not available to us in the public domain, the extent to which third parties may be willing to out-license or sell certain drug rescue candidates to us on commercially reasonable terms;
|
|
●
|
our medicinal chemistry collaborator’s ability to design and produce proprietary drug rescue NCEs based on the novel biology and structure-function insight we provide using
CardioSafe
3D or
LiverSafe
3D; and
|
|
●
|
financial resources available to us to develop and commercialize lead drug rescue NCEs internally, or, if we out-license them to strategic partners, the resources such partners choose to dedicate to development and commercialization of any drug rescue NCEs they license from us.
|
|
●
|
decreased demand for products that we may develop;
|
|
●
|
injury to our reputation;
|
|
●
|
withdrawal of clinical trial participants;
|
|
●
|
costs to defend the related litigation;
|
|
●
|
a diversion of management's time and our resources;
|
|
●
|
substantial monetary awards to trial participants or patients;
|
|
●
|
product recalls, withdrawals or labeling, marketing or promotional restrictions;
|
|
●
|
loss of revenue;
|
|
●
|
the inability to commercialize our product candidates; and
|
|
●
|
a decline in our stock price.
|
|
•
|
initiate and successfully complete clinical trials that meet their clinical endpoints;
|
|
•
|
initiate and successfully complete all safety studies required to obtain U.S. and foreign marketing approval for our product candidates;
|
|
•
|
commercialize our product candidates, if approved, by developing a sales force or entering into collaborations with third parties; and
|
|
•
|
achieve market acceptance of our product candidates in the medical community and with third-party payors.
|
|
•
|
Absent our entering into a collaboration or partnership agreement, we expect to incur significant sales and marketing costs as we prepare to commercialize our product candidates. Even if we initiate and successfully complete pivotal clinical trials of our product candidates, and our product candidates are approved for commercial sale, and despite expending these costs, our product candidates may not be a commercially successful drug. We may not achieve profitability soon after generating product sales, if ever. If we are unable to generate product revenue, we will not become profitable and may be unable to continue operations without continued funding.
|
|
●
|
the number and characteristics of the product candidates we pursue, including AV-101 or drug rescue NCEs;
|
|
●
|
the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical and clinical studies;
|
|
●
|
the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates;
|
|
●
|
the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs;
|
|
●
|
the cost of manufacturing our product candidates and any products we successfully commercialize;
|
|
●
|
our ability to establish and maintain strategic partnerships, licensing or other arrangements and the financial terms of such agreements;
|
|
●
|
market acceptance of our products;
|
|
●
|
the effect of competing technological and market developments;
|
|
●
|
our ability to obtain government funding for our programs;
|
|
●
|
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims necessary to preserve our freedom to operate in the stem cell industry, including litigation costs associated with any claims that we infringe third-party patents or violate other intellectual property rights and the outcome of such litigation;
|
|
●
|
the timing, receipt and amount of potential future licensee fees, milestone payments, and sales of, or royalties on, our future products, if any; and
|
|
●
|
the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions.
|
|
•
|
any of our AV-101 or other pending patent applications, if issued, will include claims having a scope sufficient to protect AV-101 or any other products or product candidates;
|
|
•
|
any of our pending patent applications will issue as patents at all;
|
|
•
|
we will be able to successfully commercialize our product candidates, if approved, before our relevant patents expire;
|
|
•
|
we were the first to make the inventions covered by each of our patents and pending patent applications;
|
|
•
|
we were the first to file patent applications for these inventions;
|
|
•
|
others will not develop similar or alternative technologies that do not infringe our patents;
|
|
•
|
others will not use pre-existing technology to effectively compete against us;
|
|
•
|
any of our patents, if issued, will be found to ultimately be valid and enforceable;
|
|
•
|
any patents issued to us will provide a basis for an exclusive market for our commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties;
|
|
•
|
we will develop additional proprietary technologies or product candidates that are separately patentable; or
|
|
•
|
that our commercial activities or products will not infringe upon the patents or proprietary rights of others.
|
|
•
|
cease developing, selling or otherwise commercializing our product candidates;
|
|
•
|
pay substantial damages for past use of the asserted intellectual property;
|
|
•
|
obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable terms, if at all; and
|
|
•
|
in the case of trademark claims, redesign, or rename, some or all of our product candidates to avoid infringing the intellectual property rights of third parties, which may not be possible and, even if possible, could be costly and time-consuming.
|
|
•
|
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
•
|
whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
|
•
|
our right to sublicense patent and other rights to third parties under collaborative development relationships;
|
|
•
|
our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our product candidates, and what activities satisfy those diligence obligations; and
|
|
•
|
the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners.
|
|
•
|
others may be able to develop and/or practice technology that is similar to our technology or aspects of our technology but that is not covered by the claims of patents, should such patents issue from our patent applications;
|
|
•
|
we might not have been the first to make the inventions covered by a pending patent application that we own;
|
|
•
|
we might not have been the first to file patent applications covering an invention;
|
|
•
|
others may independently develop similar or alternative technologies without infringing our intellectual property rights;
|
|
•
|
pending patent applications that we own or license may not lead to issued patents;
|
|
•
|
patents, if issued, that we own or license may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors;
|
|
•
|
third parties may compete with us in jurisdictions where we do not pursue and obtain patent protection;
|
|
•
|
we may not be able to obtain and/or maintain necessary or useful licenses on reasonable terms or at all;
|
|
•
|
third parties may assert an ownership interest in our intellectual property and, if successful, such disputes may preclude us from exercising exclusive rights over that intellectual property;
|
|
•
|
we may not develop or in-license additional proprietary technologies that are patentable; and
|
|
•
|
the patents of others may have an adverse effect on our business.
|
|
•
|
plans for, progress of or results from non-clinical studies and clinical trials of our product candidates;
|
|
•
|
the failure of the FDA to approve our product candidates;
|
|
•
|
announcements of new products, technologies, commercial relationships, acquisitions or other events by us or our competitors;
|
|
•
|
the success or failure of other CNS therapies;
|
|
•
|
regulatory or legal developments in the United States and other countries;
|
|
•
|
failure of our product candidates, if approved, to achieve commercial success;
|
|
•
|
fluctuations in stock market prices and trading volumes of similar companies;
|
|
•
|
general market conditions and overall fluctuations in U.S. equity markets;
|
|
•
|
variations in our quarterly operating results;
|
|
•
|
changes in our financial guidance or securities analysts’ estimates of our financial performance;
|
|
•
|
changes in accounting principles;
|
|
•
|
our ability to raise additional capital and the terms on which we can raise it;
|
|
•
|
sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders;
|
|
•
|
additions or departures of key personnel;
|
|
•
|
discussion of us or our stock price by the press and by online investor communities; and
|
|
•
|
other risks and uncertainties described in these risk factors.
|
|
High
|
Low
|
|
|
Year Ending March 31, 2015
|
||
|
First quarter ending June 30, 2014
|
$14.80
|
$5.60
|
|
Second quarter ending September 30, 2014
|
$15.00
|
$7.99
|
|
Third quarter ending December 31, 2014
|
$10.50
|
$8.00
|
|
Fourth quarter ending March 31, 2015
|
$12.00
|
$3.16
|
|
Year Ending March 31, 2014
|
||
|
First quarter ending June 30, 2013
|
$18.00
|
$12.00
|
|
Second quarter ending September 30, 2013
|
$17.80
|
$11.00
|
|
Third quarter ending December 31, 2013
|
$12.20
|
$5.20
|
|
Fourth quarter ending March 31, 2014
|
$10.00
|
$5.60
|
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Operating expenses:
|
||||||||
|
Research and development
|
$ | 2,433 | $ | 2,481 | ||||
|
General and administrative
|
4,344 | 2,548 | ||||||
|
Total operating expenses
|
6,777 | 5,029 | ||||||
|
Loss from operations
|
(6,777 | ) | (5,029 | ) | ||||
|
Other expenses, net:
|
||||||||
|
Interest expense, net
|
(4,549 | ) | (1,503 | ) | ||||
|
Change in warrant liabilities
|
(35 | ) | 3,567 | |||||
|
Loss on extinguishment of debt
|
(2,388 | ) | - | |||||
|
Other expense
|
(135 | ) | - | |||||
|
Loss before income taxes
|
(13,884 | ) | (2,965 | ) | ||||
|
Income taxes
|
(2 | ) | (3 | ) | ||||
|
Net loss
|
$ | (13,886 | ) | $ | (2,968 | ) | ||
|
•
|
Salaries and benefits, including stock-based compensation costs, travel and related expense for personnel associated with internal research and development activities;
|
|
•
|
fees to contract research organizations and other professional service providers for services related to the conduct and analysis of clinical trials and other drug development activities;
|
|
•
|
fees to third parties for access to licensed technology and costs associated with securing and maintaining patents related to our internally generated inventions:
|
|
•
|
laboratory supplies and materials;
|
|
•
|
leasing and depreciation of laboratory equipment; and
|
|
•
|
allocated costs of facilities and infrastructure.
|
|
·
|
Collaborative arrangements typically consist of non-refundable and/or exclusive technology access fees, cost reimbursements for specific research and development spending, and various milestone and future product royalty payments. If the delivered technology does not have stand-alone value, the amount of revenue allocable to the delivered technology is deferred. Non-refundable upfront fees with stand-alone value that are not dependent on future performance under these agreements are recognized as revenue when received, and are deferred if we have continuing performance obligations and have no objective and reliable evidence of the fair value of those obligations. We recognize non-refundable upfront technology access fees under agreements in which we have a continuing performance obligation ratably, on a straight-line basis, over the period in which we are obligated to provide services. Cost reimbursements for research and development spending are recognized when the related costs are incurred and when collectability is reasonably assured. Payments received related to substantive, performance-based “at-risk” milestones are recognized as revenue upon achievement of the milestone event specified in the underlying contracts, which represent the culmination of the earnings process. Amounts received in advance are recorded as deferred revenue until the technology is transferred, costs are incurred, or a milestone is reached.
|
|
·
|
Technology license agreements typically consist of non-refundable upfront license fees, annual minimum access fees and/or royalty payments. Non-refundable upfront license fees and annual minimum payments received with separable stand-alone values are recognized when the technology is transferred or accessed, provided that the technology transferred or accessed is not dependent on the outcome of the continuing research and development efforts. Otherwise, revenue is recognized over the period of our continuing involvement.
|
|
·
|
Government grant awards, which support our research efforts on specific projects, generally provide for reimbursement of approved costs as defined in the terms of grant awards. We recognize grant revenue when associated project costs are incurred.
|
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Salaries and benefits
|
$ | 889 | $ | 902 | ||||
|
Stock-based compensation
|
849 | 453 | ||||||
|
UHN research under SRCA
|
- | 160 | ||||||
|
Consulting services
|
109 | 53 | ||||||
|
Technology licenses and royalties
|
217 | 484 | ||||||
|
Project-related third-party research and supplies:
|
||||||||
|
AV-101
|
51 | 51 | ||||||
|
All other including CardioSafe and LiverSafe
|
54 | 145 | ||||||
| 105 | 196 | |||||||
|
Rent
|
220 | 185 | ||||||
|
Depreciation
|
44 | 44 | ||||||
|
All other
|
- | 4 | ||||||
|
Total Research and Development Expense
|
$ | 2,433 | $ | 2,481 | ||||
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Salaries and benefits
|
$ | 714 | $ | 675 | ||||
|
Stock-based compensation
|
1,611 | 684 | ||||||
|
Consulting Services
|
112 | 94 | ||||||
|
Legal, accounting and other professional fees
|
1,197 | 340 | ||||||
|
Investor relations
|
132 | 120 | ||||||
|
Insurance
|
136 | 130 | ||||||
|
Travel and entertainment
|
71 | 18 | ||||||
|
Rent and utilities
|
155 | 139 | ||||||
|
Warrant modification expense
|
98 | 205 | ||||||
|
All other expenses
|
118 | 143 | ||||||
|
Total General and Administrative Expense
|
$ | 4,344 | $ | 2,548 | ||||
To conserve cash resources, during fiscal 2015 and 2014, both Shawn Singh, our Chief Executive Officer ( CEO ), and Jerrold Dotson, our Chief Financial Officer ( CFO ), have accepted voluntary cash pay reductions to substantially less than their contractual pay rates. For fiscal 2015, the CEO’s and CFO’s actual cash pay represented approximately 24% and 62%, respectively, of contractual rates. In fiscal 2014, the CEO and CFO voluntarily agreed to accept cash pay rates of approximately 72% and 80%, respectively, of their contractual rates, not all of which amounts were paid. In fiscal 2015, we have accrued the difference between the CEO’s and CFO’s contractual pay rate and his actual cash pay, $264,700 and $96,100, respectively, for future payment. In fiscal 2014, we accrued the difference between the CEO’s and CFO’s reduced pay rates and his actual cash pay, $125,000 and $56,700, respectively, for future payment. Such accrued amounts for both fiscal 2014 and fiscal 2015 remain unpaid. Offsetting the impact of the accrual to contractual pay rates for the CEO and CFO for fiscal 2015 is the annual impact of the voluntary resignations of two administrative employees in August and November 2013 who have not been replaced. Pay rates for other administrative employees remained stable between the periods presented.
