|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year period ended December 31, 2018
|
|
|
OR
|
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from _______ to _______
|
|
|
Commission File Number: 001-38536
|
|
|
Delaware
|
20-3352427
|
|||
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(I.R.S. Employer
Identification Number)
|
|||
|
180 N. LaSalle Street, Suite 1810
Chicago, Illinois 60601
|
||||
|
(Address of Principal Executive Offices)
|
||||
|
(844) 445-5704
|
||||
|
(Registrant's Telephone Number, Including Area Code)
|
||||
|
Securities Registered Pursuant to Section 12(b) of the Exchange Act:
|
||||
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|||
|
Common Stock, $0.0001 par value per share
|
The Nasdaq Global Select Market
|
|||
|
Securities Registered Pursuant to Section 12(g) of the Exchange Act: None
|
||||
|
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined by Rule 405 of the Securities Act. Yes
¨
No
þ
|
|||||||
|
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Act. Yes
¨
No
þ
|
|||||||
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes
þ
No
¨
|
|||||||
|
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes
þ
No
¨
|
|||||||
|
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of Registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
¨
|
|||||||
|
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
|||||||
|
Large accelerated filer
¨
|
Accelerated filer
¨
|
||||||
|
Non-accelerated filer
þ
|
Smaller reporting company
þ
|
||||||
|
Emerging growth company
þ
|
|||||||
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
¨
|
|||||||
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
¨
No
þ
|
|||||||
|
As of June 30, 2018, the aggregate market value of the Registrant's common stock held by non-affiliates of the Registrant was approximately $281.9 million based on the closing sales price as reported on the Nasdaq Exchange. As of February 28, 2019, 26,809,851 shares, par value $0.0001 per share, of common stock were outstanding.
|
|||||||
|
DOCUMENTS INCORPORATED BY REFERENCE
|
|||||||
|
Part III incorporates certain information by reference from the Registrant's Definitive Proxy Statement to be filed with the Commission in connection with the Registrant's 2019 Annual Meeting of Shareholders. Such Definitive Proxy Statement will be filed not later than 120 days after the conclusion of the Registrant’s fiscal year ended December 31, 2018.
|
|||||||
|
Page
|
|||||
|
Cautionary Statements for Forward-Looking Information
|
|||||
|
Part I.
|
|||||
|
Item 1. Business
|
|||||
|
Item 1A. Risk Factors
|
|||||
|
Item 1B. Unresolved Staff Comments
|
|||||
|
Item 2. Properties
|
|||||
|
Item 3. Legal Proceedings
|
|||||
|
Item 4. Mine Safety Disclosures
|
|||||
|
Part II.
|
|||||
|
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases
of Equity Securities
|
|||||
|
Item 6. Selected Financial Data
|
|||||
|
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
|
|||||
|
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
|
|||||
|
Item 8. Financial Statements and Supplementary Data
|
|||||
|
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
|||||
|
Item 9A. Controls and Procedures
|
|||||
|
Item 9B. Other Information
|
|||||
|
Part III.
|
|||||
|
Item 10. Directors, Executive Officers and Corporate Governance
|
|||||
|
Item 11. Executive Compensation
|
|||||
|
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
|||||
|
Item 13. Certain Relationships and Related Transactions, and Director Independence
|
|||||
|
Item 14. Principal Accountant Fees and Services
|
|||||
|
Part IV.
|
|||||
|
Item 15. Exhibits, Financial Statement Schedules
|
|||||
|
Index to Exhibits
|
|||||
|
Signatures
|
|||||
|
•
|
the timing or likelihood of approval by the U.S. Food & Drug Administration of our New Drug Application for our Gvoke HypoPen;
|
|
•
|
our estimates regarding the market opportunities for our product candidates;
|
|
•
|
the commercialization, marketing and manufacturing of our product candidates, if approved;
|
|
•
|
the pricing and reimbursement of our Gvoke HypoPen or any other of our product candidates, if approved;
|
|
•
|
the rate and degree of market acceptance and clinical utility of our Gvoke HypoPen or any other of our product candidates for which we receive marketing approval;
|
|
•
|
the initiation, timing, progress and results of our research and development programs and future preclinical and clinical studies;
|
|
•
|
our ability to advance any other product candidates into, and successfully complete, clinical studies and obtain regulatory approval for them;
|
|
•
|
our ability to identify additional product candidates;
|
|
•
|
the implementation of our strategic plans for our business, product candidates and technology;
|
|
•
|
the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology;
|
|
•
|
our ability to use the proceeds of our initial public offering in ways that increase the value of your investment;
|
|
•
|
our expectations related to the use of proceeds from our initial public offering, or any subsequent public equity offerings, and estimates of our expenses, future revenues, capital requirements and our needs for additional financing;
|
|
•
|
our ability to manufacture, or the ability of third parties to deliver, sufficient quantities of components and drug product for commercialization of our Gvoke HypoPen or any other of our product candidates;
|
|
•
|
our ability to maintain and establish collaborations;
|
|
•
|
our financial performance;
|
|
•
|
our ability to effectively manage our anticipated growth;
|
|
•
|
developments relating to our competitors and our industry, including the impact of government regulation;
|
|
•
|
our ability to avoid any findings of material weakness or significant deficiencies by our independent registered public accounting firm in the future; and
|
|
•
|
other risks and uncertainties, including those listed under the caption “Risk Factors.”
|
|
|
<
|
|
Post-Bariatric Hypoglycemia, or PBH, a serious complication of bariatric surgery that can arise from excessive insulin, or hyperinsulinism, due to the change in gastric anatomy resulting from bariatric surgery.
|
|
<
|
Congenital Hyperinsulinism, or CHI, a condition caused by several genetic defects that result in severe, persistent hypoglycemia in infants and children, which can lead to brain damage and death.
|
||
|
<
|
Hypoglycemia-Associated Autonomic Failure, or HAAF, in which chronic hypoglycemia impairs the body’s natural response to restore blood sugar levels and can lead to an individual becoming unaware of the onset of a severe hypoglycemic event and result in cardiovascular disease, seizure, coma, and, if left untreated, death.
|
||
|
<
|
Exercise-Induced Hypoglycemia, or EIH, in people with diabetes. Exercise, particularly aerobic exercise, often results in a significant drop in blood glucose levels for people on insulin.
|
||
|
<
|
Management of diabetes via glucagon in a fully-integrated, bi-hormonal artificial pancreas closed-loop system.
|
||
|
|
<
|
|
Rapidly secure regulatory approval for our lead product candidate, Gvoke HypoPen for severe hypoglycemia.
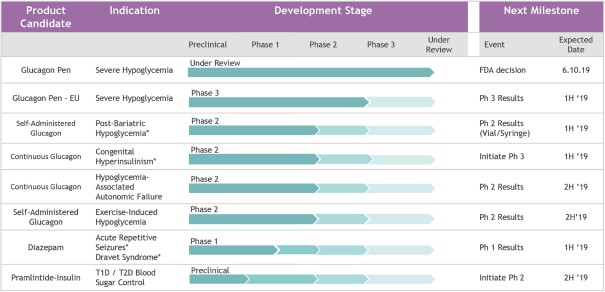
We have completed three Phase 3 clinical trials for our Gvoke HypoPen and submitted an NDA to the FDA early in August 2018 utilizing the 505(b)(2) regulatory pathway, which has been accepted for review by the FDA with a PDUFA action goal date of June 10, 2019. Additionally, through our interactions with the European Medicines Agency, or EMA, regarding our development path in Europe, we have finalized our clinical study plan and initiated a Phase 3 pivotal trial to support EMA registration of an MAA.
|
|
<
|
Maximize the commercial potential for Gvoke HypoPen
. If approved, we plan to commercially launch our Gvoke HypoPen in the United States in the second half of 2019. We expect to initially target approximately 8,000 healthcare professionals who are high prescribers of current glucagon kits and/or mealtime insulin products, using an expected initial sales force of 60-70 individuals, and activate demand through targeted direct-to-patient promotion. We have accelerated our build of our commercial organization and critical infrastructure, including individuals in operations, supply chain, medical affairs, pharmacovigilance, compliance, regulatory, marketing, sales leadership, market access and sales operations, as well as our medical affairs organization. Outside of the United States, we plan to pursue development and commercialization partnerships.