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Interest expense on promissory notes
|
$ | 1,238 | $ | 907 | ||||
|
Amortization of discount on promissory notes
|
3,372 | 640 | ||||||
|
Other interest expense, including on capital leases and premium financing
|
7 | 15 | ||||||
| 4,617 | 1,562 | |||||||
|
Effect of foreign currency fluctuations on notes payable
|
(63 | ) | (49 | ) | ||||
|
Interest income
|
(5 | ) | (10 | ) | ||||
|
Interest expense, net
|
$ | 4,549 | $ | 1,503 | ||||
|
Fiscal Years Ended
|
||||||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Net cash used in operating activities
|
$ | (2,769 | ) | $ | (2,126 | ) | ||
|
Net cash used in investing activities
|
- | (10 | ) | |||||
|
Net cash provided by financing activities
|
2,839 | 1,498 | ||||||
|
Net increase (decrease) in cash and cash equivalents
|
70 | (638 | ) | |||||
|
Cash and cash equivalents at beginning of period
|
- | 638 | ||||||
|
Cash and cash equivalents at end of period
|
$ | 70 | $ | - | ||||
Off-Balance Sheet Arrangements
|
Page
|
|
|
95
|
|
|
96
|
|
|
97
|
|
|
98
|
|
|
99
|
|
|
100
|
|
VISTAGEN THERAPEUTICS
|
||||||||
|
|
||||||||
|
Amounts in Dollars
|
||||||||
|
March 31,
|
March 31,
|
|||||||
|
2015
|
2014
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 70,000 | $ | - | ||||
|
Prepaid expenses and other current assets
|
35,700 | 40,500 | ||||||
|
Total current assets
|
105,700 | 40,500 | ||||||
|
Property and equipment, net
|
117,100 | 176,300 | ||||||
|
Security deposits and other assets
|
46,900 | 46,900 | ||||||
|
Total assets
|
$ | 269,700 | $ | 263,700 | ||||
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 2,251,100 | $ | 2,443,900 | ||||
|
Accrued expenses
|
1,206,500 | 625,600 | ||||||
|
Advance from officer
|
- | 3,600 | ||||||
|
Current maturities of senior secured convertible promissory notes and accrued interest
|
4,146,100 | - | ||||||
|
Current portion of notes payable, net of discount of $474,500 at March 31, 2014 and accrued interest
|
4,117,000 | 1,442,300 | ||||||
|
Current portion of notes payable to related parties, net of discount of $54,500 at March 31, 2015
|
||||||||
|
and accrued interest
|
1,508,800 | 290,400 | ||||||
|
Convertible promissory notes and accrued interest, net of discount of $180,000 at March 31, 2015 and
|
||||||||
|
$697,400 at March 31, 2014, respectively
|
4,157,600 | 396,000 | ||||||
|
Capital lease obligations
|
1,000 | 3,900 | ||||||
|
Total current liabilities
|
17,388,100 | 5,205,700 | ||||||
|
Non-current liabilities:
|
||||||||
|
Senior secured convertible promissory notes, net of discount of $0 at March 31, 2015 and
|
||||||||
|
$2,085,900 at March 31, 2014, respectively, and accrued interest
|
296,200 | 1,929,800 | ||||||
|
Notes payable, net of discount of $0 at March 31, 2015 and $848,100 at March 31, 2014, and
|
||||||||
|
and accrued interest
|
35,600 | 1,797,600 | ||||||
|
Notes payable to related parties, net of discount of $103,200 at March 31, 2014 and accrued interest
|
- | 1,057,100 | ||||||
|
Warrant liability
|
3,008,500 | 2,973,900 | ||||||
|
Deferred rent liability
|
83,000 | 97,400 | ||||||
|
Capital lease obligations
|
1,100 | 2,100 | ||||||
|
Total non-current liabilities
|
3,424,400 | 7,857,900 | ||||||
|
Total liabilities
|
20,812,500 | 13,063,600 | ||||||
|
Commitments and contingencies
|
||||||||
|
Stockholders’ deficit:
|
||||||||
|
Preferred stock, $0.001 par value; 10,000,000 shares, including 500,000 Series A shares, authorized
|
||||||||
|
at March 31, 2015 and March 31, 2014, respectively; 500,000 Series A shares issued and
|
||||||||
|
outstanding at March 31, 2015 and March 31, 2014, respectively
|
500 | 500 | ||||||
|
Common stock, $0.001 par value; 10,000,000 shares authorized at March 31, 2015 and March 31, 2014,
|
||||||||
|
respectively; 1,677,110 shares and 1,310,093 shares issued at March 31, 2015 and March 31,
|
||||||||
|
2014, respectively
|
1,700 | 1,300 | ||||||
|
Additional paid-in capital
|
67,945,800 | 62,001,400 | ||||||
|
Treasury stock, at cost, 135,665 shares of common stock held at March 31, 2015 and March 31, 2014,
|
||||||||
|
respectively
|
(3,968,100 | ) | (3,968,100 | ) | ||||
|
Note receivable from sale of common stock
|
- | (198,100 | ) | |||||
|
Accumulated deficit
|
(84,522,700 | ) | (70,636,900 | ) | ||||
|
Total stockholders’ deficit
|
(20,542,800 | ) | (12,799,900 | ) | ||||
|
Total liabilities and stockholders’ deficit
|
$ | 269,700 | $ | 263,700 | ||||
|
Fiscal Years Ended
|
||||||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Operating expenses:
|
||||||||
|
Research and development
|
$ | 2,432,700 | $ | 2,480,600 | ||||
|
General and administrative
|
4,344,400 | 2,548,300 | ||||||
|
Total operating expenses
|
6,777,100 | 5,028,900 | ||||||
|
Loss from operations
|
(6,777,100 | ) | (5,028,900 | ) | ||||
|
Other expenses, net:
|
||||||||
|
Interest expense, net
|
(4,548,700 | ) | (1,503,000 | ) | ||||
|
Change in warrant liability
|
(34,600 | ) | 3,566,900 | |||||
|
Loss on extinguishment of debt
|
(2,388,000 | ) | - | |||||
|
Other expense
|
(135,000 | ) | - | |||||
|
Loss before income taxes
|
(13,883,400 | ) | (2,965,000 | ) | ||||
|
Income taxes
|
(2,400 | ) | (2,700 | ) | ||||
|
Net loss and comprehensive loss
|
$ | (13,885,800 | ) | $ | (2,967,700 | ) | ||
|
Basic net loss per common share
|
$ | (10.53 | ) | $ | (2.70 | ) | ||
|
Diluted net loss per common share
|
$ | (10.61 | ) | $ | (3.81 | ) | ||
|
Weighted average shares used in computing
|
||||||||
|
Basic net loss per common share
|
1,318,797 | 1,098,742 | ||||||
|
Diluted net loss per common share
|
1,318,797 | 1,099,216 | ||||||
See accompanying notes to consolidated financial statements.
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Cash flows from operating activities:
|
||||||||
|
Net loss
|
$ | (13,885,800 | ) | $ | (2,967,700 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||
|
Depreciation and amortization
|
59,100 | 54,600 | ||||||
|
Amortization of discounts on convertible and promissory notes
|
3,372,000 | 640,000 | ||||||
|
Change in warrant liability
|
34,600 | (3,566,900 | ) | |||||
|
Stock-based compensation
|
2,460,100 | 1,137,300 | ||||||
|
Expense related to modification of warrants
|
98,400 | 204,300 | ||||||
|
Non-cash rent and relocation expense
|
(14,400 | ) | 56,800 | |||||
|
Interest income on note receivable for stock purchase
|
2,800 | (1,200 | ) | |||||
|
Loss on settlement of note receivable for stock purchase
|
134,900 | - | ||||||
|
Fair value of common stock granted for services
|
469,000 | - | ||||||
|
Fair value of warrants granted for services and interest
|
44,500 | 60,700 | ||||||
|
Gain on currency fluctuation
|
(63,600 | ) | (48,600 | ) | ||||
|
Loss on extinguishment of debt
|
2,388,000 | - | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Prepaid expenses and other current assets
|
107,400 | 92,700 | ||||||
|
Security deposits and other assets
|
- | (17,900 | ) | |||||
|
Accounts payable and accrued expenses, including accrued interest
|
2,024,100 | 2,229,900 | ||||||
|
Net cash used in operating activities
|
(2,768,900 | ) | (2,126,000 | ) | ||||
|
Cash flows from investing activities:
|
||||||||
|
Purchases of equipment, net
|
- | (9,600 | ) | |||||
|
Net cash used in investing activities
|
- | (9,600 | ) | |||||
|
Cash flows from financing activities:
|
||||||||
|
Net proceeds from issuance of common stock and warrants, including Units
|
3,146,600 | 1,075,500 | ||||||
|
Proceeds from exercise of modified warrants
|
- | 264,200 | ||||||
|
Proceeds from sale of note and warrant to Platinum
|
- | 250,000 | ||||||
|
Advance from officer
|
- | 64,000 | ||||||
|
Repayment of capital lease obligations
|
(3,900 | ) | (7,600 | ) | ||||
|
Repayment of notes
|
(303,800 | ) | (148,600 | ) | ||||
|
Net cash provided by financing activities
|
2,838,900 | 1,497,500 | ||||||
|
Net increase (decrease) in cash and cash equivalents
|
70,000 | (638,100 | ) | |||||
|
Cash and cash equivalents at beginning of period
|
- | 638,100 | ||||||
|
Cash and cash equivalents at end of period
|
$ | 70,000 | $ | - | ||||
|
Supplemental disclosure of cash flow activities:
|
||||||||
|
Cash paid for interest
|
$ | 35,700 | $ | 21,000 | ||||
|
Cash paid for income taxes
|
$ | 2,400 | $ | 2,700 | ||||
|
Supplemental disclosure of noncash activities:
|
||||||||
|
Insurance premiums settled by issuing note payable
|
$ | 105,300 | $ | 98,300 | ||||
|
Accounts payable settled by issuance of stock or notes payable and stock
|
$ | 438,400 | $ | - | ||||
|
Recognition of warrant liability upon issuance to Platinum of July 2013
|
||||||||
|
Senior Secured Convertible Note
|
$ | - | $ | 146,800 | ||||
|
Series A Preferred Stock
|
Common Stock
|
Additional
Paid-in
|
Treasury
|
Note
Receivable
|
Accumulated
|
Total
Stockholders’
|
||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Stock
|
Stock
|
Deficit
|
Deficit
|
||||||||||||||||||||||||||||
|
Balances at March 31, 2013
|
500,000 | $ | 500 | 1,174,092 | $ | 1,200 | $ | 59,288,300 | $ | (3,968,100 | ) | $ | (209,100 | ) | $ | (67,669,200 | ) | $ | (12,556,400 | ) | ||||||||||||||||
|
Share-based compensation expense
|
- | - | - | - | 1,137,300 | - | - | - | 1,137,300 | |||||||||||||||||||||||||||
|
Proceeds from sale of common stock for cash, including exercises
of warrants under Discount Warrant Exercise Program
|
- | - | 32,751 | - | 335,900 | - | - | - | 335,900 | |||||||||||||||||||||||||||
|
Beneficial conversion feature on note issued to Platinum in
July 2013
|
- | - | - | - | 100,700 | - | - | - | 100,700 | |||||||||||||||||||||||||||
|
Payment on note receivable from sale of stock
|
- | - | - | - | - | - | 11,000 | - | 11,000 | |||||||||||||||||||||||||||
|
Allocated proceeds from sale of Units for cash under 2013 Unit Private Placement, including beneficial conversion feature
|
- | - | 100,750 | 100 | 838,100 | - | - | - | 838,200 | |||||||||||||||||||||||||||
|
Allocated proceeds from sale of Units for cash under 2014 Unit Private Placement, including beneficial conversion feature
|
- | - | 2,500 | - | 36,000 | 36,000 | ||||||||||||||||||||||||||||||
|
Incremental fair value of warrant modifications
|
- | - | - | - | 204,300 | - | - | - | 204,300 | |||||||||||||||||||||||||||
|
Fair value of warrants issued to Morrison & Foerster, Cato Research Ltd. and University Health Network in connection with accrued interest on underlying notes
|
- | - | - | - | 60,800 | - | - | - | 60,800 | |||||||||||||||||||||||||||
|
Net loss for fiscal year 2014
|
- | - | - | - | - | - | - | (2,967,700 | ) | (2,967,700 | ) | |||||||||||||||||||||||||
|
Balances at March 31, 2014
|
500,000 | $ | 500 | 1,310,093 | $ | 1,300 | $ | 62,001,400 | $ | (3,968,100 | ) | $ | (198,100 | ) | $ | (70,636,900 | ) | $ | (12,799,900 | ) | ||||||||||||||||
|
Allocated proceeds from sale of Units for cash under 2014 Unit Private Placement, including beneficial conversion feature
|
- | - | 280,350 | 300 | 2,746,800 | - | - | - | 2,747,100 | |||||||||||||||||||||||||||
|
Share-based compensation expense
|
- | - | - | 2,460,100 | - | - | - | 2,460,100 | ||||||||||||||||||||||||||||
|
Payment on and settlement of note receivable from sale of stock
|
- | - | - | - | - | - | 198,100 | - | 198,100 | |||||||||||||||||||||||||||
|
Incremental fair value of modified warrants
|
- | - | - | - | 98,400 | - | - | - | 98,400 | |||||||||||||||||||||||||||
|
Fair Value of common stock issued for services
|
- | - | 71,667 | 100 | 635,600 | - | - | - | 635,700 | |||||||||||||||||||||||||||
|
Fair value of common stock and warrants issued in settlement of technology license expenses
|
- | - | 15,000 | - | 230,200 | - | - | - | 230,200 | |||||||||||||||||||||||||||
|
Fair value of warrants issued to Morrison & Foerster, Cato Research Ltd. and University Health Network in connection with accrued
interest on underlying notes
|
- | - | - | - | 44,400 | - | - | - | 44,400 | |||||||||||||||||||||||||||
|
Effect of amendments of 2013 Unit Notes and warrants, including repurchase of beneficial conversion feature
|
- | - | - | - | 109,300 | - | - | - | 109,300 | |||||||||||||||||||||||||||
|
Effect of amendments of Platinum Senior Secured Promissory Notes, including repurchase of beneficial conversion feature
|
- | - | - | - | (380,400 | ) | - | - | - | (380,400 | ) | |||||||||||||||||||||||||
|
Net loss for fiscal year 2015
|
- | - | - | - | - | - | - | (13,885,800 | ) | (13,885,800 | ) | |||||||||||||||||||||||||
|
Balances at March 31, 2015
|
500,000 | $ | 500 | 1,677,110 | $ | 1,700 | $ | 67,945,800 | $ | (3,968,100 | ) | $ | - | $ | (84,522,700 | ) | $ | (20,542,800 | ) | |||||||||||||||||
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Numerator:
|
||||||||
|
Net loss attributable to common stockholders for basic earnings per share
|
$ | (13,885,800 | ) | $ | (2,967,700 | ) | ||
|
less: change in fair value of warrant liability attributable to Exchange, Investment and Bridge Warrants issued to Platinum
|
(105,200 | ) | (1,219,500 | ) | ||||
|
Net loss for diluted earnings per share attributable to common stockholders
|
$ | (13,991,000 | ) | $ | (4,187,200 | ) | ||
|
Denominator:
|
||||||||
|
Weighted average basic common shares outstanding
|
1,318,797 | 1,098,742 | ||||||
|
Assumed conversion of dilutive securities:
|
||||||||
|
Warrants to purchase common stock
|
- | 474 | ||||||
|
Potentially dilutive common shares assumed converted
|
- | 474 | ||||||
|
Denominator for diluted earnings per share - adjusted weighted average shares
|
1,318,797 | 1,099,216 | ||||||
|
Basic net loss attributable to common stockholders per common share
|
$ | (10.53 | ) | $ | (2.70 | ) | ||
|
Diluted net loss attributable to common stockholders per common share
|
$ | (10.61 | ) | $ | (3.81 | ) | ||
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Series A preferred stock issued and outstanding
(1)
|
750,000 | 750,000 | ||||||
|
Warrant shares issuable to Platinum upon exercise of common stock warrants by Platinum
|
||||||||
|
upon exchange of Series A Preferred under the terms of the October 11, 2012 Note
|
||||||||
|
Exchange and Purchase Agreement
|
375,000 | 375,000 | ||||||
|
Outstanding options under the 2008 and 1999 Stock Incentive Plans
|
207,638 | 212,486 | ||||||
|
Outstanding warrants to purchase common stock
|
1,544,474 | 854,782 | ||||||
|
10% convertible Exchange Note and Investment Notes issued to Platinum in October 2012,
|
||||||||
|
February 2013 and March 2013, including accrued interest through March 31, 2015
|
||||||||
|
and 2014, respectively
(2)
|
414,615 | 374,798 | ||||||
|
10% convertible note issued to Platinum on July 26, 2013, including accrued interest
|
||||||||
|
through March 31, 2015 and 2014, respectively
|
29,620 | 26,776 | ||||||
|
10% convertible notes issued as a component of Unit Private Placements, including accrued interest through March 31, 2014
|
||||||||
|
accrued interest through March 31, 2015 and 2014, respectively
(3)
|
433,758 | 109,341 | ||||||
|
Total
|
3,755,105 | 2,703,183 | ||||||
|
____________
|
||||||||
|
(1)
Assumes exchange under the terms of the October 11, 2012 Note Exchange and Purchase Agreement with Platinum
|
||||||||
|
(2)
Assumes conversion under the terms of the October 11, 2012 Note Exchange and Purchase Agreement with Platinum and the terms of the individual notes
|
||||||||
|
(3)
Excludes effect of conversion premium upon conversion into securities which may be issued in a Qualified Financing, as defined in the notes
|
||||||||
|
|
•
|
Level 1
— Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
|
|
|
•
|
Level 2
— Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
|
•
|
Level 3
— Unobservable inputs (
i.e.,
inputs that reflect the reporting entity’s own assumptions about the assumptions that market participants would use in estimating the fair value of an asset or liability) are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs.