|
||
|
<
|
Continue to advance our ready-to-use glucagon portfolio to address hypoglycemia associated with other conditions
. We plan to apply our ready-to-use, room-temperature stable liquid glucagon to address multiple conditions that could benefit from intermittent or chronic administration, such as PBH and CHI as well as in diabetes for HAAF and EIH. We are also evaluating our liquid-stable glucagon as the glucagon component of a fully-integrated, bi-hormonal artificial pancreas. During 2018, all five of these programs either produced positive clinical trial results or advanced into clinical trials. Through these programs, our primary goal is to secure FDA approval of a vial of our liquid glucagon for self-administration via a syringe, or transfer to a pump reservoir for continuous infusion. We plan to leverage efficiencies across our portfolio, such as our supply chain, research and development, and our commercial and medical organizations. We plan to use commercially available drug delivery devices for our liquid-stable glucagon formulation and associated intermittent and chronic glucagon programs.
|
||
|
<
|
Continue to leverage our technology and expertise to develop a portfolio of additional product candidates
. We are exploring the application of our formulation technology platforms to other commercially available drugs for multiple conditions. We are developing an improved formulation of diazepam for the treatment of ARS in patients with epilepsy as well as patients with Dravet syndrome, to be administered through a ready-to-use auto-injector. We have completed formulation development and preclinical pharmacokinetic studies. An IND application for our ready-to-use diazepam rescue pen for ARS went into effect on November 28, 2018. This IND authorized us to initiate a study evaluating the pharmacokinetics and pharmacodynamics of our ready-to-use, room-temperature stable liquid diazepam formulation in normal volunteers. We initiated this trial in December 2018 and expect top-line results in the first half of 2019. If results are positive, we plan to initiate a Phase 2 clinical trial in the second half of 2019. In addition, we formulated and completed several preclinical studies of a fixed-ratio pramlintide-insulin coformulation combination product for the treatment of diabetes and plan to begin a Phase 2 clinical trial in the second half of 2019. Finally, we have advanced several additional formulation programs in the past year and plan to continue to advance these programs to clinical trials.
|
||
|
<
|
Collaborate with pharmaceutical and biotechnology companies to apply our technology platforms to enhance the formulations of their proprietary products and candidates
. We are pursuing formulation and development partnerships to apply our XeriSol and XeriJect technology platforms to enhance the formulation, delivery and clinical profile of other companies’ proprietary drugs and biologics. We currently are working with several companies on feasibility programs to evaluate the formulation of their proprietary therapeutics with, depending on the type of molecule, XeriSol or XeriJect. Active programs include feasibility evaluations with Regeneron Pharmaceuticals, Inc., Asahi Kasei Pharma Corporation, Hawaii Biotech, Inc. and Islet Sciences, Inc. on XeriJect monoclonal antibody formulations, a XeriJect biologic product formulation, a XeriJect vaccine formulation, and a XeriSol co-formulation of a peptide and small molecule for insulin-dependent diabetes, respectively. We plan to continue to explore the application of our formulation technology platforms to proprietary drugs and biologics from additional pharmaceutical and biotechnology companies.
|
||

|
|
Step-by-Step Instructions for GEK
|
|
|
1.
|
|
Flip off the seal from the vial of Glucagon powder.
|
|
2.
|
|
Remove the needle cover from the syringe. DO NOT REMOVE THE PLASTIC CLIP FROM THE SYRINGE, as this may allow the push rod to come out of the syringe.
|
|
3.
|
|
Insert the needle into the rubber stopper on the vial, then inject the entire contents of the syringe into the vial of Glucagon powder.
|
|
4.
|
|
Remove the syringe from the vial, then swirl the vial until the liquid becomes clear. Glucagon should not be used unless the solution is clear and of a water-like consistency.
|
|
5.
|
|
Insert the same syringe into the vial and slowly withdraw all the liquid. To use on children weighing less than 44 pounds, withdraw half of the liquid (0.5 mark on the syringe).
|
|
6.
|
|
Cleanse site on buttock, arm or thigh and inject Glucagon immediately after mixing, and then withdraw the needle. Apply light pressure against the injection site.
|
|
7.
|
|
Turn the person on his/her side. When an unconscious person awakens, he/she may vomit.
Call 911 immediately after administering Glucagon. If the person does not awaken within 15 minutes, you may administer a second dose of Glucagon, if previously instructed to do so by a healthcare professional.
|
|
8.
|
|
As soon as the person is awake and able to swallow, give him/her a fast-acting source of sugar (such as fruit juice), followed by a snack or meal containing both protein and carbohydrates (such as cheese and crackers, or a peanut butter sandwich).
|
|
9.
|
|
Discard any unused reconstituted Glucagon. Remember to notify your healthcare professional that an episode of severe hypoglycemia has occurred. These are not the complete instructions. Go to “Information for the User” for complete instructions on how to administer Glucagon.
|
|
|
<
|
|
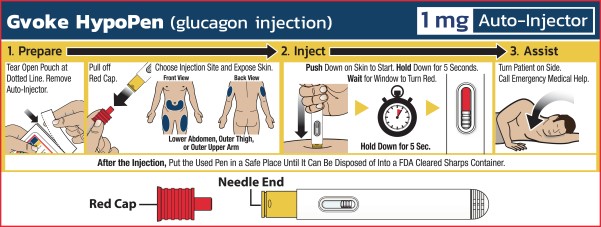
Ready-to-use:
With its easy two-step administration process, the user simply pulls off the red cap and pushes the Gvoke HypoPen down on the skin for five seconds, until the window turns red. There is no reconstitution required at the time of emergency.
|
|
<
|
Easy-to-use:
In our human factors study, 99% of users were able to successfully administer the full dose with our Gvoke HypoPen.
|
||
|
<
|
No dose calibration required:
The Gvoke HypoPen will be offered in two pre-measured doses, 0.5 mg for pediatric administration and 1 mg for adolescents and adults.
|
||
|
<
|
No visible needle:
The needle in the Gvoke HypoPen is not visible to the user.
|
||
|
<
|
Auto-retraction:
The needle auto-retracts after administration for safety.
|
||
|
<
|
Auto-locks:
The device auto-locks after use for safety.
|
||
|
<
|
Two-year room-temperature stability:
No refrigeration is required at any time.
|
||



|
|
|
SEVERE ALLERGIC REACTION
(EPINEPHRINE)
|
|
SEVERE HYPOGLYCEMIA
(GLUCAGON)
|
|
Clinically Appropriate Patient
Population in the United States
|
|
5.2 million patients
|
|
3.5 million patients
|
|
No. of Units Sold in the United States
(2018)
|
|
~8.2 million auto-injectors
|
|
~978,000 kits*
|
|
* Single-dose units of Eli Lilly’s Glucagon Emergency Kits and Novo Nordisk’s GlucaGen
|
||||
|
|
<
|
Create awareness and anticipation prior to launch.
We plan to use the FDA’s NDA review period to both better understand the market and create excitement and anticipation for our company and our technology. We expect to hire ten regional medical affairs directors prior to commercial launch to establish additional relationships with key opinion leaders and gain insight into current practice patterns and burdens. We also plan to begin to raise awareness in the market on the incidence, prevalence and impact of severe hypoglycemic events.
|
||
|
<
|
Drive awareness and adoption of our Gvoke HypoPen
. If approved by the FDA, we plan to drive awareness and adoption of our Gvoke HypoPen to replace current emergency glucagon kits in the market.
|
|||
|
o
|
Healthcare Professionals:
At launch, our targets will consist of high glucagon prescribing healthcare professionals. Approximately 3,000 healthcare professionals issue 50% of current glucagon prescriptions. We plan to hire 60-70 sales representatives initially to reach these professionals.
|
|||
|
o
|
Patients and Caregivers:
We intend to activate patient advocacy organizations and leverage channels such as direct-to-consumer tactics, social media, digital presence, traditional offline channels and press coverage to drive awareness and communicate our value proposition to patients and caregivers. Because we do not anticipate having the first product to market in a competitive landscape, we plan to increase our spend on direct-to-consumer tactics. Because of our earlier than expected action date, we plan to accelerate these activities into late 2019. Epidemiology and census data indicate that 15 states account for almost 60% of people with diabetes, allowing us to be efficient and effective with our promotional activities.
|
|||
|
<
|
Penetrate the market
. We believe that the Gvoke HypoPen market is currently significantly underpenetrated due to the lack of, and limitations in, current treatment options. We are designing our Gvoke HypoPen to offer healthcare professionals, patients and caregivers a ready-to-use alternative that facilitates administration of the full dose of glucagon every time it is used. We believe this product offering, paired with our commercial focus, has the potential to grow the market in two ways:
|
|||
|
o
|
Healthcare Professionals:
In addition to the 3,000 healthcare professionals who issue about half of the current glucagon prescriptions, we will target approximately 5,000 healthcare professionals who are high meal time insulin prescribers but who are under-indexed in prescribing glucagon. We intend to reach these professionals using our initial sales representatives.