|
|
Fair Value Measurements at Reporting Date Using
|
||||||||||||||||
|
Total
Carrying
|
Quoted Prices in
Active Markets for
|
Significant Other
Observable Inputs
|
Significant Unobservable
Inputs
|
|||||||||||||
|
|
Value
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
||||||||||||
|
March 31, 2015:
|
||||||||||||||||
|
Warrant liability
|
$ | 3,008,500 | $ | - | $ | - | $ | 3,008,500 | ||||||||
|
March 31, 2014:
|
||||||||||||||||
|
Warrant liability
|
$ | 2,973,900 | $ | - | $ | - | $ | 2,973,900 | ||||||||
|
Fair Value Measurements
Using Significant
|
||||
|
(Level 3)
|
||||
|
Warrant Liability
|
||||
|
Balance at March 31, 2013
|
$ | 6,394,000 | ||
|
Recognition of warrant liability upon issuance of Senior Secured Convertible
|
||||
|
Promissory Note and warrant to Platinum on July 26, 2013
|
146,800 | |||
|
Mark to market gain included in net loss
|
(3,566,900 | ) | ||
|
Balance at March 31, 2014
|
2,973,900 | |||
|
Mark to market loss included in net loss
|
34,600 | |||
|
Balance at March 31, 2015
|
$ | 3,008,500 | ||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Insurance
|
$ | 27,300 | $ | 21,800 | ||||
|
Legal fees
|
3,400 | 3,400 | ||||||
|
Interest receivable on note receivable from sale
|
||||||||
|
of common stock
|
- | 2,800 | ||||||
|
Technology license fees and all other
|
5,000 | 12,500 | ||||||
| $ | 35,700 | $ | 40,500 | |||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Laboratory equipment
|
$ | 653,600 | $ | 653,600 | ||||
|
Tenant improvements
|
26,900 | 26,900 | ||||||
|
Computers and network equipment
|
32,200 | 32,200 | ||||||
|
Office furniture and equipment
|
69,500 | 69,500 | ||||||
| 782,200 | 782,200 | |||||||
|
Accumulated depreciation and amortization
|
(665,100 | ) | (605,900 | ) | ||||
|
Property and equipment, net
|
$ | 117,100 | $ | 176,300 | ||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Accrued professional services
|
$ | 213,800 | $ | 135,700 | ||||
|
Accrued compensation
|
990,700 | 489,900 | ||||||
|
All other
|
2,000 | - | ||||||
| $ | 1,206,500 | $ | 625,600 | |||||
|
March 31, 2015
|
March 31, 2014
|
|||||||||||||||||||||||
|
|
Principal
|
Accrued
|
Principal
|
Accrued
|
||||||||||||||||||||
|
Balance
|
Interest
|
Total
|
Balance
|
Interest
|
Total
|
|||||||||||||||||||
|
Senior Secured 10% Convertible Promissory Notes issued to Platinum:
|
||||||||||||||||||||||||
|
Exchange Note issued on October 11, 2012
|
$ | 1,272,600 | $ | 360,200 | $ | 1,632,800 | $ | 1,272,600 | $ | 203,400 | $ | 1,476,000 | ||||||||||||
|
Investment Note issued on October 11, 2012
|
500,000 | 141,500 | 641,500 | 500,000 | 79,900 | 579,900 | ||||||||||||||||||
|
Investment Note issued on October 19, 2012
|
500,000 | 140,100 | 640,100 | 500,000 | 78,600 | 578,600 | ||||||||||||||||||
|
Investment Note issued on February 22, 2013
|
250,000 | 59,100 | 309,100 | 250,000 | 29,400 | 279,400 | ||||||||||||||||||
|
Investment Note issued on March 12, 2013
|
750,000 | 172,600 | 922,600 | 750,000 | 84,100 | 834,100 | ||||||||||||||||||
| 3,272,600 | 873,500 | 4,146,100 | 3,272,600 | 475,400 | 3,748,000 | |||||||||||||||||||
|
Convertible promissory note issued on July 26, 2013
|
250,000 | 46,200 | 296,200 | 250,000 | 17,700 | 267,700 | ||||||||||||||||||
|
Total Senior notes
|
3,522,600 | 919,700 | 4,442,300 | 3,522,600 | 493,100 | 4,015,700 | ||||||||||||||||||
|
Aggregate note discount
|
- | - | - | (2,085,900 | ) | - | (2,085,900 | ) | ||||||||||||||||
|
Net Senior notes
|
3,522,600 | 919,700 | 4,442,300 | 1,436,700 | 493,100 | 1,929,800 | ||||||||||||||||||
|
less: current portion
|
(3,272,600 | ) | (873,500 | ) | (4,146,100 | ) | - | - | - | |||||||||||||||
|
Senior notes - non-current portion and discount
|
$ | 250,000 | $ | 46,200 | $ | 296,200 | $ | 1,436,700 | $ | 493,100 | $ | 1,929,800 | ||||||||||||
|
10% Convertible Promissory Notes (Unit Notes)
|
||||||||||||||||||||||||
|
2013 Unit Notes, due 7/31/14
|
$ | - | $ | - | $ | - | $ | 1,007,500 | $ | 35,700 | $ | 1,043,200 | ||||||||||||
|
2014 Unit Notes, including amended notes, due 3/31/15
|
4,066,900 | 270,700 | 4,337,600 | 50,000 | 200 | 50,200 | ||||||||||||||||||
| 4,066,900 | 270,700 | 4,337,600 | 1,057,500 | 35,900 | 1,093,400 | |||||||||||||||||||
|
Note discounts
|
(180,000 | ) | - | (180,000 | ) | (697,400 | ) | - | (697,400 | ) | ||||||||||||||
|
Net convertible notes (all current)
|
$ | 3,886,900 | $ | 270,700 | $ | 4,157,600 | $ | 360,100 | $ | 35,900 | $ | 396,000 | ||||||||||||
|
Notes Payable to unrelated parties:
|
||||||||||||||||||||||||
|
7.5% Notes payable to service providers for
|
||||||||||||||||||||||||
|
accounts payable converted to notes payable:
|
||||||||||||||||||||||||
|
Burr, Pilger, Mayer
|
$ | 90,400 | $ | 13,100 | $ | 103,500 | $ | 90,400 | $ | 6,800 | $ | 97,200 | ||||||||||||
|
Desjardins
|
156,300 | 24,100 | 180,400 | 178,600 | 14,100 | 192,700 | ||||||||||||||||||
|
McCarthy Tetrault
|
319,700 | 46,000 | 365,700 | 360,900 | 24,800 | 385,700 | ||||||||||||||||||
|
August 2012 Morrison & Foerster Note A
|
918,200 | 193,200 | 1,111,400 | 918,200 | 87,900 | 1,006,100 | ||||||||||||||||||
|
August 2012 Morrison & Foerster Note B
(1)
|
1,379,400 | 333,100 | 1,712,500 | 1,379,400 | 195,200 | 1,574,600 | ||||||||||||||||||
|
University Health Network
(1)
|
549,500 | 101,800 | 651,300 | 549,500 | 60,600 | 610,100 | ||||||||||||||||||
| 3,413,500 | 711,300 | 4,124,800 | 3,477,000 | 389,400 | 3,866,400 | |||||||||||||||||||
|
Note discount
|
(474,500 | ) | - | (474,500 | ) | (848,100 | ) | - | (848,100 | ) | ||||||||||||||
| 2,939,000 | 711,300 | 3,650,300 | 2,628,900 | 389,400 | 3,018,300 | |||||||||||||||||||
|
less: current portion
|
(3,413,500 | ) | (711,300 | ) | (4,124,800 | ) | (1,130,100 | ) | (133,600 | ) | (1,263,700 | ) | ||||||||||||
|
non-current portion and discount
|
$ | (474,500 | ) | $ | - | $ | (474,500 | ) | $ | 1,498,800 | $ | 255,800 | $ | 1,754,600 | ||||||||||
|
5.75% and 10.25% Notes payable to insurance
|
||||||||||||||||||||||||
|
premium financing company (current)
|
$ | 5,800 | $ | - | $ | 5,800 | $ | 4,900 | $ | - | $ | 4,900 | ||||||||||||
|
10% Notes payable to vendors for accounts
|
||||||||||||||||||||||||
|
payable converted to notes payable
|
$ | 378,300 | $ | 51,500 | $ | 429,800 | $ | 119,400 | $ | 34,700 | $ | 154,100 | ||||||||||||
|
less: current portion
|
(378,300 | ) | (51,500 | ) | (429,800 | ) | (119,400 | ) | (34,700 | ) | (154,100 | ) | ||||||||||||
|
non-current portion
|
$ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||
|
7.0% Note payable (August 2012)
|
$ | 58,800 | $ | 7,900 | $ | 66,700 | $ | 58,800 | $ | 3,800 | $ | 62,600 | ||||||||||||
|
less: current portion
|
(23,200 | ) | (7,900 | ) | (31,100 | ) | (15,800 | ) | (3,800 | ) | (19,600 | ) | ||||||||||||
|
7.0% Notes payable - non-current portion
|
$ | 35,600 | $ | - | $ | 35,600 | $ | 43,000 | $ | - | $ | 43,000 | ||||||||||||
|
Total notes payable to unrelated parties
|
$ | 3,856,400 | $ | 770,700 | $ | 4,627,100 | $ | 3,660,100 | $ | 427,900 | $ | 4,088,000 | ||||||||||||
|
less: current portion
|
(3,820,800 | ) | (770,700 | ) | (4,591,500 | ) | (1,270,200 | ) | (172,100 | ) | (1,442,300 | ) | ||||||||||||
|
non-current portion
|
35,600 | - | 35,600 | 2,389,900 | 255,800 | 2,645,700 | ||||||||||||||||||
|
less: discount (current at March 31, 2015)
|
- | - | - | (848,100 | ) | - | (848,100 | ) | ||||||||||||||||
|
Net non-current portion
|
$ | 35,600 | $ | - | $ | 35,600 | $ | 1,541,800 | $ | 255,800 | $ | 1,797,600 | ||||||||||||
|
Notes payable to related parties:
|
||||||||||||||||||||||||
|
October 2012 7.5% Note to Cato Holding Co.
|
$ | 293,600 | $ | 55,900 | $ | 349,500 | $ | 293,600 | $ | 30,800 | $ | 324,400 | ||||||||||||
|
October 2012 7.5% Note to Cato Research Ltd.
(1)
|
1,009,000 | 204,800 | 1,213,800 | 1,009,000 | 117,300 | 1,126,300 | ||||||||||||||||||
| 1,302,600 | 260,700 | 1,563,300 | 1,302,600 | 148,100 | 1,450,700 | |||||||||||||||||||
|
Note discount
|
(54,500 | ) | - | (54,500 | ) | (103,200 | ) | - | (103,200 | ) | ||||||||||||||
|
Total notes payable to related parties
|
1,248,100 | 260,700 | 1,508,800 | 1,199,400 | 148,100 | 1,347,500 | ||||||||||||||||||
|
less: current portion
|
(1,248,100 | ) | (260,700 | ) | (1,508,800 | ) | (259,600 | ) | (30,800 | ) | (290,400 | ) | ||||||||||||
|
non-current portion and discount
|
$ | - | $ | - | $ | - | $ | 939,800 | $ | 117,300 | $ | 1,057,100 | ||||||||||||
|
____________
|
||||||||||||||||||||||||
|
(1)
Note and interest payable solely in restricted shares of the Company's common stock.
|
||||||||||||||||||||||||
|
·
|
December 2011 Common Stock Exchange Agreement with Platinum
|
|
·
|
December 2011 Note and Warrant Exchange Agreement with Platinum
|
|
·
|
2012 Exchange Agreement with Platinum
|
|
Unit Warrants
|
||||||||||||
|
Weighted Average Issuance Date Valuation Assumptions
|
Per Share
Fair
|
Aggregate
Fair Value
|
Aggregate
Proceeds
|
Aggregate Allocation of Proceeds
Based on Relative Fair Value of:
|
||||||||
|
Warrant
|
Risk free
|
|||||||||||
|
Shares
|
Market
|
Exercise
|
Term
|
Interest
|
Dividend
|
Value of
|
of Unit
|
of Unit
|
Unit
|
|||
|
Issued
|
Price
|
Price
|
(Years)
|
Rate
|
Volatility
|
Rate
|
Warrant
|
Warrants
|
Sales
|
Unit Stock
|
Warrant
|
Unit Note
|
|
282,850
|
$9.28
|
$10.00
|
2.17
|
0.62%
|
72.36%
|
0.0%
|
$3.63
|
$1,027,000
|
$3,133,500
|
$1,122,400
|
$454,200
|
$1,556,900
|
|
2013Unit Warrants
|
||||||||||||
|
Weighted Average Issuance Date Valuation Assumptions
|
Per Share
Fair
|
Aggregate
Fair Value
|
Aggregate
Proceeds
|
Aggregate Allocation of Proceeds
Based on Relative Fair Value of:
|
||||||||
|
Warrant
|
Risk free
|
|||||||||||
|
Shares
|
Market
|
Exercise
|
Term
|
Interest
|
Dividend
|
Value of
|
of Unit
|
of Unit
|
Unit
|
|||
|
Issued
|
Price
|
Price
|
(Years)
|
Rate
|
Volatility
|
Rate
|
Warrant
|
Warrants
|
Sales
|
Unit Stock
|
Warrant
|
Unit Note
|
|
100,750
|
$9.01
|
$20.00
|
2.68
|
0.58%
|
76.29%
|
0.0%
|
$2.53
|
$254,700
|
$1,007,500
|
$415,000
|
$111,400
|
$481,100
|
|
Assumption:
|
Pre-modification
|
Post-modification
|
||||||
|
Market price per share
|
$
|
12.60
|
$
|
12.60
|
||||
|
Exercise price per share
|
$
|
20.00
|
$
|
10.00
|
||||
|
Risk-free interest rate
|
0.44%
|
0.62%
|
||||||
|
Remaining contractual term in years
|
2.17
|
2.59
|
||||||
|
Volatility
|
75.6%
|
76.6%
|
||||||
|
Dividend rate
|
0.0%
|
0.0%
|
||||||
|
Fair Value per share
|
$
|
3.73
|
$
|
6.65
|
||||
|
Assumption:
|
Pre-modification
|
Post-modification
|
||||||
|
Market price per share at modification date
|
$
|
8.00
|
$
|
8.00
|
||||
|
Exercise price per share (weighted average)
|
$
|
23.13
|
$
|
13.00
|
||||
|
Risk-free interest rate (weighted average)
|
0.04%
|
0.31%
|
||||||
|
Contractual term in years (weighted average)
|
0.24
|
1.24
|
||||||
|
Volatility (weighted average)
|
69.7%
|
69.8%
|
||||||
|
Dividend rate
|
0.0%
|
0.0%
|
||||||
|
Weighted Average Fair Value per share
|
$
|
0.22
|
$
|
1.31
|
||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Market price of common stock
|
$ | 10.00 | $ | 9.20 | ||||
|
Exercise price per share
|
$ | 10.00 | $ | 9.80 to $10.00 | ||||
|
Risk-free interest rate
|
0.74% to 1.37%
|
1.73% | ||||||
|
Volatility
|
73.3% to 75.9%
|
75% | ||||||
|
Term (years)
|
2.5 to 5.0
|
3.5 to 5.0
|
||||||
|
Dividend rate
|
0% | 0% | ||||||
|
Probability of Series A Preferred exchange
|
95% | 95% | ||||||
|
Fair value per share
|
$ | 4.45 to $6.17 | $ | 5.20 to $5.80 | ||||
|
Assumption:
|
Pre-modification
|
Post-modification
|
||||||
|
Market price per share at modification date
|
$
|
10.00
|
$
|
10.00
|
||||
|
Exercise price per share (weighted average)
|
$
|
31.97
|
$
|
24.68
|
||||
|
Risk-free interest rate (weighted average)
|
0.33%
|
0.44%
|
||||||
|
Contractual term in years (weighted average)
|
1.40
|
2.10
|
||||||
|
Volatility (weighted average)
|
74.4%
|
75.8%
|
||||||
|
Dividend rate
|
0.0%
|
0.0%
|
||||||
|
Weighted Average Fair Value per share
|
$
|
1.08
|
$
|
2.29
|
||||
|
Assumption:
|
Pre-modification
|
Post-modification
|
||||||
|
Market price per share at modification date
|
$
|
8.00
|
$
|
8.00
|
||||
|
Exercise price per share (weighted average)
|
$
|
33.49
|
$
|
10.00
|
||||
|
Risk-free interest rate (weighted average)
|
0.51%
|
0.57%
|
||||||
|
Contractual term in years (weighted average)
|
2.06
|
2.34
|
||||||
|
Volatility (weighted average)
|
73.6%
|
74.4%
|
||||||
|
Dividend rate
|
0.0%
|
0.0%
|
||||||
|
Weighted Average Fair Value per share
|
$
|
0.91
|
$
|
2.85
|
||||
|
Exercise
Price
per Share
|
Expiration
Date
|
Shares Subject
to Purchase at
|
|||||
| $ | 10.00 |
1/31/2016 to 1/1/2020
|
1,064,683 | ||||
| $ | 12.80 |
3/3/2023
|
147,000 | ||||
| $ | 15.00 |
1/31/2016 to 6/11/2016
|
54,477 | ||||
| $ | 20.00 |
7/30/2016 to 9/30/2017
|
186,388 | ||||
| $ | 30.00 |
2/13/2016 to 3/4/2018
|
69,426 | ||||
| $ | 40.00 |
9/15/2017
|
21,250 | ||||
| $ | 60.00 |
2/13/2016
|
1,250 | ||||
| 1,544,474 | |||||||
|
Upon exchange of all shares of Series A Preferred Stock currently issued and outstanding
(1)
|
750,000 | |||
|
Warrant shares issuable to Platinum upon exercise of common stock warrant upon exchange of Series A preferred stock under the terms of the October 11, 2012 Note Purchase and Exchange Agreement
|
375,000 | |||
|
110% of shares issuable upon conversion of 10% senior secured convertible notes issued to Platinum in October 2012, February 2013, March 2013 and July 2013, including interest accrued through maturity
(2)
|
563,871 | |||
|
Pursuant to warrants to purchase common stock:
|
||||
|
Subject to outstanding warrants
|
1,544,474 | |||
|
Issuable pursuant to accrued interest through maturity on outstanding promissory notes issued to Morrison & Foerster, Cato Research Ltd., and University Health Network
|
33,612 | |||
| 1,578,086 | ||||
|
Pursuant to stock incentive plans:
|
||||
|
Subject to outstanding options under the 2008 and 1999 Stock Incentive Plans
|
207,638 | |||
|
Available for future grants under the 2008 Stock Incentive Plan
|
40,491 | |||
| 248,129 | ||||
|
For additional issuances under the 2014 Private Placement of Units and upon conversion of notes and accrued interest pursuant to the 2013 Private Placement of Units and 2014 Private Placement of Units
|
807,800 | |||
|
Total
|
4,322,886 |
|
____________
|
||||
|
(1)
assumes exchange under the terms of the October 11, 2012 Note Exchange and Purchase Agreement with Platinum
|
||||
|
(2)
assumes conversion under the terms of the October 11, 2012 Note Exchange and Purchase Agreement with Platinum and the terms of the individual notes
|
||||
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Computed expected tax benefit
|
(34.0 | ) % | (34.0 | ) % | ||||
|
Tax effect of Warrant Liability mark to market
|
(0.1 | ) % | 41.5 | % | ||||
|
Other losses not benefitted
|
34.0 | % | (7.5 | ) % | ||||
|
Other
|
0.1 | % | 0.1 | % | ||||
|
Income tax expense
|
0.0 | % | 0.1 | % | ||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Net operating loss carryovers
|
$ | 23,054 | $ | 19,733 | ||||
|
Basis differences in fixed assets
|
24 | 37 | ||||||
|
Accruals and reserves
|
2,694 | 1,383 | ||||||
|
Total deferred tax assets
|
25,772 | 21,153 | ||||||
|
Valuation allowance
|
(25,772 | ) | (21,153 | ) | ||||
|
Net deferred tax assets
|
$ | - | $ | - | ||||
|
Fiscal Years Ended
|
||||||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Research and development expense:
|
||||||||
|
Stock option grants
|
$ | 176,200 | $ | 296,900 | ||||
|
Fully-vested warrants granted to officer and
|
||||||||
|
consultants in January 2015
|
527,500 | - | ||||||
|
Warrants granted to officer in March 2014 and 2013
|
145,100 | 156,500 | ||||||
| 848,800 | 453,400 | |||||||
|
General and administrative expense:
|
||||||||
|
Stock option grants
|
98,800 | 385,100 | ||||||
|
Fully-vested warrants granted to officers, directors
|
||||||||
|
and consultants in January 2015
|
1,229,400 | - | ||||||
|
Warrants granted to officers and directors in March
|
||||||||
|
2014 and 2013
|
283,100 | 298,800 | ||||||
| 1,611,300 | 683,900 | |||||||
|
Total stock-based compensation expense
|
$ | 2,460,100 | $ | 1,137,300 | ||||
| Fiscal Years Ended March 31, | ||||
|
2015
|
2014
|
|||
|
Exercise price
|
not applicable
|
$8.00 to $16.40
|
||
|
Market price on date of grant
|
not applicable
|
$8.00 to $16.40
|
||
|
Risk-free interest rate
|
not applicable
|
1.08% to 2.53%
|
||
|
Expected term (years)
|
not applicable
|
6.25 to 10.0
|
||
|
Volatility
|
not applicable
|
87.9% to 103.2%
|
||
|
Expected dividend yield
|
not applicable
|
0%
|
||
|
Fair value per share at grant date
|
not applicable
|
$6.38 to $13.63
|
||
|
Fiscal Years Ended March 31,
|
||||||||||||||||
|
2015
|
2014
|
|||||||||||||||
|
Weighted
|
Weighted
|
|||||||||||||||
|
Average
|
Average
|
|||||||||||||||
|
Number of
|
Exercise
|
Number of
|
Exercise
|
|||||||||||||
|
Shares
|
Price
|
Shares
|
Price
|
|||||||||||||
|
Options outstanding at beginning of period
|
212,486 | $ | 10.09 | 245,653 | $ | 26.43 | ||||||||||
|
Options granted
|
- | $ | - | 19,050 | $ | 10.89 | ||||||||||
|
Options exercised
|
- | $ | - | - | $ | - | ||||||||||
|
Options forfeited
|
(2,001 | ) | $ | 9.25 | (3,954 | ) | $ | 27.22 | ||||||||
|
Options expired
|
(2,847 | ) | $ | 10.56 | (48,263 | ) | $ | 23.94 | ||||||||
|
Options outstanding at end of period
|
207,638 | $ | 10.09 | 212,486 | $ | 10.09 | ||||||||||
|
Options exercisable at end of period
|
199,013 | $ | 10.09 | 182,775 | $ | 10.06 | ||||||||||
|
Weighted average grant-date fair value of
|
||||||||||||||||
|
options granted during the period
|
$ | - | $ | 8.36 | ||||||||||||
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||||||||
|
Weighted
|
||||||||||||||||||||||
|
Average
|
Weighted
|
Weighted
|
||||||||||||||||||||
|
Remaining
|
Average
|
Average
|
||||||||||||||||||||
|
Exercise
|
Number
|
Years until
|
Exercise
|
Number
|
Exercise
|
|||||||||||||||||
|
Price
|
Outstanding
|
Expiration
|
Price
|
Exercisable
|
Price
|
|||||||||||||||||
| $ | 8.00 | 49,590 | 7.53 | $ | 8.00 | 46,466 | $ | 8.00 | ||||||||||||||
| $ | 10.00 | 147,939 | 4.88 | $ | 10.00 | 142,751 | $ | 10.00 | ||||||||||||||
| $ | 14.40 to $36.00 | 10,109 | 4.64 | $ | 21.69 | 9,796 | $ | 21.23 | ||||||||||||||
| 207,638 | 5.51 | $ | 10.09 | 199,013 | $ | 10.09 | ||||||||||||||||
|
March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Laboratory equipment
|
$ | - | $ | 19,000 | ||||
|
Office equipment
|
4,500 | 4,500 | ||||||
| 4,500 | 23,500 | |||||||
|
Accumulated depreciation
|
(2,500 | ) | (11,100 | ) | ||||
|
Net book value
|
$ | 2,000 | $ | 12,400 | ||||
|
Fiscal Years Ending March 31,
|
Capital
Leases
|
|||
|
2016
|
$ | 1,200 | ||
|
2017
|
1,200 | |||
|
2018
|
100 | |||
|
Future minimum lease payments
|
2,500 | |||
|
Less imputed interest included in minimum lease payments
|
(400 | ) | ||
|
Present value of minimum lease payments
|
2,100 | |||
|
Less current portion
|
(1,000 | ) | ||
|
Non-current capital lease obligation
|
$ | 1,100 | ||
|
Fiscal Years Ending March 31,
|
Amount
|
|||
|
2016
|
264,000 | |||
|
2017
|
277,100 | |||
|
2018
|
93,800 | |||
| $ | 634,900 | |||
|
Fiscal Years Ending March 31,
|
Amount
|
|||
|
2016
|
$ | 2,185,700 | ||
|
2017
|
9,800 | |||
|
2018
|
10,500 | |||
|
2019
|
11,300 | |||
|
Thereafter through June 2019
|
4,000 | |||
| $ | 2,221,300 | |||
|
Three Months Ended
|
Total
|
|||||||||||||||||||
|
June 30, 2014
|
September 30, 2014
|
December 31, 2014
|
March 31, 2015
|
Fiscal Year 2015
|
||||||||||||||||
|
Revenues:
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
474 | 558 | 445 | 956 | 2,433 | |||||||||||||||
|
General and administrative
|
797 | 556 | 671 | 2,320 | 4,344 | |||||||||||||||
|
Total operating expenses
|
1,271 | 1,114 | 1,116 | 3,276 | 6,777 | |||||||||||||||
|
Loss from operations
|
(1,271 | ) | (1,114 | ) | (1,116 | ) | (3,276 | ) | (6,777 | ) | ||||||||||
|
Other expenses, net:
|
||||||||||||||||||||
|
Interest expense, net
|
(785 | ) | (606 | ) | (792 | ) | (2,366 | ) | (4,549 | ) | ||||||||||
|
Change in warrant liabilities
|
(1,727 | ) | 1,302 | 953 | (563 | ) | (35 | ) | ||||||||||||
|
Loss on extinguishment of debt
|
(768 | ) | (1,603 | ) | - | (17 | ) | (2,388 | ) | |||||||||||
|
Other expense, net
|
- | - | (135 | ) | - | (135 | ) | |||||||||||||
|
Loss before income taxes
|
(4,551 | ) | (2,021 | ) | (1,090 | ) | (6,222 | ) | (13,884 | ) | ||||||||||
|
Income taxes
|
(2 | ) | - | - | - | (2 | ) | |||||||||||||
|
Net loss
|