|
|||
|
o
|
Patients and Caregivers:
We believe there is an opportunity to activate patient and caregiver demand for our Gvoke HypoPen. Our Gvoke HypoPen is designed as an easy-to-use solution for a segment of patients and caregivers who currently lack the confidence in administering current emergency glucagon kits and would rather rely on emergency responders for treatment.
|
|||
|
<
|
Promote access:
Current emergency glucagon kits have favorable market access, and current trends indicate a relatively low level of management of these products by payors. For example, Eli Lilly’s GEK is covered at or above 94% with unrestricted access across commercial, Medicare, Managed Medicaid and State Medicaid plans. A Diabetes Health Coverage: State Laws and Programs report reviewing state insurance mandated coverage, Medicaid coverage and state-sponsored diabetes programs showed that 46 states and the District of Columbia have a diabetes statutory mandate for coverage, whether as medication or supply. Of our target patient population, approximately 50% are commercially-insured, one-third are covered by Medicare and approximately 15% are covered by Medicaid. However, gaining market access and formulary coverage for new products takes substantial time and resources. As a result, we plan to increase our focus on promoting access to our Gvoke HypoPen. We plan to engage with payors to more fully understand their drivers and barriers and convey the health and pharmacoeconomic value of our Gvoke HypoPen prior to launch.
|
|||
|
PROTOCOL NO./TITLE
|
|
PHASE OF
DEVELOPMENT
|
|
DESIGN/OBJECTIVES
|
|
STUDY POPULATION
AND
DEMOGRAPHICS
|
|
DOSE (NO. EXPOSED
EACH TREATMENT) AND
DOSAGE FORM/
PRODUCT
CONFIGURATION
|
|
Completed
|
||||||||
|
XSGP-302
A Phase 3 Study to Evaluate the Glucose Response of Glucagon Rescue Pen (Glucagon Injection) In Pediatric Patients With Type 1 Diabetes
|
|
Phase 3a
|
|
Non-randomized,
open-label, single
dose/Efficacy, PD, PK,
safety and tolerability
|
|
Children
(2<6,
6<12 and
12<18
years) with
T1D
n=31
|
|
Ages 2<6 years (n=7),
single dose of 0.5mg
Glucagon Rescue Pen; ages 6<12 years
(n=13), single dose of
0.5mg Glucagon
Rescue Pen; ages
12<18 years (n=11),
single dose of 1mg
Glucagon Rescue Pen
followed by single
dose of 0.5mg
Glucagon Rescue Pen
7 to 28 days later/
Rescue Pen
|
|
XSGP-301
Glucagon Rescue Pen (Glucagon Injection) Compared To Eli Lilly Glucagon (Glucagon For Injection [rDNA Origin]) For Induced Hypoglycemia Rescue In Adult Patients With T1D: A Phase 3, Randomized, Blinded, 2-Way Crossover Study To Evaluate Efficacy and Safety
|
|
Phase 3a
|
|
Double-blind,
randomized, two-way
crossover/Efficacy
(return to plasma
glucose >70.0 mg/dL)
of Glucagon Rescue
Pen 1 mg to be non-
inferior to Eli Lilly’s
glucagon; compare
the PD characteristics
of Glucagon Rescue
Pen versus Eli Lilly’s
glucagon; safety and
tolerability; PK.
|
|
Adult
patients
with T1D
n=80
|
|
Glucagon Rescue Pen
1mg (n=78), Eli
Lilly’s glucagon 1mg
(n=79)/Rescue Pen
|
|
PROTOCOL NO./TITLE
|
|
PHASE OF
DEVELOPMENT
|
|
DESIGN/OBJECTIVES
|
|
STUDY POPULATION
AND
DEMOGRAPHICS
|
|
DOSE (NO. EXPOSED
EACH TREATMENT)
AND DOSAGE
FORM/PRODUCT
CONFIGURATION
|
|
XSGP-303
Glucagon Rescue Pen (Glucagon Injection) Compared To Eli Lilly Glucagon (Glucagon For Injection [rDNA Origin]) For Induced Hypoglycemia Rescue In Adults With T1D: A Phase 3B Multi-Centered, Randomized, Controlled, Single-Blind, 2-Way Crossover Study To Evaluate Efficacy And Safety
|
|
Phase 3b
|
|
Non-inferiority, multi-
centered, randomized,
controlled, single-
blind, two-period,
two-way crossover/
Efficacy and safety
|
|
T1D adult
male/female patients
18-75 years of age
n=81
|
|
Glucagon Rescue
Pen 1 mg, Eli Lilly’s
glucagon 1 mg/
Rescue Pen
|
|
XSGP-202
Glucagon Rescue Pen (Glucagon Injection) For Induced Hypoglycemia Rescue In Adult Patients With T1D: A Phase 2A Pilot Study To Evaluate Protocol Design Issues For An Upcoming Phase 3 Clinical Study
|
|
Phase 2
|
|
Open-label
2-way crossover
Explore safety efficacy
in treatment of
insulin-induced
hypoglycemia
|
|
T1D adult
male/female
patients
18-65 years
of age
n=7
|
|
Glucagon Rescue
Pen 0.5 mg (n=6)
and 1 mg (n=7),
subcutaneous
injections given one
week apart/Pre-Filled
Syringe
|
|
XSGP-201
A Randomized, Phase 2, Double-Blind, 3-Way Crossover Study With Glucagon Rescue Pen (Glucagon For Injection) To Evaluate Safety, Tolerability and Comparative Pharmacokinetics and Pharmacodynamics To Eli Lilly Glucagon (Glucagon For Injection [rDNA Origin]) In Healthy Volunteers
|
|
Phase 2
|
|
Double-blind,
randomized, 3-way
crossover/Safety,
tolerability, PK and
efficacy vs. marketed
comparator
|
|
Healthy
male/female
volunteers
18-60 years
of age
n=28
|
|
Subcutaneous
injection of:
Glucagon Rescue
Pen 0.5 mg (n=29) and
1 mg (n=28) and Eli
Lilly’s glucagon
(rDNA origin) 1 mg/
Pre-Filled Syringe
|
|
XSGP-101
A Two-Way Crossover Comparative PD/PK Study Of Glucagon Rescue Pen (Glucagon Injection) Administered By Auto-Injector And Pre-Filled Syringe
|
|
Phase 1
|
|
Two-way crossover
comparative
bioequivalence,
safety, tolerability and
PD/PK of Glucagon
Rescue Pen
administered via auto-
injector vs. pre-filled
syringe
|
|
Healthy
male/female
volunteers
18-64 years
of age
n=32
|
|
Glucagon Rescue
Pen 1 mg/Pre-Filled
Syringe 1 mg
|
|
GLUCAGON DOSE
|
0.5 MG DOSE
|
1 MG DOSE
|
||||||||||
|
SUBJECT AGES
|
2 TO
< 6 YEARS
|
6 TO
< 12 YEARS
|
12 TO
< 18 YEARS
|
12 TO
< 18 YEARS
|
||||||||
|
n
|
7
|
|
13
|
|
11
|
|
11
|
|
||||
|
% with >25 mg/dL rise in glucose within 30 minutes
|
100
|
|
100
|
|
100
|
|
100
|
|
||||
|
Glucose C
max
(mg/dL)
Mean (SD)
|
207.8 (35.9)
|
|
206.9 (49.6)
|
|
212.1 (40.6)
|
|
198.9 (60.0)
|
|
||||
|
Glucose T
max
(minutes)
Mean (SD)
|
67.7 (11.1)
|
|
66.4 (15.7)
|
|
78.2 (11.5)
|
|
81.8 (15.6)
|
|
||||
|
% with nausea
|
42.9
|
|
53.8
|
|
36.4
|
|
36.4
|
|
||||
|
% with emesis
|
14.3
|
|
23.1
|
|
0
|
|
18.2
|
|
||||
|
CLINICAL COMPARISON
|
|
mITT RESPONSE RATE
|
||
|
|
GVOKE
HYPOPEN
|
|
ELI LILLY
GLUCAGON
|
|
|
Subjects successfully rescued from induced hypoglycemia without other
rescue therapy (e.g., intravenous dextrose)
|
|
100% (78/78)
|
|
100% (79/79)
|
|
Plasma glucose >70 mg/dL within 30 minutes of glucagon (primary
endpoint)
|
|
Intent-to-treat
†
94.9% (74/78)
|
|
Intent-to-treat
100% (79/79)
|
|
|
Per-protocol
96.1% (74/77)
|
|
Per-protocol
100% (78/78)
|
|
|
Plasma glucose of >70 mg/dL or
>
20 mg/dL increase within 30 minutes
of glucagon (additional primary endpoint)
|
|
Intent-to-treat
97.4% (76/78)
|
|
Intent-to-treat
100% (79/79)
|
|
|
Per-protocol
97.4% (75/77)
|
|
Per-protocol
100% (78/78)
|
|
|
Plasma glucose of >70 mg/dL or resolution of all induced neuroglycopenic
symptoms within 30 minutes of glucagon
|
|
100% (78/78)
|
|
100% (79/79)
|
|
Resolution of hypoglycemia symptoms
|
|
100% (78/78)
|
|
100% (79/79)
|
|
Global feeling of hypoglycemia improvement pre/post injection
|
|
100% (77/77)
|
|
100% (79/79)
|
|
Sustained glucose elevation from 0-90 minutes post-injection
|
|
100% (78/78)
|
|
100% (79/79)
|
|
†
one (1) additional endpoint failure exceeded the non-inferiority threshold of N=3; all other comparisons demonstrate non-inferiority vs.