$ | (4,553 | ) | $ | (2,021 | ) | $ | (1,090 | ) | $ | (6,222 | ) | $ | (13,886 | ) | |||||
|
Basic net (loss) per common share
|
$ | (3.70 | ) | $ | (1.58 | ) | $ | (0.84 | ) | $ | (4.24 | ) | $ | (10.53 | ) | |||||
|
Diluted net loss per common share
|
$ | (3.70 | ) | $ | (1.90 | ) | $ | (1.08 | ) | $ | (4.24 | ) | $ | (10.61 | ) | |||||
|
Weighted average shares used in computing:
|
||||||||||||||||||||
|
Basic net (loss) per common share
|
1,229,488 | 1,279,251 | 1,302,300 | 1,466,386 | 1,318,797 | |||||||||||||||
|
Diluted net loss per common share
|
1,229,488 | 1,299,099 | 1,302,300 | 1,466,386 | 1,318,797 | |||||||||||||||
|
Three Months Ended
|
Total
|
|||||||||||||||||||
|
June 30, 2013
|
September 30, 2013
|
December 31, 2013
|
March 31, 2014
|
Fiscal Year 2014
|
||||||||||||||||
|
Revenues:
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
695 | 669 | 551 | 566 | 2,481 | |||||||||||||||
|
General and administrative
|
605 | 546 | 897 | 500 | 2,548 | |||||||||||||||
|
Total operating expenses
|
1,300 | 1,215 | 1,448 | 1,066 | 5,029 | |||||||||||||||
|
Loss from operations
|
(1,300 | ) | (1,215 | ) | (1,448 | ) | (1,066 | ) | (5,029 | ) | ||||||||||
|
Other expenses, net:
|
||||||||||||||||||||
|
Interest expense, net
|
(316 | ) | (323 | ) | (361 | ) | (503 | ) | (1,503 | ) | ||||||||||
|
Change in warrant liabilities
|
1,805 | 79 | 1,940 | (257 | ) | 3,567 | ||||||||||||||
|
Income (loss) before income taxes
|
189 | (1,459 | ) | 131 | (1,826 | ) | (2,965 | ) | ||||||||||||
|
Income taxes
|
(3 | ) | - | - | - | (3 | ) | |||||||||||||
|
Net income (loss)
|
186 | $ | (1,459 | ) | $ | 131 | $ | (1,826 | ) | $ | (2,968 | ) | ||||||||
|
Basic net income (loss) per common share
|
$ | 0.18 | $ | (1.35 | ) | $ | 0.12 | $ | (1.57 | ) | $ | (2.70 | ) | |||||||
|
Diluted net loss per common share
|
$ | (0.44 | ) | $ | (1.37 | ) | $ | (0.44 | ) | $ | (1.57 | ) | $ | (3.81 | ) | |||||
|
Weighted average shares used in computing:
|
||||||||||||||||||||
|
Basic net income (loss) per common share
|
1,042,081 | 1,081,529 | 1,110,529 | 1,162,636 | 1,098,742 | |||||||||||||||
|
Diluted net loss per common share
|
1,061,544 | 1,128,152 | 1,110,529 | 1,162,636 | 1,099,216 | |||||||||||||||
|
Name
|
Age
|
Position
|
|||
|
Shawn K. Singh, J.D.
|
52
|
Chief Executive Officer and Director
|
|||
|
H. Ralph Snodgrass, Ph.D.
|
65
|
Founder, President, Chief Scientific Officer and Director
|
|||
|
Jerrold D. Dotson
|
61
|
Vice President, Chief Financial Officer and Secretary
|
|||
|
Jon S. Saxe
(1)
|
78
|
Director
|
|||
|
Brian J. Underdown, PhD.
(2)
|
74
|
Director
|
|||
|
(1)
|
Chairman of the audit committee and member of the compensation committee and corporate governance and nominating committee
|
|
(2)
|
Member of the audit committee and chairman of the compensation committee and corporate governance and nominating committee
|
|
·
|
overseeing our accounting and financial reporting process;
|
|
·
|
selecting, retaining and replacing our independent auditors and evaluating their qualifications, independence and performance;
|
|
·
|
reviewing and approving scope of the annual audit and audit fees;
|
|
·
|
monitoring rotation of partners of independent auditors on engagement team as required by law;
|
|
·
|
discussing with management and independent auditors the results of annual audit and review of quarterly financial statements;
|
|
·
|
reviewing adequacy and effectiveness of internal control policies and procedures;
|
|
·
|
approving retention of independent auditors to perform any proposed permissible non-audit services;
|
|
·
|
overseeing internal audit functions and annually reviewing audit committee charter and committee performance; and
|
|
·
|
preparing the audit committee report that the SEC requires in our annual proxy statement.
|
|
·
|
Reviewing and approving our compensation programs and arrangements applicable to our executive officers (as defined in Rule I 6a-I (f) of the Exchange Act), including all employment-related agreements or arrangements under which compensatory benefits are awarded or paid to, or earned or received by, our executive officers, including, without limitation, employment, severance, change of control and similar agreements or arrangements;
|
|
·
|
Determining the objectives of our executive officer compensation programs;
|
|
·
|
Ensuring corporate performance measures and goals regarding executive officer compensation are set and determining the extent to which they are achieved and any related compensation earned;
|
|
·
|
Establishing goals and objectives relevant to CEO compensation, evaluating CEO performance in light of such goals and objectives, and determining CEO compensation based on the evaluation; and
|
|
·
|
Endeavoring to ensure that our executive compensation programs are effective in attracting and retaining key employees and reinforcing business strategies and objectives for enhancing stockholder value
,
monitoring the administration of incentive-compensation plans and equity-based incentive plans as in effect and as adopted from time to time by the board.
|
|
·
|
Reviewing and approving any new equity compensation plan or any material change to an existing plan.
|
|
·
|
Reviewing and approving any stock option award or any other type of award as may be required for complying with any tax, securities, or other regulatory requirement, or otherwise determined to be appropriate or desirable by the committee or board.
|
|
·
|
Monitoring the size and composition of the board;
|
|
·
|
Making recommendations to the board with respect to the nominations or elections of our directors;
|
|
·
|
Reviewing the adequacy of our corporate governance policies and procedures and our Code of Business Conduct and Ethics, and recommending any proposed changes to the board for approval; and
|
|
·
|
Considering any requests for waivers from our Code of Business Conduct and Ethics and ensure that we disclose such waivers as may be required by the exchange on which we are listed, if any, and rules and regulations of the SEC.
|
|
·
|
compiling names of potentially eligible candidates;
|
|
·
|
conducting background and reference checks;
|
|
·
|
conducting interviews with candidates and/or others;
|
|
·
|
meeting to consider and approve final candidates; and, as appropriate,
|
|
·
|
preparing and presenting to the full Board an analysis with regard to particular recommended candidates.
|
|
·
|
personal and professional integrity;
|
|
·
|
broad experience in business, finance or administration;
|
|
·
|
familiarity with our industry; and
|
|
·
|
prominence and reputation.
|
| Name and Principal Position |
Fiscal
Year
|
Salary
($)
|
Bonus
($)
|
Option and Warrant
Awards
(10)
($)
|
All Other Compensation
($)
|
Total
($)
|
|||||||||||||||
|
Shawn K. Singh, J.D.
(1)
|
2015
|
347,500 | (4) | - | 688,050 | (11) | - | 1,035,550 | |||||||||||||
|
Chief Executive Officer
|
2014
|
250,000 | (5) | - | 159,802 | (12) | - | 409,802 | |||||||||||||
|
H. Ralph Snodgrass, Ph.D.
(2)
|
2015
|
305,000 | (6) | - | 458,700 | (11) | - | 763,700 | |||||||||||||
|
President, Chief Scientific Officer
|
2014
|
250,000 | (7) | - | 102,353 | (12) | - | 352,353 | |||||||||||||
|
Jerrold D. Dotson
(3)
|
2015
|
250,000 | (8) | - | 229,350 | (11) | - | 479,350 | |||||||||||||
| Vice President, Chief Financial Officer, Secretary |
2013
|
200,000 | (9) | - | 36,846 | (12) | - | 236,846 | |||||||||||||
|
(1)
|
Mr. Singh became VistaGen California’s Chief Executive Officer on August 20, 2009 and our Chief Executive Officer in May 2011, in connection with the Merger. In our fiscal years ended March 31, 2015 and 2014, Mr. Singh’s annual base cash salary, pursuant to his January 2010 employment agreement, was contractually set at $347,500. However, to conserve cash for our operations during those years, Mr. Singh voluntarily agreed to receive cash payments of less than his contractual base cash salary. Further, in fiscal 2014 Mr. Singh voluntarily reduced his base cash salary to the amount indicated. The figures reported above reflect the amount of Mr. Singh’s salary that we expensed for accounting purposes in our financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for the respective fiscal years. See notes (4) and (5) for amounts actually paid in cash to Mr. Singh. The difference between the amounts expensed for accounting purposes and the amounts actually paid to Mr. Singh has been accrued for payment in the future. Additionally, pursuant to his employment agreement, Mr. Singh is eligible to receive an annual cash incentive bonus of up to fifty percent (50%) of his base cash salary. Again, to conserve cash for our operations during our fiscal years ended March 31, 2015 and 2014, Mr. Singh voluntarily refrained from receiving any cash bonus.
|
|
(2)
|
Through August 20, 2009, Dr. Snodgrass served as VistaGen California’s President and Chief Executive Officer, at which time he became its President and Chief Scientific Officer. He became our President and Chief Scientific Officer in May 2011, in connection with the Merger. In our fiscal years ended March 31, 2015 and 2014, Dr. Snodgrass’ annual base cash salary, pursuant to his January 2010 employment agreement, was contractually set at $305,000. However, to conserve cash for our operations during those years, Dr. Snodgrass voluntarily agreed to receive cash payments of less than his contractual base cash salary. Further, in fiscal 2014 Dr. Snodgrass voluntarily reduced his base cash salary to the amount indicated. The figures reported above reflect the amount of Dr. Snodgrass’ salary that we expensed for accounting purposes in our financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for the respective fiscal years. See notes (6) and (7) for amounts actually paid in cash to Dr. Snodgrass. The difference between the amounts expensed for accounting purposes and the amounts actually paid to Dr. Snodgrass has been accrued for payment in the future. Additionally, pursuant to his employment agreement, Dr. Snodgrass is eligible to receive an annual cash incentive bonus of up to fifty percent (50%) of his base cash salary. Again, to conserve cash for our operations during our fiscal years ended March 31, 2015 and 2014, Dr. Snodgrass voluntarily refrained from receiving any cash bonus.
|
|
(3)
|
Mr. Dotson served as Chief Financial Officer on a part-time contract basis from September 19, 2011 through August 2012, at which time he became our full-time employee. In our fiscal years ended March 31, 2015 and 2014, Mr. Dotson’s annual base cash salary was $250,000 and $200,000, respectively. However, to conserve cash for our operations during those years, Mr. Dotson voluntarily agreed to receive cash payments of less than his base cash salary. The figures reported above reflect the amount of Mr. Dotson’s salary that we expensed for accounting purposes in our financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for the respective fiscal years. See notes (8) and (9) for amounts actually paid in cash to Mr. Dotson. The difference between the amounts expensed for accounting purposes and the amounts actually paid to Mr. Dotson has been accrued for payment in the future. Mr. Dotson did not receive a cash bonus in either of our fiscal years ended March 31, 2015 or 2014.
|
|
(4)
|
Mr. Singh received only $82,813 in cash compensation in our fiscal year ended March 31, 2015 and the remaining balance has been accrued for future payment and remains unpaid at the date of this report.
|
|
(5)
|
Mr. Singh received only $125,000 in cash compensation in our fiscal year ended March 31, 2014. At March 31, 2014, the remaining balance was accrued for future payment and remained outstanding at March 31, 2015 and through the date of this report.
|
|
(6)
|
Dr. Snodgrass received only $157,292 in cash compensation in our fiscal year ended March 31, 2015 and the remaining balance has been accrued for future payment and remains unpaid at the date of this report.
|
|
(7)
|
Dr. Snodgrass received only $149,606 in cash compensation in our fiscal year ended March 31, 2014. At March 31, 2014, the remaining balance was accrued for future payment and remained outstanding at March 31, 2015 and through the date of this report.
|
|
(8)
|
Mr. Dotson received only $153,917 in cash compensation in our fiscal year ended March 31, 2015 and the remaining balance has been accrued for future payment and remains unpaid at the date of this report.
|
|
(9)
|
Mr. Dotson received only $143,333 in cash compensation in our fiscal year ended March 31, 2014. At March 31, 2014, the remaining balance was accrued for future payment and remained outstanding at March 31, 2015 and through the date of this report.
|
|
(10)
|
The amounts in the Option and Warrant Awards column represent the aggregate grant date fair value of options or warrants to purchase restricted shares of our common stock awarded to Mr. Singh, Dr. Snodgrass and Mr. Dotson, or the effect of modifications to prior grants of options or warrants occurring during the fiscal year presented, computed in accordance with the Financial Accounting Standards Board’s Accounting Standards Codification Topic 718, Compensation – Stock Compensation ("
ASC 718
”). The amounts in this column do
not
represent any cash payments actually received by Mr. Singh, Dr. Snodgrass or Mr. Dotson with respect to any of such options or warrants to purchase restricted shares of our common stock awarded to them or modified during the periods presented. To date, Mr. Singh, Dr. Snodgrass and Mr. Dotson have not exercised any of such options or warrants to purchase common stock, and there can be no assurance that any of them will ever realize any of the ASC 718 grant date fair value amounts presented in the Option and Warrant Awards column.
|
|
(11)
|
We used the Black Scholes Option Pricing Model and the following assumptions for determining the grant date fair value of the warrants to purchase shares of our common stock granted in January 2015. |
|
Market price per share
|
$ | 8.00 | |||
|
Exercise price per share
|
$ | 10.00 | |||
|
Risk-free interest rate
|
1.45 | % | |||
|
Expected Term (years)
|
5.0 | ||||
|
Volatility
|
75.86 | % | |||
|
Dividend rate
|
0.0 | % | |||
|
Grant date fair value per share
|
$ | 4.59 |
| Mr. Singh, Dr. Snodgrass and Mr. Dotson were granted warrants to purchase 150,000, 100,000 and 50,000 restricted shares of our common stock, respectively | |
| (12) |
The table below provides information regarding the option and warrant awards and modifications we granted to Mr. Singh, Dr. Snodgrass and Mr. Dotson during fiscal 2014 and the assumptions used in the Black Scholes Option Pricing Model to determine the grant date fair values of the respective awards and modifications.
|
|
NEO and Board Stock Compensation Summary for FY 2014
|
Restated for Reverse split
|
|||||||||||||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||
|
Option
Grant
|
Warrant
Grant
|
Option Modification
12/20/2013
|
Warrant Modification
12/20/2013
|
Option/Warrant Exchange (a)
3/19/2014
|
Total
|
|||||||||||||||||||||||||||||||
|
Singh
|
$ | - | $ | - | $ | 134,436 | $ | 25,366 | $ | - | $ | 159,802 | ||||||||||||||||||||||||
|
Snodgrass
|
- | 14,560 | 56,835 | - | 30,958 | 102,353 | ||||||||||||||||||||||||||||||
|
Dotson
|
6,380 | 29,120 | 1,346 | - | - | 36,846 | ||||||||||||||||||||||||||||||
| $ | 6,380 | $ | 43,680 | $ | 192,617 | $ | 25,366 | $ | 30,958 | $ | 299,001 | |||||||||||||||||||||||||
|
Before
|
After
|
Before
|
After
|
Before
|
After
|
|||||||||||||||||||||||||||||||
|
Market price per share
|
$ | 8.00 | $ | 9.20 | $ | 8.00 | $ | 8.00 | $ | 8.00 | $ | 8.00 | $ | 9.20 | $ | 9.20 | ||||||||||||||||||||
|
Exercise price per share
|
$ | 8.00 | $ | 10.00 | $ | 15.00 to $42.00 | $ | 0.50 | $ | 30.00 to $35.00 | $ | 0.50 | $ | 10.00 | $ | 10.00 | ||||||||||||||||||||
|
Risk-free interest rate
|
1.675 | % | 1.750 | % |
0.7% to 2.68%
|
0.12% to 2.68%
|
0.07% to 1.18%
|
0.75% to 1.18%
|
0.106 | % | 1.750 | % | ||||||||||||||||||||||||
|
Volatility
|
99.53 | % | 80.57 | % |
68.8% to 97.6%
|
68.8% to 97.6%
|
68.76% to 78.21%
|
76.51% to 78.21%
|
68.96 | % | 80.57 | % | ||||||||||||||||||||||||
|
Expected term (years)
|
6.25 | 5.00 |
0.25 to 8.86
|
0.87 to 8.86
|
0.03 to 3.96
|
3.03 to 3.96
|
0.63 | 5.00 | ||||||||||||||||||||||||||||
|
Dividend rate
|
0 | % | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||||||||||||||
|
Fair value per share
|
$ | 6.38 | $ | 5.82 | $ | 0.00 to $6.49 | $ | 1.40 to $6.76 | $ | 0.00 to $2.29 | $ | 3.55 to $4.20 | $ | 1.70 | $ | 5.82 | ||||||||||||||||||||
|
Aggregate shares
|
1,000 | 7,500 | 116,125 | 116,125 | 8,303 | 8,303 | 7,500 | 7,500 | ||||||||||||||||||||||||||||
|
Stock Options
|
|||||||||||||
|
Name
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
|
Option
Exercise
Price
($)
|
Option
Expiration
Date
|
|||||||||
|
Shawn K. Singh, J.D.
|
1,000
|
-
|
16.00
|
12/21/2016
|
|||||||||
|
2,000
|
-
|
14.40
|
5/17/2017
|
||||||||||
|
1,000
|
-
|
10.00
|
1/17/2018
|
||||||||||
|
1,000
|
-
|
10.00
|
1/17/2018
|
||||||||||
|
3,000
|
-
|
10.00
|
3/24/2019
|
||||||||||
|
1,125
|
-
|
10.00
|
6/17/2019
|
||||||||||
|
50,000
|
-
|
10.00
|
11/4/2019
|
||||||||||
|
21,250
|
-
|
10.00
|
12/30/2019
|
||||||||||
|
4,896
|
104
|
10.00
|
4/26/2021
|
||||||||||
|
4,017
|
-
|
10.00
|
12/31/2016
|
||||||||||
|
1,786
|
-
|
10.00
|
12/31/2016
|
||||||||||
|
2,500
|
-
|
10.00
|
12/6/2017
|
||||||||||
|
5,000
|
-
|
20.00
|
7/30/2016
|
||||||||||
|
53,250
|
18,750
|
(1)
|
12.80
|
3/3/2023
|
|||||||||
|
150,000
|
-
|
(3)
|
10.00
|
1/11/2020
|
|||||||||
|
Total:
|
301,824
|
18,854
|
|||||||||||
|
H. Ralph Snodgrass, Ph.D.
|
2,500
|
-
|
10.00
|
3/24/2019
|
|||||||||
|
1,250
|
-
|
10.00
|
6/17/2014
|
||||||||||
|
319
|
-
|
17.60
|
12/20/2016
|
||||||||||
|
12,500
|
-
|
10.00
|
12/30/2019
|
||||||||||
|
4,896
|
104
|
10.00
|
4/25/2021
|
||||||||||
|
37,500
|
12,500
|
(1)
|
12.80
|
3/3/2023
|
|||||||||
|
1,250
|
1,250
|
(2)
|
10.00
|
3/19/2024
|
|||||||||
|
3,750
|
3,750
|
(2)
|
10.00
|
3/19/2024
|
|||||||||
|
100,000
|
-
|
(3)
|
10.00
|
1/11/2020
|
|||||||||
|
Total:
|
163,965
|
17,604
|
|||||||||||
|
Jerrold D. Dotson
|
5,001
|
-
|
10.00
|
10/30/2022
|
|||||||||
|
708
|
292
|
8.00
|
10/27/2023
|
||||||||||
|
7,500
|
2,500
|
(1)
|
12.80
|
3/3/2023
|
|||||||||
|
2,500
|
2,500
|
(2)
|
10.00
|
3/19/2024
|
|||||||||
|
50,000
|
-
|
(3)
|
10.00
|
1/11/2020
|
|||||||||
|
Total:
|
65,709
|
5,292
|
|||||||||||
|
(1)
|
Represents warrant to purchase restricted shares of our common stock granted on March 3, 2013 at the market price of our common stock on the grant date. At March 31, 2015, the warrant is exercisable for 75% of the shares and became exercisable for the remaining 25% of the shares on April 1, 2015.
|
|
(2)
|
Represents warrant to purchase restricted shares of our common stock granted on March 19, 2014 when the market price of our common stock was $9.20 per share. The warrant became exercisable for 50% of the shares on April 1, 2014, and became exercisable for an additional 25% of the shares on April 1, 2015. It becomes exercisable for the remaining 25% of the shares on April 1, 2016, provided that the warrant will become fully vested upon a change in control of the Company, as defined, or upon the consummation by the Company and a third party of a license or sale transaction involving at least one new Drug Rescue Variant.
|
|
(3)
|
Represents warrant to purchase restricted shares of our common stock granted as fully-exercisable on January 11, 2015 when the market price of our common stock was $8.00 per share.
|
|
•
|
twelve months of his then-current base salary payable in the form of salary continuation;
|
|
•
|
a pro-rated portion of the incentive cash bonus that the Board of Directors determines in good faith that Mr. Singh earned prior to his termination; and
|
|
•
|
such amounts required to reimburse him for Consolidated Omnibus Budget Reconciliation Act (
COBRA
) payments for continuation of his medical health benefits for such twelve-month period.
|
|
•
|
twelve months of his then-current base salary payable in the form of salary continuation;
|
|
•
|
a pro-rated portion of the incentive bonus that the Board of Directors determines in good faith that Dr. Snodgrass earned prior to his termination; and
|
|
•
|
such amounts required to reimburse him for COBRA payments for continuation of his medical health benefits for such twelve-month period.
|
|
•
|
a material reduction in the executive’s responsibility; or
|
|
•
|
a material reduction in the executive’s base salary except for reductions that are comparable to reductions generally applicable to similarly situated executives of VistaGen.
|
|
Fees Earned or
Paid in Cash (1)
|
Option and Warrant
Awards(2)
|
Other
Compensation
|
Total
|
|||||||||||||
|
Name
|
($)
|
($)
|
($)
|
($)
|
||||||||||||
|
Jon S. Saxe
(3)
|
$
|
55,000
|
$
|
91,734
|
(5)
|
-
|
$
|
146,734
|
||||||||
|
Brian J. Underdown, Ph.D
. (4)
|
$
|
57,500
|
$
|
91,734
|
(5)
|
-
|
$
|
149,234
|
||||||||
|
(1)
|
The amounts shown represent fees earned for service on our Board of Directors, and Audit Committee, Compensation Committee and Corporate Governance and Nominating Committee during the fiscal year ended March 31, 2015 which we have accrued but have not paid to the director during that period or through the date of this report.
|
|
|
(2)
|
The amounts in this column represent the aggregate grant date fair value of warrants to purchase restricted shares of our common stock awarded to Mr. Saxe and Dr. Underdown occurring during the fiscal year ended March 31, 2015, computed in accordance with the Financial Accounting Standards Board’s Accounting Standards Codification Topic 718, Compensation – Stock Compensation ("
ASC 718
”). The amounts in this column do not represent any cash payment actually received by Mr. Saxe or Dr. Underdown with respect to any of such warrants to purchase restricted shares of our common stock awarded to them during the fiscal year ended March 31, 2015. To date, Mr. Saxe and Dr. Underdown have not exercised such warrants to purchase common stock, and there can be no assurance that either of them will ever realize any of the ASC 718 grant date fair value amounts presented in the Option and Warrant Awards column.
|
|
|
(3)
|
Mr. Saxe has served as the Chairman of our Board of Directors, the Chairman of our Audit Committee and a member of our Compensation Committee and Corporate Governance and Nominating Committee throughout our fiscal year ended March 31, 2015. At March 31, 2015, Mr. Saxe holds: (i) 1,875 restricted shares of our common stock; (ii) options to purchase 12,500 restricted shares of our common stock, of which options to purchase 12,448 restricted shares are vested; and (iii) warrants to purchase 33,250 restricted shares of our common stock, of which 29,750 are exercisable and of which an additional 2,687 shares became exercisable on April 1, 2015.
|
|
|
(4)
|
Dr. Underdown has served as a member of our Board of Directors, as the Chairman of our Compensation Committee and Corporate Governance and Nominating Committee and as a member of our Audit Committee throughout our fiscal year ended March 31, 2015. At March 31, 2015, Dr. Underdown holds: (i) options to purchase 9,250 restricted shares of our common stock, of which options to purchase 9,198 restricted shares are vested; and (ii) warrants to purchase 32,500 restricted shares of our common stock, of which 29,375 are exercisable and of which an additional 2,500 shares became exercisable on April 1, 2015.
|
|
|
(5)
|
The table below provides information regarding the warrant awards we granted to Mr. Saxe and Dr. Underdown during fiscal 2015 and the assumptions used in the Black Scholes Option Pricing Model to determine the grant date fair values of the awards as reported in the table above.
|
|
|
Market price per share
|
$ | 8.00 | ||
|
Exercise price per share
|
$ | 10.00 | ||
|
Risk-free interest rate
|
1.450 | % | ||
|
Volatility
|
75.86 | % | ||
|
Expected term (years)
|
5.00 | |||
|
Dividend rate
|
0 | % | ||
|
Fair value per share
|
$ | 4.59 | ||
|
Aggregate shares
|
40,000 |
|
●
|
each stockholder known by us to be the beneficial owner of more than 5% of our common stock;
|
|
●
|
each of our directors;
|
|
●
|
each of our named executive officers; and
|
|
●
|
all of our directors and executive officers as a group.
|
|
Name and address of beneficial owner
|
Number of shares beneficially owned
|
Percent of shares beneficially owned
(1)
|
||||
|
Executive officers and directors:
|
||||||
|
Shawn K. Singh, JD (2)
|
341,287
|
17.82
|
%
|
|||
|
H. Ralph Snodgrass, PhD (3)
|
239,293
|
13.49
|
%
|
|||
|
Jerrold D. Dotson (4)
|
70,302
|
4.22
|
%
|
|||
|
Jon S. Saxe (5)
|
46,563
|
2.84
|
%
|
|||
|
Brian J. Underdown, PhD (6)
|
41,125
|
2.51
|
%
|
|||
|
5% Stockholders:
|
||||||
|
Cato BioVentures (7)
|
238,734
|
14.32
|
%
|
|||
|
Platinum Long Term Growth Fund VII/Montsant Partners, LLC (8)
|
120,250
|
7.54
|
%
|
|||
|
University Health Network (9)
|
150,678
|
8.93
|
%
|
|||
|
Brio Capital Master Fund Ltd. (10)
|
128,594
|
7.64
|
%
|
|||
|
Lincoln Park Capital Fund LLC (11)
|
153,053
|
8.97
|
%
|
|||
|
Morrison & Foerster LLP (12)
|
120,448
|
7.06
|
%
|
|||
|
All executive officers and directors as a group (5 persons) (13)
|
738,570
|
32.82
|
%
|
|||
|
(1)
|
Assumes 1,594,461 shares of common stock are issued and outstanding as of June 26, 2015.