Eli Lilly’s glucagon.
|
||||
|
Clinical Comparison
|
|
RESPONSE RATE
|
||
|
|
|
GVOKE HYPOPEN
|
|
ELI LILLY GLUCAGON
|
|
Subjects successfully rescued from induced hypoglycemia without other rescue therapy (e.g., D50).
|
|
100%
(76/76)
|
|
100%
(78/78)
|
|
Plasma glucose >70 mg/dl in 30 minutes.
(primary endpoint)
|
|
Intent-to-treat
100%
(76/76)
|
|
Intent-to-treat
100%
(78/78)
|
|
|
Per-protocol
100%
(73/73)
|
|
Per-protocol
100%
(73/73)
|
|
|
Plasma glucose of >70 mg/dl or
>
20 mg/dl increase within 30 minutes of glucagon.
|
|
Intent-to-treat
100%
(76/76)
|
|
Intent-to-treat
100%
(78/78)
|
|
|
Per-protocol
100%
(73/73)
|
|
Per-protocol
100%
(73/73)
|
|
|
Plasma glucose of >70 mg/dl or resolution of all induced neuroglycopenic symptoms within 30 minutes of glucagon.
|
|
100%
(76/76)
|
|
100%
(78/78)
|
|
Resolution of hypoglycemia symptoms
|
100%
(76/76)
|
100%
(78/78)
|
||
|
Global feeling of hypoglycemia improvement pre/post injection†
|
100%
(73/73)
|
100%
(76/76)
|
||
|
Sustained glucose elevation from 0-90 minutes post-injection
|
100%
(76/76)
|
100%
(78/78)
|
||
|
† Population of subjects only includes those reporting hypoglycemic symptoms at baseline.
|
||||
|
|
<
|
|
Chemistry, manufacturing and controls, or CMC
|
|
<
|
Nonclinical toxicology program
|
||
|
<
|
Clinical supplies manufacturing
|
||
|
|
<
|
|
Offers a direct effect of increasing glucose levels compared to indirect mechanisms of glucose control.
|
|
<
|
Enables release of patient’s excess glycogen stores.
|
||
|
<
|
Avoids the side effects related to octreotide, nifedipine and diazoxide.
|
||
|
|
<
|
|
Provides an approach to wean the patient off a central glucose line, such as an IV, to enable discharge from the hospital.
|
|
<
|
Eliminates bloating observed with the high-volume glucose infusions often required to maintain normal blood glucose levels.
|
||
|
PER PROTOCAL
(4 SUBJECTS)
|
|
BASELINE
STABILIZATION
|
|
RANDOMIZED BLINDED TREATMENT (PLACEBO)
|
|
RANDOMIZED BLINDED TREATMENT
(CSI GLUCAGON)
|
|
OPEN-LABEL
CSI-GLUCAGON
(CSI GLUCAGON)
|
|
Stable GIR
|
|
5/5
a
|
|
0/2
|
|
2/2
|
|
4/4
|
|
GIR Response
(reduction
>
20% at 24 hrs.)
|
|
N/A
|
|
1/2
|
|
2/2
|
|
N/A
|
|
GIR Response
(reduction
>
33% at 48 hrs.)
|
|
N/A
|
|
0/2
|
|
2/2
|
|
4/4
b
|
|
GIR change
(range)
|
|
N/A
|
|
31% reduction, to
34% increase
|
|
53%-65%
reduction
|
|
44%-66%
reduction
|
|
a:
|
The first enrolled subject, 01-01, was not deemed evaluable for responsive to glucagon based upon prior history of glucagon resistance, per investigator. Selection criteria were changed after this first patient enrollment to exclude glucagon resistance.
|
|
b:
|
Treatment time varied from 4-52 hours.
|




|
|
<
|
|
completion of extensive preclinical laboratory tests, animal studies and formulation studies in accordance with applicable regulations, including the FDA’s Good Laboratory Practice, or GLP, regulations;
|
|
<
|
submission to the FDA of an IND, which must become effective before human clinical trials may begin;
|
||
|
<
|
approval by an independent institutional review board, or IRB, representing each clinical site before each clinical trial may be initiated;
|
||
|
<
|
performance of adequate and well-controlled human clinical trials in accordance with an applicable IND and other clinical study related regulations, sometimes referred to as good clinical practices, or GCPs, to establish the safety and efficacy of the proposed drug or biologic for its proposed indication;
|
||
|
<
|
submission to the FDA of an NDA or BLA;
|
||
|
<
|
satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with the FDA’s current good manufacturing practice requirements, or cGMP;
|
||
|
<
|
potential FDA audit of the clinical trial sites that generated the data in support of the NDA or BLA and payment of associated user fees;
|
||
|
<
|
review by an FDA advisory committee, where appropriate or if applicable;
|
||
|
<
|
FDA review and approval of the NDA or BLA prior to any commercial marketing or sale; and
|
||
|
<
|
compliance with any post-approval requirements, including the potential requirement to implement a Risk Evaluation and Mitigation Strategy, or REMS, and the potential requirement to conduct post-approval studies.
|
||
|
|
<
|
|
Phase 1. The product is initially introduced into a small number of healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, especially when the product is suspected or known to be unavoidably toxic, the initial human testing may be conducted in patients.
|
|
<
|
Phase 2. Involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule.
|
||
|
<
|
Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit relationship of the product and provide an adequate basis for product labeling.
|
||
|
|
<
|
|
the required patent information has not been filed;
|
|
<
|
the listed patent has expired;
|
||
|
<
|
the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or
|
||
|
<
|
the listed patent is invalid, unenforceable or will not be infringed by the new product.
|
||
|
|
<
|
|
a product comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced as a single entity;
|
|
<
|
two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products;
|
||
|
<
|
a drug, or device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, or device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or
|
||
|
<
|
any investigational drug, or device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect.
|
||
|
|
<
|
|
the federal Anti-Kickback Statute, or AKS, which makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, receive or pay any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, in exchange for, or intended to induce or reward, including arranging for or recommending, either the referral of an individual, or the purchase, lease, order, prescription or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs; a person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act (see below) or federal civil money penalties statute. Violations of the AKS carry potentially significant civil and criminal penalties, including imprisonment, fines, administrative civil monetary penalties, and exclusion from participation in federal healthcare programs;
|
|
<
|
federal civil and criminal false claims laws and civil monetary penalties laws, such as the federal False Claims Act, which impose criminal and civil penalties and authorizes civil whistleblower or qui tam actions, against individuals or entities (including manufacturers) for, among other things: knowingly presenting, or causing to be presented, to a federal government healthcare program, claims for payment that are false or fraudulent; making, using or causing to be made or used, a false statement or record material to payment of a false or fraudulent claim or obligation to pay or transmit money or property to the federal government; or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay money to the federal government. The government may deem manufacturers to have “caused” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Our marketing and activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state and third-party reimbursement for our products, and the sale and marketing of our products and any future product candidates, are subject to scrutiny under this law;
|
||
|
<
|
the anti-inducement law, which prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program;
|
||
|
<
|
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program (including private payors) or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
|
||
|
<
|
HIPAA, as amended by HITECH, and their respective implementing regulations, which impose specified requirements on certain covered healthcare providers, health plans, and healthcare clearinghouse (“covered entities”) as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information, including mandatory contractual terms and required implementation of technical safeguards of such information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associate with pursing federal civil actions;
|
||
|
<
|
the federal false statements statute, which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
|
||
|
<
|
the federal transparency requirements under the Affordable Care Act, including the Physician Payments Sunshine Act, which requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the U.S. Department of Health and Human Services information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members;
|
||
|
<
|
federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs;
|
||
|
<
|
federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and
|
||
|
<
|
The Foreign Corrupt Practices Act, or FCPA, which prohibits companies and their intermediaries from making, or offering or promising to make improper payments to non-U.S. officials for the purpose of obtaining or retaining business or otherwise seeking favorable treatment.