|
|
(2)
|
Includes options to purchase 85,375 restricted shares of common stock exercisable within 60 days of June 26, 2015 and warrants to purchase 235,303 restricted shares of common stock exercisable within 60 days of June 26, 2015.
|
|
(3)
|
Includes options to purchase 21,569 restricted shares of common stock exercisable within 60 days of June 26, 2015 and warrants to purchase 157,500 restricted shares of common stock exercisable within 60 days of June 26, 2015.
|
|
|
(4)
|
Includes options to purchase 6,552 restricted shares of common stock exercisable within 60 days of June 26, 2015, including options to purchase 676 shares of common stock held by Mr. Dotson’s wife, and warrants to purchase 63,750 restricted shares of common stock exercisable within 60 days of June 26, 2015.
|
|
|
(5)
|
Includes options to purchase 12,250 restricted shares of common stock exercisable within 60 days of June 26, 2015 and warrants to purchase 32,438 restricted shares of common stock exercisable within 60 days of June 26, 2015.
|
|
|
(6)
|
Includes options to purchase 9,250 restricted shares of common stock exercisable within 60 days of June 26, 2015 and warrants to purchase 31,875 restricted shares of common stock exercisable within 60 days of June 26, 2015.
|
|
|
(7)
|
Based upon information contained in Form 4 filed on January 9, 2012, as updated to give effect to transactions through June 26, 2015 as recorded on our books. Includes currently exercisable warrants to purchase 73,191 shares of restricted common stock. The reported number of shares beneficially owned excludes 328,571 restricted shares of our Series B Preferred stock currently exchangeable for 328,571 restricted shares of our common stock. Pursuant to the terms of the Certificate of Designation of Rights and Preferences of the Series B Preferred, there is a limitation on conversion of the Series B Preferred such that the number of shares of common stock that Cato may beneficially acquire upon such conversion is limited to the extent necessary to ensure that, following such conversion, the total number of shares of common stock then beneficially owned by Cato does not exceed 9.99% of the total number of issued and outstanding shares of our common stock without providing us with 61 days’ prior notice thereof. Including the shares otherwise excluded due to the beneficial ownership restrictions noted above, Cato beneficially owns 567,305 shares or 28.42% of our common stock. Dr. Allen E. Cato, Ph.D., M.D. is deemed to have voting and investment authority over the shares held by Cato Holding Company. The primary business address of Cato BioVentures is 4364 South Alston Avenue, Durham, North Carolina 27713.
|
|
|
(8)
|
Based upon information contained in Schedule 13G/A filed on February 18, 2015 by Platinum Long Term Growth Fund VII (
Platinum
) and adjusted to give effect to the transactions consummated between Platinum, Montsant Partners, LLC (
Montsant
), a Platinum affiliate, and us pursuant to the Agreement between the Company and Platinum effective May 12, 2015 (
Agreement
)
including Montsant’s investment of $100,000 in our Series B Preferred Unit offering pursuant to the Agreement on June 19, 2015.
The number of beneficially owned shares reported includes 134,536 restricted shares of common stock owned by Montsant.
The reported number of shares beneficially owned excludes 637,500 restricted shares of common stock and a warrant to purchase 455,357 restricted shares of common stock that may currently be acquired by Montsant upon exchange of 425,000 restricted shares of our Series A Preferred Stock (
Series A Preferred
). Pursuant to the October 11, 2012 Note Exchange and Purchase Agreement by and between us and Platinum, there is a limitation on exchange such that the number of shares of our common stock that may be acquired by Platinum or its affiliates upon exchange of the Series A Preferred is limited to the extent necessary to ensure that, following such exchange, the total number of shares of our common stock then beneficially owned by Platinum or its affiliates does not exceed 9.99% of the total number of our issued and outstanding shares of common stock without providing us with 61 days’ prior notice thereof.
Further, the reported number of shares beneficially owned also excludes 1,454,878 shares of our Series B 10% Convertible Preferred Stock (
Series B Preferred
), immediately convertible into a like number of shares of our restricted common stock, and currently exercisable warrants to purchase 2,459,723 shares of our restricted common stock owned by Montsant. Pursuant to the terms of the Certificate of Designation of Rights and Preferences of the Series B Preferred and of the respective common stock purchase warrant agreements, there is a limitation on conversion of the Series B Preferred and exercise of the warrants such that the number of shares of common stock that Platinum or Montsant may beneficially acquire upon such conversion or exercise is limited to the extent necessary to ensure that, following such conversion or exercise, the total number of shares of common stock then beneficially owned by Platinum or Montsant does not exceed 9.99% of the total number of issued and outstanding shares of our common stock without providing us with 61 days’ prior notice thereof.
Including the shares otherwise excluded due to the beneficial ownership restrictions noted above, Platinum and Montsant beneficially own 4,672,351 shares or 76.02% of our common stock. The primary business address of Platinum Long Term Growth Fund VII and Montsant Partners, LLC is c/o Platinum Partners, 250 West 55th Street, 14th Floor, New York, NY 10019. Mark Nordlicht has voting and investment control over the shares held by Platinum and Montsant.
|
|
|
(9)
|
Includes 93,775 restricted shares of our Series B Preferred currently exchangeable for 93,775 restricted shares of our common stock. The primary business address of University Health Network is 101 College Street, Suite 150, Toronto, Ontario Canada M5G 1L7..
|
|
(10)
|
Includes currently exercisable warrants to purchase 61,378 restricted shares of common stock and 27,107 restricted shares of our Series B Preferred currently exchangeable for 27,107 restricted shares of our common stock. The primary business address of Brio Capital Master Fund Ltd. is 100 Merrick Road, Suite 401W, Rockville Centre, NY 11570. Shaye Hirsch has voting and investment control over the shares held by Brio Capital Master Fund Ltd.
|
|
(11)
|
Includes currently exercisable warrants to purchase 72,740 restricted shares of common stock and 38,347 restricted shares of our Series B Preferred currently exchangeable for 38,347 restricted shares of our common stock. The primary business address of Lincoln Park Capital Fund LLC is 440 N. Wells Street, Chicago, IL 60654. Joshua Scheinfeld has voting and investment control over the shares held by Lincoln Park Capital Fund LLC.
|
|
(12)
|
Includes currently exercisable warrants to purchase 110,448 restricted shares of common stock. The reported number of shares beneficially owned excludes 257,143 restricted shares of our Series B Preferred stock currently exchangeable for 257,143 restricted shares of our common stock. Pursuant to the terms of the Certificate of Designation of Rights and Preferences of the Series B Preferred, there is a limitation on conversion of the Series B Preferred such that the number of shares of common stock that Morrison & Foerster may beneficially acquire upon such conversion is limited to the extent necessary to ensure that, following such conversion, the total number of shares of common stock then beneficially owned by Morrison & Foerster does not exceed 9.99% of the total number of issued and outstanding shares of our common stock without providing us with 61 days’ prior notice thereof. Effective on June 12, 2015, Morison & Foerster provided us with such 61 day advance notice. Including the shares otherwise excluded due to the beneficial ownership restrictions noted above, Morrison & Foerster beneficially owns 377,591 shares or 19.24% of our common stock. The primary business address of Morrison & Foerster is 555 Market Street, San Francisco, California 94105. Mark Blumenthal has voting and investment control over the shares held by Morrison & Foerster.
|
|
(13)
|
Includes options to purchase an aggregate of 134,996 restricted shares of common stock exercisable within 60 days of June 26, 2015 and warrants to purchase an aggregate of 520,866 restricted shares of common stock exercisable within 60 days of June 26, 2015.
|
|
Plan category
|
Number of securities
to be issued upon
exercise of
outstanding options,
warrants and rights
(a)
|
Weighted-average
exercise price of
outstanding
options, warrants
and rights
(b)
|
Number of securities
remaining available for
future issuance under
equity compensation plans
(excluding securities
reflected in column (a))
(c)
|
|||||||||
|
Equity compensation plans approved by security holders
|
194,509
|
$
|
9.99
|
40,491
|
||||||||
|
Equity compensation plans not approved by security holders
|
13,129
|
11.67
|
--
|
|||||||||
|
Total
|
207,638
|
$
|
10.09
|
40,491
|
||||||||
|
•
|
by will and by the laws of descent and distribution; and
|
|
•
|
during the lifetime of the participant, to the extent and in the manner authorized by the Administrator by gift or pursuant to a domestic relations order to members of the participant’s immediate family.
|
|
•
|
increase in share price;
|
|
•
|
earnings per share;
|
|
•
|
total shareholder return;
|
|
•
|
operating margin;
|
|
•
|
gross margin;
|
|
•
|
return on equity;
|
|
•
|
return on assets;
|
|
•
|
return on investment;
|
|
•
|
operating income;
|
|
•
|
net operating income;
|
|
•
|
pre-tax profit;
|
|
•
|
cash flow;
|
|
•
|
revenue;
|
|
•
|
expenses;
|
|
•
|
earnings before interest, taxes and depreciation;
|
|
•
|
economic value added; and
|
|
•
|
market share.
|
|
•
|
an acquisition of securities possessing more than fifty percent (50%) of the total combined voting power of our outstanding securities but excluding any such transaction or series of related transactions that the Administrator determines shall not be a Corporate Transaction;
|
|
•
|
a reverse merger in which we remain the surviving entity but: (i) the shares of common stock outstanding immediately prior to such merger are converted or exchanged by virtue of the merger into other property, whether in the form of securities, cash or otherwise; or (ii) in which securities possessing more than fifty percent (50%) of the total combined voting power of our outstanding securities are transferred to a person or persons different from those who held such securities immediately prior to such merger;
|
|
•
|
a sale, transfer or other disposition of all or substantially all of the assets of our Corporation;
|
|
•
|
a merger or consolidation in which our Corporation is not the surviving entity; or
|
|
•
|
a complete liquidation or dissolution.
|
|
Fiscal Years Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Audit fees
|
$ | 182,500 | $ | 172,500 | ||||
|
Audit-related fees
|
53,952 | 4,600 | ||||||
|
Tax fees
|
10,960 | 12,643 | ||||||
|
All other fees
|
- | - | ||||||
|
Total fees
|
$ | 247,412 | $ | 189,743 | ||||
Audit Fees:
|
|
Respectfully Submitted by:
|
|
|
MEMBERS OF THE AUDIT COMMITTEE
|
|
|
Jon S. Saxe, Audit Committee Chairman
|
|
|
Brian J. Underdown
|
|
Exhibit No.
|
Description*
|
|
|
2.1 *
|
Agreement and Plan of Merger by and among Excaliber Enterprises, Ltd., VistaGen Therapeutics, Inc. and Excaliber Merger Subsidiary, Inc.
|
|
|
3.1 *
|
Articles of Incorporation, dated October 6, 2005.
|
|
|
3.2
|
Certificate of Amendment filed with the Nevada Secretary of State on December 6, 2011, incorporated by reference from Exhibit 3.3 to the Company's Annual Report on Form 10-K, filed July 2, 2012.
|
|
|
3.3
|
Amended and Restated Bylaws as of February 5, 2014, incorporated by reference from the Company’s Report on Form 8-K filed on February 7, 2014.
|
|
|
3.4
|
Articles of Merger filed with the Nevada Secretary of State on May 24, 2011, incorporated by reference from Exhibit 3.1 to the Company's Current Report on Form 8-K filed on May 31, 2011.
|
|
|
3.5
|
Certificate of Designations Series A Preferred, incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on December 23, 2011.
|
|
|
3.6
|
Certificate of Change filed with the Nevada Secretary of State on August 11, 2014 incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on August 14, 2014.
|
|
|
3.7
|
Certificate of Designation of the Relative Rights and Preferences of the Series B 10% Convertible Preferred Stock of VistaGen Therapeutics, Inc., filed with the Nevada Secretary of State on May 7, 2015, incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on May 13, 2015.
|
|
|
10.1 *
|
VistaGen’s 1999 Stock Incentive Plan.
|
|
|
10.2 *
|
Form of Option Agreement under VistaGen’s 1999 Stock Incentive Plan.
|
|
|
10.5 *
|
VistaGen’s 2008 Stock Incentive Plan.
|
|
|
10.6 *
|
Form of Option Agreement under VistaGen’s 2008 Stock Incentive Plan.
|
|
|
10.20 *
|
Strategic Development Services Agreement, dated February 26, 2007, by and between VistaGen and Cato Research Ltd.
|
|
|
10.21 *
|
License Agreement by and between National Jewish Medical and Research Center and VistaGen, dated July 12, 1999, as amended by that certain Amendment to License Agreement dated January 25, 2001, as amended by that certain Second Amendment to License Agreement dated November 6, 2002, as amended by that certain Third Amendment to License Agreement dated March 1, 2003, and as amended by that certain Fourth Amendment to License Agreement dated April 15, 2010.
|
|
|
10.22 *
|
License Agreement by and between Mount Sinai School of Medicine of New York University and the Company, dated October 1, 2004.
|
|
|
10.23 *
|
Non-Exclusive License Agreement, dated December 5, 2008, by and between VistaGen and Wisconsin Alumni Research Foundation, as amended by that certain Wisconsin Materials Addendum, dated February 2, 2009.
|
|
|
10.24 *
|
Sponsored Research Collaboration Agreement, dated September 18, 2007, between VistaGen and University Health Network, as amended by that certain Amendment No. 1 and Amendment No. 2, dated April 19, 2010 and December 15, 2010, respectively.
|
|
|
10.26 *
|
License Agreement, dated October 24, 2001, by and between the University of Maryland, Baltimore, Cornell Research Foundation and Artemis Neuroscience, Inc.
|
|
|
10.27 *
|
Non-exclusive License Agreement, dated September 1, 2010, by and between VistaGen and TET Systems GmbH & Co. KG.
|
|
|
10.31 *
|
Unsecured Promissory Note dated April 28, 2011 issued by VistaGen to Desjardins Securities.
|
|
|
10.32 *
|
Unsecured Promissory Note dated April 28, 2011 issued by VistaGen to McCarthy Tetrault LLP.
|
|
|
10.34 *
|
Promissory Note dated February 25, 2010 issued by VistaGen to The Regents of the University of California.
|
|
10.40 *
|
Employment Agreement, by and between, VistaGen and Shawn K. Singh, dated April 28, 2010, as amended May 9, 2011.
|
|
|
10.41 *
|
Employment Agreement, by and between, VistaGen and H. Ralph Snodgrass, PhD, dated April 28, 2010, as amended May 9, 2011.
|
|
|
10.46
|
Notice of Award by National Institutes of Health, Small Business Innovation Research Program, to VistaGen Therapeutics, Inc. for project, Clinical Development of 4-CI-KYN to Treat Pain dated June 22, 2009, with revisions dated July 19, 2010 and August 9, 2011, incorporated by reference from Exhibit 10.46 to the Company’s Current Report on Form 8-K/A filed on December 20, 2011.
|
|
|
10.47
|
Notice of Grant Award by California Institute of Regenerative Medicine and VistaGen Therapeutics, Inc. for Project: Development of an hES Cell-Based Assay System for Hepatocyte Differentiation Studies and Predictive Toxicology Drug Screening, dated April 1, 2009, incorporated by reference from Exhibit 10.47 to the Company’s Current Report on Form 8-K/A filed on December 20, 2011.
|
|
|
10.48
|
Amendment No. 4, dated October 24, 2011, to Sponsored Research Collaboration Agreement between VistaGen and University Health Network, incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on November 30, 2011.
|
|
|
10.49
|
License Agreement No. 1, dated as of October 24, 2011 between University Health Network and VistaGen Therapeutics, Inc., incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 30, 2011.
|
|
|
10.50
|
Strategic Medicinal Chemistry Services Agreement, dated as of December 6, 2011, between Synterys, Inc. and VistaGen Therapeutics, Inc., incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 7, 2011.
|
|
|
10.51
|
Common Stock Exchange Agreement, dated as of December 22, 2011 between Platinum Long Term Growth VII, LLC and VistaGen Therapeutics, Inc., incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 23, 2011.
|
|
|
10.52
|
Note and Warrant Exchange Agreement, dated as of December 28, 2011 between Platinum Long Term Growth VII, LLC and VistaGen Therapeutics, Inc., incorporated by reference from Exhibit 10.1 to the Current Report on Form 8-K filed on January 4, 2012.
|
|
|
10.55
|
Form of Warrant to Purchase Common Stock, dated as of February 28, 2012, incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on March 2, 2012.
|
|
|
10.57
|
License Agreement No. 2, dated as of March 19, 2012 between University Health Network and VistaGen Therapeutics, Inc., incorporated by reference from Exhibit 10.57 to the Company’s Annual Report on Form 10-K filed on July 2, 2012.
|
|
|
10.58
|
Exchange Agreement dated as of June 29, 2012 between Platinum Long Term Growth VII, LLC and VistaGen Therapeutics. Inc., incorporated by reference from Exhibit 10.58 to the Company’s Annual Report on Form 10-K filed on July 2, 2012.
|
|
|
10.63
|
Unsecured Promissory Note in the face amount of $1,000,000 issued to Morrison & Foerster LLP on August 31, 2012 (Replacement Note A), incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on September 6, 2012.
|
|
|
10.64
|
Unsecured Promissory Note in the face amount of $1,379,376 issued to Morrison & Foerster LLP on August 31, 2012 (Replacement Note B), incorporated by reference from Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on September 6, 2012.
|
|
|
10.65
|
Stock Purchase Warrant issued to Morrison & Foerster LLP on August 31, 2012 to purchase 1,379,376 shares of the Company’s common stock (New Morrison & Foerster Warrant), incorporated by reference from Exhibit 10.5 to the Company’s Current Report on Form 8-K filed on September 6, 2012.
|
|
|
10.66
|
Warrant to Purchase Common Stock issued to Morrison & Foerster LLP on August 31, 2012 to purchase 425,000 shares of the Company’s common stock (Amended Morrison & Foerster Warrant), incorporated by reference from Exhibit 10.6 to the Company’s Current Report on Form 8-K filed on September 6, 2012.
|
|
|
10.67
|
Note Exchange and Purchase Agreement dated as of October 11, 2012 by and between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 16, 2012.
|
|
|
10.68
|
Form of Senior Secured Convertible Promissory Note issued to Platinum Long Term Growth VII, LLP under the Note Exchange and Purchase Agreement, incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on October 16, 2012.
|
|
|
10.69
|
Form of Warrant to Purchase Shares of Common Stock issued to Platinum Long Term Growth VII, LLP under the Note Exchange and Purchase Agreement, incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on October 16, 2012.
|
|
10.70
|
Amended and Restated Security Agreement as of October 11, 2012 between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on October 16, 2012.
|
|
|
10.71
|
Intellectual Property Security and Stock Pledge Agreement as of October 11, 2012 between VistaGen California and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.5 to the Company’s Current Report on Form 8-K filed on October 16, 2012.
|
|
|
10.72
|
Negative Covenant Agreement dated October 11, 2012 between VistaGen California, Artemis Neuroscience, Inc. and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.6 to the Company’s Current Report on Form 8-K filed on October 16, 2012.
|
|
|
10.73
|
Amendment to Note Exchange and Purchase Agreement as of November 14, 2012 between VistaGen Therapeutics Inc. and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 20, 2012.
|
|
|
10.75
|
Amendment No. 2 to Note Exchange and Purchase Agreement as of January 31, 2013 between VistaGen Therapeutics Inc. and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on February 14, 2013.
|
|
|
10.76
|
Amendment No. 3 to Note Exchange and Purchase Agreement as of February 22, 2013 between VistaGen Therapeutics Inc. and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 28, 2013.
|
|
|
10.77
|
Form of Warrant to Purchase Common Stock issued to independent members of the Company’s Board of Directors and its executive officers on March 3, 2013, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 6, 2013.
|
|
|
10.78
|
Securities Purchase Agreement between VistaGen Therapeutics, Inc., and Autilion AG dated April 8, 2013, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 10, 2013.
|
|
|
10.79
|
Voting Agreement between VistaGen Therapeutics, Inc., and Autilion AG dated April 8, 2013, incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 10, 2013.
|
|
|
10.80
|
Note Conversion Agreement as of April 4, 2013 between VistaGen Therapeutics Inc. and Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on April 10, 2013.
|
|
|
10.81
|
Assignment and Assumption Agreement between Autilion AG and Bergamo Acquisition Corp. PTE LTD dated April 12, 2013, incorporated by reference from Exhibit 10.81 to the Company’s Annual Report on Form 10-K filed July 18, 2013.
|
|
|
10.82
|
Amendment No. 1 to Securities Purchase Agreement dated April 30, 2013 between VistaGen Therapeutics, Inc. and Bergamo Acquisition Corp. PTE LTD, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 1, 2013.
|
|
|
10.83
|
Lease between Bayside Area Development, LLC and VistaGen Therapeutics, Inc. (California) dated April 24, 2013, incorporated by reference from Exhibit 10.83 to the Company’s Annual Report on Form 10-K filed July 18, 2013.
|
|
|
10.84
|
Indemnification Agreement effective May 20, 2013 between the Company and Jon S. Saxe, incorporated by reference from Exhibit 10.84 to the Company's Annual Report on Form 10-K filed on July 18, 2013.
|
|
|
10.85
|
Indemnification Agreement effective May 20, 2013 between the Company and Shawn K. Singh, incorporated by reference from Exhibit 10.85 to the Company’s Annual Report on Form 10-K filed on July 18, 2013.
|
|
|
10.86
|
Indemnification Agreement effective May 20, 2013 between the Company and H. Ralph Snodgrass, incorporated by reference from Exhibit 10.86 to the Company’s Annual Report on Form 10-K filed on July 18, 2013.
|
|
|
10.87
|
Indemnification Agreement effective May 20, 2013 between the Company and Brian J. Underdown, incorporated by reference from Exhibit 10.87 to the Company’s Annual Report on Form 10-K filed on July 18, 2013.
|
|
|
10.88
|
Indemnification Agreement effective May 20, 2013 between the Company and Jerrold D. Dotson, incorporated by reference from Exhibit 10.88 to the Company’s Annual Report on Form 10-K filed on July 18, 2013.
|
|
|
10.89
|
Amendment and Waiver effective May 24, 2013 between the Company and Platinum Long Term Growth VII, LLC, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 3, 2013.
|
|
|
10.90
|
Amendment No 2 to Securities Purchase Agreement dated June 27, 2013 between the Company, Autilion AG and Bergamo Acquisition Corp. PTE LTD, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 28, 2013.
|
|
|
10.91
|
Senior Secured Convertible Promissory Note, dated July 26, 2013 issued to Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on August 2, 2013.
|
|
|
10.92
|
Common Stock Warrant, dated July 26, 2013 issued to Platinum Long Term Growth VII, LLP, incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on August 2, 2013.
|
|
10.93
|
Form of Subscription Agreement between the Company and investors in the Fall 2013 Unit Private Placement, incorporated by reference from Exhibit 10.93 to the Company’s Annual Report on Form 10-K filed on June 24, 2014.
|
|
|
10.94
|
Form of Convertible Promissory Note between the Company and investors in the Fall 2013 Unit Private Placement, incorporated by reference from Exhibit 10.94 to the Company’s Annual Report on Form 10-K filed on June 24, 2014.
|
|
|
10.95
|
Form of Common Stock Purchase Warrant between the Company and investors in the Fall 2013 Unit Private Placement, incorporated by reference from Exhibit 10.95 to the Company’s Annual Report on Form 10-K filed on June 24, 2014.
|
|
|
10.96
|
Form of Amendment to Convertible Promissory Note and Warrant between the Company and investors in the Fall 2013 Unit Private Placement, effective May 31, 2014, incorporated by reference from Exhibit 10.96 to the Company’s Annual Report on Form 10-K filed on June 24, 2014.
|
|
|
10.97
|
Form of Unit Subscription Agreement between the Company and investors in the Spring 2014 Unit Private Placement dated April 1, 2014, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 8, 2014.
|
|
|
10.98
|
Form of Subordinate Convertible Promissory Note between the Company and investors in the Spring 2014 Unit Private Placement dated April 1, 2014, incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 8, 2014.
|
|
|
10.99
|
Form of Common Stock Purchase Warrant between the Company and investors in the Spring 2014 Unit Private Placement dated April 1, 2014, incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on April 8, 2014.
|
|
|
10.100
|
Common Stock Purchase Warrant between the Company and Platinum Long Term Growth Fund VII dated May 14, 2014, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 19, 2014.
|
|
|
10.101
|
Subordinate Convertible Promissory Note between the Company and Platinum Long Term Growth Fund VII dated May 14, 2014, incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on May 19, 2014.
|
|
|
10.102
|
Form of Promissory Note and Form of Warrant issued by the Company to Icahn School of Business at Mount Sinai effective April 10, 2014 in satisfaction of technology license maintenance fees and reimbursable patent costs, incorporated by reference from Exhibit 10.102 to the Company’s Annual Report on Form 10-K filed on June 24, 2014.
|
|
|
10.103
|
Amendment No. 3 to Sponsored Research Collaboration Agreement, dated April 25, 2011, by and between VistaGen and University Health Network, incorporated by reference from Exhibit 10.103 to the Company’s Annual Report on Form 10-K filed on June 24, 2014.
|
|
|
10.104
|
Amendment No. 5 to Sponsored Research Collaboration Agreement, dated October 10, 2012, by and between VistaGen and University Health Network, incorporated by reference from Exhibit 10.104 to the Company’s Annual Report on Form 10-K filed on June 24, 2014.
|
|
|
10.105
|
Amended and Restated Note Conversion Agreement and Warrant Amendment, by and between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLC, dated July 18, 2014, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 22, 2014.
|
|
|
10.106
|
Amendment No. 1 to Amended and Restated Note Conversion Agreement and Warrant Amendment, by and between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLC, dated September 2, 2014, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 4, 2014.
|
|
|
10.107
|
Amendment No. 2 to Amended and Restated Note Conversion Agreement and Warrant Amendment, by and between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLC, dated September 30, 2014, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 3, 2014.
|
|
|
10.108
|
Agreement, by and between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLC, dated May 5, 2015, incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 13, 2015.
|
|
|
10.109
|
Acknowledgement and Agreement, by and between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLC, dated May 12, 2015, incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on May 13, 2015.
|
|
|
10.110
|
Form of Securities Purchase Agreement by and between VistaGen Therapeutics, Inc. and Platinum Long Term Growth VII, LLC, dated May 12, 2015, incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on May 13, 2015.
|
|
|
21.1*
|
List of Subsidiaries.
|
|
|
24.1
|
Power of Attorney
|
|
|
31.1
|
Certification of the Company’s Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
31.2
|
Certification of the Company’s Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.1
|
Certification of the Company’s Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS
|
XBRL Instance Document
|
|
|
101.SCH
|
XBRL Taxonomy Schema
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase
|
|
|
|
|
VistaGen Therapeutics, Inc.
|
||
|
By:
|
/s/
Shawn K. Singh
|
|
|
Shawn K. Singh, J.D.
Chief Executive Officer
|
||
|
Signature
|
|
Title
|
Date
|
|
|
/s/ Shawn K. Singh
Shawn K. Singh, JD
|
|
Chief Executive Officer, and Director
(Principal Executive Officer)
|
June 29, 2015
|
|
|
/s/ Jerrold D. Dotson
Jerrold D. Dotson
|
|
Vice President and Chief Financial Officer
(Principal Financial and Accounting Officer)
|
June 29, 2015
|
|
|
/s/ H. Ralph Snodgrass
H. Ralph Snodgrass, Ph.D
|
|
President, Chief Scientific Officer and Director
|
June 29, 2015
|
|
|
/s/ Jon S. Saxe
Jon S. Saxe
|
|
Chairman of the Board of Directors
|
June 29, 2015
|
|
|
/s/ Brian J. Underdown
Brian J. Underdown, Ph. D
|
|
Director
|
June 29 2015
|
|