|
||
|
|
<
|
|
The Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2027 unless additional Congressional action is taken.
|
|
<
|
The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
|
||
|
|
<
|
|
continue our research and development efforts;
|
|
<
|
seek regulatory approval for new product candidates and product enhancements;
|
||
|
<
|
build commercial infrastructure to support sales and marketing for our product candidates;
|
||
|
<
|
hire and retain additional personnel and add operational, financial and management information systems; and
|
||
|
<
|
continue to operate as a public company.
|
||
|
|
<
|
|
obtain marketing approval for our product candidates, including our Gvoke HypoPen;
|
|
<
|
obtain commercial quantities of our product candidates, if approved, at acceptable cost levels;
|
||
|
<
|
commercialize our product candidates, if approved, by developing our own sales force for commercialization in the United States or in other key territories by entering into partnership or co-promotion arrangements with third parties;
|
||
|
<
|
set an acceptable price for our product candidates, if approved;
|
||
|
<
|
obtain and maintain third-party coverage and adequate reimbursement for our product candidates, if approved; and
|
||
|
<
|
achieve an adequate level of market acceptance of our product candidates, if approved, in the medical community and with third-party payors, including placement in accepted clinical guidelines for the conditions for which our product candidates are intended to target.
|
||
|
|
<
|
|
could determine that we cannot rely on the Section 505(b)(2) regulatory pathway for our product candidates;
|
|
<
|
could determine that the information provided by us was inadequate, contained clinical deficiencies or otherwise failed to demonstrate the safety and effectiveness of our Gvoke HypoPen or any of our product candidates for any indication;
|
||
|
<
|
may not find the data from bioequivalence studies and/or clinical trials sufficient to support the submission of an NDA or to obtain marketing approval in the United States, including any findings that the clinical and other benefits of our product candidates outweigh their safety risks;
|
||
|
<
|
may disagree with our trial design or our interpretation of data from preclinical studies, bioequivalence studies and/or clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our trials;
|
||
|
<
|
may determine that there are unacceptable risks associated with the device component of our Gvoke HypoPen or that there are deficiencies with the information submitted to demonstrate the safety, effectiveness and reliability of the device component;
|
||
|
<
|
may determine that we have identified the wrong listed drug or drugs or that approval of our Section 505(b)(2) application for our Gvoke HypoPen or any of our other product candidates is blocked by patent or non-patent exclusivity of the listed drug or drugs or of other previously-approved drugs with the same conditions of approval as those of our Gvoke HypoPen or any of our other product candidates (as applicable);
|
||
|
<
|
may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for the manufacturing of our product candidates;
|
||
|
<
|
may audit some or all of our clinical research and human factors study sites to determine the integrity of our data and may reject any or all of such data;
|
||
|
<
|
may approve our product candidates for fewer or more limited indications than we request, or may grant approval contingent on the performance of costly post-approval clinical trials;
|
||
|
<
|
may change its approval policies or adopt new regulations; or
|
||
|
<
|
may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of our product candidates.
|
||
|
|
<
|
|
delays or failures in obtaining regulatory authorization to commence a trial because of safety concerns of regulators relating to our product candidates or similar product candidates, competitive or comparator products or supportive care products or failure to follow regulatory guidelines;
|
|
<
|
delays or failures in obtaining clinical materials and manufacturing sufficient quantities of the product candidate for use in a trial;
|
||
|
<
|
delays or failures in reaching agreement on acceptable terms with prospective study sites or other contract research organizations, or CROs;
|
||
|
<
|
delays or failures in obtaining approval of our clinical trial protocol from an institutional review board, or IRB, to conduct a clinical trial at a prospective study site;
|
||
|
<
|
receipt by a competitor of marketing approval for a product targeting an indication that our product candidate targets, such that we are not “first to market” with our product candidate;
|
||
|
<
|
delays in recruiting or enrolling subjects to participate in a clinical trial, particularly with respect to our product candidates for certain rare indications, including those for which we have obtained, or plan to seek, orphan drug designation;
|
||
|
<
|
failure of a clinical trial or clinical investigators to be in compliance with current Good Clinical Practices, or cGCPs;
|
||
|
<
|
unforeseen safety issues;
|
||
|
<
|
|
inability to monitor subjects adequately during or after treatment;
|
|
|
<
|
difficulty monitoring multiple study sites;
|
||
|
<
|
the FDA requiring alterations to any of our study designs, our nonclinical strategy or our manufacturing plans;
|
||
|
<
|
failure of our third-party clinical trial managers to satisfy their contractual duties, comply with regulations, or meet expected deadlines; and
|
||
|
<
|
determination by regulators that the clinical design of a trial is not adequate.
|
||
|
|
<
|
|
failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols;
|
|
<
|
inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities;
|
||
|
<
|
unforeseen safety issues, including serious adverse events associated with a product candidate, or lack of effectiveness; and
|
||
|
<
|
lack of adequate funding to continue the clinical trial.
|
||
|
|
<
|
|
regulatory authorities may require the addition of labeling statements, including “black box” warnings, contraindications or dissemination of field alerts to physicians and pharmacies;
|
|
<
|
we may be required to change instructions regarding the way the product is administered, conduct additional clinical trials or change the labeling of the product;
|
||
|
<
|
we may be subject to limitations on how we may promote the product;
|
||
|
<
|
sales of the product may decrease significantly;
|
||
|
<
|
regulatory authorities may require us to take our approved product off the market;
|
||
|
<
|
we may be subject to litigation or product liability claims; and
|
||
|
<
|
our reputation may suffer.
|
||
|
|
<
|
|
exposure to unknown liabilities;
|
|
<
|
disruption of our business and diversion of our management’s time and attention to develop acquired products or technologies;
|
||
|
<
|
incurrence of substantial debt, dilutive issuances of securities or depletion of cash to pay for acquisitions;
|
||
|
<
|
higher than expected acquisition and integration costs;
|
||
|
<
|
difficulty in combining the operations and personnel of any acquired businesses with our operations and personnel;
|
||
|
<
|
increased amortization expenses;
|
||
|
<
|
impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and
|
||
|
<
|
inability to motivate key employees of any acquired businesses.
|
||
|
|
<
|
|
the scope of regulatory approvals, including limitations or warnings contained in a product candidate’s regulatory-approved labeling;
|
|
<
|
our ability to produce, through a validated process, sufficiently large quantities of our product candidates to permit successful commercialization;
|
||
|
<
|
our ability to establish and maintain commercial manufacturing arrangements with third-party manufacturers;
|
||
|
<
|
our ability to build and maintain sales, distribution and marketing capabilities sufficient to launch commercial sales of our product candidates;
|
||
|
<
|
the acceptance in the medical community of the potential advantages of the product candidate, including with respect to our efforts to increase adoption of our product candidates such as our Gvoke HypoPen by patients and healthcare providers;
|
||
|
<
|
the incidence, prevalence and severity of adverse side effects of our product candidates;
|
||
|
<
|
the willingness of physicians to prescribe our product candidates and of the target patient population to try these therapies;
|
||
|
<
|
the price and cost-effectiveness of our product candidates;
|
||
|
<
|
the extent to which each product is approved for use at, or included on formularies of, hospitals and managed care organizations;
|
||
|
<
|
any negative publicity related to our or our competitors’ products or other formulations of products that we administer, including as a result of any related adverse side effects;
|
||
|
<
|
alternative treatment methods and potentially competitive products;
|
||
|
<
|
the potential advantages of the product candidate over existing and future treatment methods;
|
||
|
<
|
the strength of our sales, marketing and distribution support; and
|
||
|
<
|
the availability of sufficient third-party coverage and reimbursement.
|
||
|
|
<
|
|
regulatory authorities may withdraw approvals of such products, require us to take our approved product off the market or ask us to voluntarily remove the product from the market;
|
|
<
|
regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication or field alerts to physicians and pharmacies;
|
||
|
<
|
regulatory authorities may impose conditions under a risk evaluation and mitigation strategy, or REMS, including distribution of a medication guide to patients outlining the risks of such side effects or imposing distribution or use restrictions;
|
||
|
<
|
we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product;
|
||
|
<
|
we may be subject to limitations on how we may promote the product;
|
||
|
<
|
sales of the product may decrease significantly;
|
||
|
<
|
we may be subject to litigation or product liability claims; and
|
||
|
<
|
our reputation may suffer.
|
||
|
|
<
|
|
our inability to recruit and train adequate numbers of sales and marketing personnel;
|
|
<
|
the inability of sales personnel to obtain access to or to persuade adequate numbers of physicians to prescribe any of our product candidates that receive regulatory approval; and
|
||
|
<
|
unforeseen costs and expenses associated with creating an independent sales and marketing organization.
|
||
|
|
<
|
|
restrict the marketing or manufacturing of such products;
|
|
<
|
restrict the labeling of a product;
|
||
|
<
|
issue warning letters or untitled letters which may require corrective action;
|
||
|
<
|
mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners;
|
||
|
<
|
require us to enter into a consent decree or permanent injunction, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance;
|
||
|
<
|
impose other administrative or judicial civil or criminal penalties including fines, imprisonment and disgorgement of profits;
|
||
|
<
|
suspend or withdraw regulatory approval;
|
||
|
<
|
refuse to approve pending applications or supplements to approved applications filed by us;
|
||
|
<
|
close the facilities of our third-party suppliers;
|
||
|
<
|
suspend ongoing clinical trials;
|
||
|
<
|
impose restrictions on operations, including costly new manufacturing requirements; or
|
||
|
<
|
seize or detain products or recommend or require a product recall.
|
||
|
|
<
|
|
an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs;
|
|
<
|
a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
|
||
|
<
|
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13.0% of the average manufacturer price for branded and generic drugs, respectively;
|
||
|
<
|
expansion of healthcare fraud and abuse laws, including the False Claims Act and the federal Anti-Kickback Statute, or AKS, which include, among other things, new government investigative powers and enhanced penalties for non-compliance;
|
||
|
<
|
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 70% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D;
|
||
|
<
|
extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations;
|
||
|
<
|
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals, thereby potentially increasing manufacturers’ Medicaid rebate liability;
|
||
|
<
|
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
||
|
<
|
the requirements under the federal open payments program and its implementing regulations;
|
||
|
<
|
a requirement to annually report drug samples that manufacturers and distributors provide to physicians; and
|
||
|
<
|
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
|
||
|
<
|
Anti-Kickback Statute. The federal AKS makes it illegal for any person or entity (including a prescription drug manufacturer or a party acting on its behalf) to knowingly and willfully solicit, offer, receive or pay remuneration, directly or indirectly, in cash or in kind, in exchange for or intended to induce or reward either the referral of an individual for, or the purchase, order, prescription or recommendation or arranging of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are several statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, they are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. A person or entity can be found guilty of violating the AKS without actual knowledge of the statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the federal False Claims Act or federal civil money penalties statute. Violations of the AKS carry potentially significant civil and criminal penalties, including imprisonment, fines, administrative civil monetary penalties, and exclusion from participation in federal healthcare programs.
|
||
|
<
|
False Claims Laws.
The federal false claims and civil monetary penalties laws, including the federal civil False Claims Act, impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, false or fraudulent claims for payment by a federal healthcare program or making a false statement or record material to payment of a false claim or knowingly avoiding, decreasing or concealing an obligation to pay money to the federal government, with potential liability including mandatory treble damages and significant per-claim penalties.
|
||
|
<
|
Anti-Inducement Law.
The anti-inducement law prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program.
|
||
|
<
|
HIPAA.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program (including private payors) or making false or fraudulent statements relating to healthcare matters. Similar to the federal AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Additionally, HIPAA, as amended by HITECH and its implementing regulations, also imposes obligations on covered entities and their business associates, including mandatory contractual terms and technical safeguards, with respect to maintaining the privacy, security and transmission of individually identifiable health information.
|
||
|
<
|
Transparency Requirements.
The federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to report annually to the CMS information related to payments or transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as information regarding ownership and investment interests held by the physicians described above and their immediate family members.
|
||
|
<
|
Analogous State and Foreign Laws
. Analogous state and foreign fraud and abuse laws and regulations, such as state anti-kickback and false claims laws, can apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements, and claims involving healthcare items or services reimbursed by non-governmental third-party payors, and are generally broad and are enforced by many different federal and state agencies as well as through private actions. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources, and some state laws require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing information. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts.
|
||
|
|
<
|
|
our suppliers may fail to comply with regulatory requirements or make errors in manufacturing raw materials, components or products that could negatively affect the efficacy or safety of our products or cause delays in shipments of our products;
|
|
<
|
we may be subject to price fluctuations by suppliers due to terms within long-term supply arrangements or lack of long-term supply arrangements for key materials and products;
|
||
|
<
|
our suppliers may lose access to critical services or sustain damage to a facility, including losses due to natural disasters or geo-political events, that may result in a sustained interruption in the manufacture and supply of our products;
|
||
|
<
|
fluctuations in demand for our products or a supplier’s demand from other customers may affect their ability or willingness to deliver materials or products in a timely manner or may lead to long-term capacity constraints at the supplier;
|
||
|
<
|
we may not be able to find new or alternative sources or reconfigure our products and manufacturing processes in a timely manner if a necessary raw material or components becomes unavailable; and
|
||
|
|
<
|
|
our suppliers may encounter financial or other hardships unrelated to our demand for materials, products and services, which could inhibit their ability to fulfill our orders and meet our requirements.
|
|
<
|
the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case;
|
||
|
<
|
patent applications may not result in any patents being issued;
|
||
|
<
|
patents that may be issued may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable or otherwise may not provide any competitive advantage;
|
||
|
<
|
our competitors, many of whom have substantially greater resources and many of whom have made significant investments in competing technologies, may seek or may have already obtained patents that will limit, interfere with or eliminate our ability to make, use, and sell our potential product candidates;
|
||
|
<
|
there may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns; and
|
||
|
<
|
countries other than the United States may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop and market competing product candidates in such countries.
|
||
|
|
<
|
|
we may not be able to generate sufficient data to support full patent applications that protect the entire breadth of developments in one or more of our programs;
|
|
<
|
it is possible that one or more of our pending patent applications will not become an issued patent or, if issued, that the patent(s) will not: (a) be sufficient to protect our technology, (b) provide us with a basis for commercially viable products or (c) provide us with any competitive advantages;
|
||
|
<
|
if our pending applications issue as patents, they may be challenged by third parties as not infringed, invalid or unenforceable under U.S. or foreign laws; or
|
||
|
<
|
if issued, the patents under which we hold rights may not be valid or enforceable.
|
||
|
|
<
|
|
stop selling products or using technology that contains the allegedly infringing intellectual property;
|
|
<
|
lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property rights against others;
|
||
|
<
|
incur significant legal expenses;
|
||
|
<
|
pay substantial damages to the party whose intellectual property rights we may be found to be infringing;
|
||
|
<
|
redesign those products that contain the allegedly infringing intellectual property, which could be costly, disruptive and/or infeasible; or
|
||
|
<
|
attempt to obtain a license to the relevant intellectual property from third parties, which may not be available on reasonable terms or at all.
|
||
|
|
<
|
|
faulty human judgment and simple errors, omissions or mistakes;
|
|
<
|
fraudulent action of an individual or collusion of two or more people;
|
||
|
<
|
inappropriate management override of procedures; and
|
||
|
<
|
the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial control.
|
||
|
|
<
|
|
problems assimilating the purchased technologies, products or business operations;
|
|
<
|
issues maintaining uniform standards, procedures, controls and policies;
|
||
|
<
|
unanticipated costs associated with acquisitions;
|
||
|
<
|
diversion of management’s attention from our core business;
|
||
|
<
|
adverse effects on existing business relationships with suppliers and customers;
|
||
|
<
|
risks associated with entering new markets in which we have limited or no experience;
|
||
|
<
|
potential loss of key employees of acquired businesses; and
|
||
|
<
|
increased legal and accounting compliance costs.
|
||
|
|
<
|
|
the timing and results of applications for FDA review and approval of our Gvoke HypoPen and other regulatory actions with respect to our product candidates;
|
|
<
|
regulatory actions with respect to our competitors’ products and product candidates;
|
||
|
<
|
the success of existing or new competitive products or technologies;
|
||
|
<
|
results of clinical trials of product candidates of our competitors;
|
||
|
<
|
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments;
|
||
|
<
|
the timing and results of clinical trials of our pipeline product candidates;
|
||
|
<
|
commencement or termination of collaborations for our development programs;
|
||
|
<
|
the results of our efforts to develop additional product candidates or products;
|
||
|
<
|
the level of expenses related to any of our product candidates or clinical development programs;
|
||
|
<
|
failure or discontinuation of any of our development programs;
|
||
|
<
|
the pricing and reimbursement of our Gvoke HypoPen, if approved, and of other product candidates that may be approved;
|
||
|
<
|
regulatory or legal developments in the United States and other countries;
|
||
|
<
|
developments or disputes concerning patent applications, issued patents or other proprietary rights;
|
||
|
<
|
the recruitment or departure of key personnel;
|
||
|
<
|
actual or anticipated changes in estimates as to financial results or development timelines;
|
||
|
<
|
announcement or expectation of additional financing efforts;
|
||
|
<
|
sales of our common stock by us, our insiders or other stockholders;
|
||
|
<
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
||
|
<
|
changes in estimates or recommendations by securities analysts, if any, that cover our stock;
|
||
|
<
|
changes in the structure of healthcare payment systems;
|
||
|
<
|
market conditions in the pharmaceutical and biotechnology sectors;
|
||
|
<
|
general economic, industry and market conditions; and
|
||
|
<
|
the other factors described in this “Risk Factors” section.
|
||
|
|
<
|
|
establish a classified board of directors such that all members of the board are not elected at one time;
|
|
<
|
allow the authorized number of our directors to be changed only by resolution of our board of directors;
|
||
|
<
|
limit the manner in which stockholders can remove directors from the board;
|
||
|
<
|
establish advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on at stockholder meetings;
|
||
|
<
|
require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;
|
||
|
<
|
limit who may call a special meeting of stockholders;
|
||
|
<
|
authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors;
|
||
|
<
|
require the approval of the holders of at least two-thirds of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws;
|
||
|
<
|
provide that the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty by one or more of our directors, officers or employees, any action asserting a claim against us pursuant to the Delaware General Corporation Law, or any action asserting a claim against us that is governed by the internal affairs doctrine.
|
||
|
|
Years Ended December 31,
|
|||||||||||
|
(in thousands, except share and per share data)
|
2018
|
2017
|
2016
|
|||||||||
|
Statements of Operations Data
|
||||||||||||
|
Grant income
|
$
|
2,365
|
|
$
|
1,540
|
|
$
|
1,022
|
|
|||
|
Service revenue
|
100
|
|
16
|
|
53
|
|
||||||
|
Cost of revenue
|
42
|
|
4
|
|
8
|
|
||||||
|
Gross profit
|
2,423
|
|
1,552
|
|
1,067
|
|
||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
40,654
|
|
20,166
|
|
10,238
|
|
||||||
|
Selling, general and administrative
|
21,113
|
|
8,015
|
|
4,060
|
|
||||||
|
Expense from operations
|
61,767
|
|
28,181
|
|
14,298
|
|
||||||
|
Loss from operations
|
(59,344
|
)
|
(26,629
|
)
|
(13,231
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Interest income
|
1,613
|
|
124
|
|
5
|
|
||||||
|
Interest expense
|
(2,545
|
)
|
(2
|
)
|
(2
|
)
|
||||||
|
Change in fair value of warrants
|
196
|
|
(46
|
)
|
24
|
|
||||||
|
Other expense
|
—
|
|
(1
|
)
|
(5
|
)
|
||||||
|
Total other income (expense)
|
(736
|
)
|
75
|
|
22
|
|
||||||
|
Net loss
|
$
|
(60,080
|
)
|
$
|
(26,554
|
)
|
$
|
(13,209
|
)
|
|||
|
Net loss per share - basic and diluted
(1)
|
$
|
(4.99
|
)
|
$
|
(13.09
|
)
|
$
|
(7.17
|
)
|
|||
|
Weighted average number of common
shares outstanding, basic and diluted
(1)
|
12,045,999
|
|
2,028,224
|
|
1,842,416
|
|
||||||
|
|
As of December 31,
|
|||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
|||||||||
|
Balance Sheets Data
|
||||||||||||
|
Cash and cash equivalents
|
$
|
45,716
|
|
$
|
42,045
|
|
$
|
32,269
|
|
|||
|
Short-term investments
|
66,917
|
|
—
|
|
—
|
|
||||||
|
Working capital
(2)
|
107,727
|
|
39,193
|
|
30,647
|
|
||||||
|
Total assets
|
120,028
|
|
44,998
|
|
33,533
|
|
||||||
|
Long-term debt, net of deferred costs
|
31,890
|
|
—
|
|
—
|
|
||||||
|
Other long-term liabilities
|
2,560
|
|
90
|
|
42
|
|
||||||
|
Total liabilities
|
44,622
|
|
4,950
|
|
2,569
|
|
||||||
|
Total convertible preferred stock
|
—
|
|
97,878
|
|
62,898
|
|
||||||
|
Total stockholders' equity (deficit)
|
75,406
|
|
(57,830
|
)
|
(31,934
|
)
|
||||||
|
(1)
|
Refer to Note 2, "Summary of Significant Accounting Policies", of the notes to financial statements for an explanation of the calculations of our basic and diluted net loss per share and the shares used in computing basic and diluted net loss per share.
|
|
(2)
|
We define working capital as current assets less current liabilities.
|
|
<
|
expenses incurred under agreements with contract research organizations, or CROs, as well as investigative sites and consultants that conduct our preclinical studies and clinical trials;
|
|
<
|
manufacturing scale-up expenses, the cost of acquiring and manufacturing preclinical and clinical trial materials, including manufacturing validation batches, and the cost of manufacturing commercial supplies in advance of regulatory approval;
|
|
|
Years Ended December 31,
|
||||||||||
|
(in thousands)
|
2018
|
2017
|
$ Change
|
||||||||
|
Grant income
|
$
|
2,365
|
|
$
|
1,540
|
|
$
|
825
|
|
||
|
Service revenue
|
100
|
|
16
|
|
84
|
|
|||||
|
Cost of revenue
|
42
|
|
4
|
|
38
|
|
|||||
|
Gross profit
|
2,423
|
|
1,552
|
|
871
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
40,654
|
|
20,166
|
|
20,488
|
|
|||||
|
Selling, general and administrative
|
21,113
|
|
8,015
|
|
13,098
|
|
|||||
|
Expense from operations
|
61,767
|
|
28,181
|
|
33,586
|
|
|||||
|
Loss from operations
|
(59,344
|
)
|
(26,629
|
)
|
(32,715
|
)
|
|||||
|
Other income (expense):
|
|||||||||||
|
Interest income
|
1,613
|
|
124
|
|
1,489
|
|
|||||
|
Interest expense
|
(2,545
|
)
|
(2
|
)
|
(2,543
|
)
|
|||||
|
Change in fair value of warrants
|
196
|
|
(46
|
)
|
242
|
|
|||||
|
Other expense
|
—
|
|
(1
|
)
|
1
|
|
|||||
|
Total other income (expense)
|
(736
|
)
|
75
|
|
(811
|
)
|
|||||
|
Net loss
|
$
|
(60,080
|
)
|
$
|
(26,554
|
)
|
$
|
(33,526
|
)
|
||
|
|
Years Ended December 31,
|
||||||||||
|
(in thousands)
|
2018
|
2017
|
$ Change
|
||||||||
|
|
|||||||||||
|
Clinical and preclinical
|
$
|
13,295
|
|
$
|
9,233
|
|
$
|
4,062
|
|
||
|
Product development
|
18,909
|
|
6,654
|
|
12,255
|
|
|||||
|
Compensation and related personnel costs
|
7,932
|
|
4,217
|
|
3,715
|
|
|||||
|
Stock-based compensation
|
518
|
|
62
|
|
456
|
|
|||||
|
Total research and development expenses
|
$
|
40,654
|
|
$
|
20,166
|
|
$
|
20,488
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
(in thousands)
|
2018
|
2017
|
$ Change
|
||||||||
|
Gvoke HypoPen
|
$
|
20,865
|
|
$
|
10,339
|
|
$
|
10,526
|
|
||
|
Other ready-to-use glucagon programs
|
4,741
|
|
4,013
|
|
728
|
|
|||||
|
Pipeline product programs
|
2,434
|
|
60
|
|
2,374
|
|
|||||
|
Overhead (personnel, facilities and other expenses)
|
12,614
|
|
5,754
|
|
6,860
|
|
|||||
|
Total research and development expenses
|
$
|
40,654
|
|
$
|
20,166
|
|
$
|
20,488
|
|
||
|
<
|
the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property-related claims;
|
|
|
Years Ended December 31,
|
||||||
|
(
in thousands
)
|
2018
|
2017
|
|||||
|
Net cash used in operating activities
|
$
|
(56,279
|
)
|
$
|
(24,663
|
)
|
|
|
Net cash used in investing activities
|
(68,261
|
)
|
(700
|
)
|
|||
|
Net cash provided by financing activities
|
128,211
|
|
35,139
|
|
|||
|
Increase in cash and cash equivalents
|
$
|
3,671
|
|
$
|
9,776
|
|
|
|
(
in thousands
)
|
Total
|
2019
|
2020-2021
|
2022-2024
|
2025 and Beyond
|
||||||||||||||
|
Operating leases
|
$
|
18,566
|
|
$
|
891
|
|
$
|
3,798
|
|
$
|
5,318
|
|
$
|
8,559
|
|
||||
|
Future principal payments under
Loan and Security Agreement
|
35,000
|
|
—
|
|
21,000
|
|
14,000
|
|
—
|
|
|||||||||
|
|
Years Ended December 31,
|
||||||
|
(in thousands)
|
2018
|
2017
|
|||||
|
Research and development
|
$
|
518
|
|
$
|
62
|
|
|
|
Selling, general and administrative
|
1,210
|
|
437
|
|
|||
|
Total stock-based compensation expense
|
$
|
1,728
|
|
$
|
499
|
|
|
|
<
|
Expected Term.
We do not believe we are able to rely on our historical exercise and post-vesting termination activity to provide accurate data for estimating the expected term for use in determining the fair value-based measurement of our options. Therefore, we have opted to use the “simplified method” for estimating the expected term of options, which is the average of the weighted-average vesting period and contractual term of the option.
|
|
∎
|
Expected Volatility.
As we have limited trading history for our common stock, the expected stock price volatility assumption is determined based on the historical volatilities of a peer group of publicly traded companies as well as the historical volatility of our own common stock since we began trading subsequent to our IPO in June 2018. In evaluating similarity, we consider factors such as stage of development, risk profile, enterprise value and position within the industry. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own common stock share price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case more suitable companies whose share prices are publicly available would be utilized in the calculation.
|
|
∎
|
Risk-Free Interest Rate.
The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of the grant for zero-coupon U.S. Treasury notes with remaining terms similar to the expected term of the options.
|
|
∎
|
Expected Dividends.
The expected dividend yield is 0% because we have not historically paid, and do not expect for the foreseeable future to pay, a dividend on our common stock.
|
|
Index to Financial Statements
|
|||||
|
Page
|
|||||
|
Report of Independent Registered Public Accounting Firm
|
|||||
|
Financial Statements
|
|||||
|
Balance Sheets as of December 31, 2018 and December 31, 2017
|
|||||
|
Statements of Operations and Comprehensive Loss for the years ended December 31, 2018 and 2017
|
|||||
|
Statements of Convertible Preferred Stock and Stockholders' Equity (Deficit) for the years ended
December 31, 2018 and 2017
|
|||||
|
Statements of Cash Flows for the years ended December 31, 2018 and 2017
|
|||||
|
Notes to Financial Statements
|
|||||
|
December 31, 2018
|
December 31, 2017
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
45,716
|
|
$
|
42,045
|
|
|
|
Short-term investments
|
66,917
|
|
—
|
|
|||
|
Accounts receivable, net
|
2,869
|
|
1,199
|
|
|||
|
Prepaid expenses and other current assets
|
2,397
|
|
809
|
|
|||
|
Total current assets
|
117,899
|
|
44,053
|
|
|||
|
Property and equipment, net
|
2,034
|
|
788
|
|
|||
|
Other assets
|
95
|
|
157
|
|
|||
|
Total assets
|
$
|
120,028
|
|
$
|
44,998
|
|
|
|
Liabilities, Convertible Preferred Stock and Stockholders’ Equity (Deficit)
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
866
|
|
$
|
1,976
|
|
|
|
Accrued expenses
|
8,214
|
|
2,557
|
|
|||
|
Warrant liabilities
|
860
|
|
93
|
|
|||
|
Deferred grant awards
|
232
|
|
234
|
|
|||
|
Total current liabilities
|
10,172
|
|
4,860
|
|
|||
|
Long-term debt, net of unamortized deferred costs
|
31,890
|
|
—
|
|
|||
|
Other long-term liabilities
|
2,560
|
|
90
|
|
|||
|
Total liabilities
|
44,622
|
|
4,950
|
|
|||
|
Commitments and Contingencies (Note 9)
|
|
|
|
|
|||
|
Convertible Preferred Stock:
|
|||||||
|
Series A Convertible Preferred Stock—par value $0.0001, 1,864,797 shares authorized and 1,843,965 shares issued and outstanding as of December 31, 2017
|
—
|
|
1,945
|
|
|||
|
Series B Convertible Preferred Stock—par value $0.0001, 5,732,338 shares authorized and 5,696,834 shares issued and outstanding as of December 31, 2017
|
—
|
|
18,536
|
|
|||
|
Series C Convertible Preferred Stock—par value $0.0001, 14,353,859 shares authorized and 12,834,912 shares issued and outstanding as of December 31, 2017
|
—
|
|
77,397
|
|
|||
|
Total convertible preferred stock
|
—
|
|
97,878
|
|
|||
|
Stockholders’ Equity (Deficit):
|
|||||||
|
Preferred stock—par value $0.0001, 10,000,000 shares authorized as of December 31, 2018 and no shares issued and outstanding as of December 31, 2018
|
—
|
|
—
|
|
|||
|
Common stock—par value $0.0001, 150,000,000 and 30,450,994 shares authorized as of December 31, 2018 and 2017, respectively; 20,808,366 and 2,159,068 shares issued and outstanding as of December 31, 2018 and 2017, respectively
|
2
|
|
1
|
|
|||
|
Additional paid in capital
|
196,121
|
|
2,754
|
|
|||
|
Accumulated deficit
|
(120,665
|
)
|
(60,585
|
)
|
|||
|
Accumulated other comprehensive loss
|
(52
|
)
|
—
|
|
|||
|
Total stockholders’ equity (deficit)
|
75,406
|
|
(57,830
|
)
|
|||
|
Total liabilities, convertible preferred stock and stockholders’ equity (deficit)
|
$
|
120,028
|
|
$
|
44,998
|
|
|
|
Years Ended December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Grant income
|
$
|
2,365
|
|
$
|
1,540
|
|
|
|
Service revenue
|
100
|
|
16
|
|
|||
|
Cost of revenue
|
42
|
|
4
|
|
|||
|
Gross profit
|
2,423
|
|
1,552
|
|
|||
|
Operating expenses:
|
|||||||
|
Research and development
|
40,654
|
|
20,166
|
|
|||
|
Selling, general and administrative
|
21,113
|
|
8,015
|
|
|||
|
Expense from operations
|
61,767
|
|
28,181
|
|
|||
|
Loss from operations
|
(59,344
|
)
|
|
(26,629
|
)
|
||
|
Other income (expense):
|
|||||||
|
Interest income
|
1,613
|
|
124
|
|
|||
|
Interest expense
|
(2,545
|
)
|
(2
|
)
|
|||
|
Change in fair value of warrants
|
196
|
|
(46
|
)
|
|||
|
Other expense
|
—
|
|
(1
|
)
|
|||
|
Total other income (expense)
|
(736
|
)
|
75
|
|
|||
|
Net loss
|
$
|
(60,080
|
)
|
$
|
(26,554
|
)
|
|
|
Other comprehensive loss, net of tax:
|
|||||||
|
Unrealized losses on short-term investments
|
(52
|
)
|
—
|
|
|||
|
Comprehensive loss
|
$
|
(60,132
|
)
|
$
|
(26,554
|
)
|
|
|
Net loss per common share - basic and diluted
|
$
|
(4.99
|
)
|
$
|
(13.09
|
)
|
|
|
Weighted average common shares outstanding, basic and diluted
|
12,045,999
|
|
2,028,224
|
|
|||
|
|
CONVERTIBLE PREFERRED STOCK
|
STOCKHOLDERS’ EQUITY (DEFICIT)
|
|||||||||||||||||||||||||||||||||||||||||||
|
|
SERIES A
|
SERIES B
|
SERIES C
|
COMMON STOCK
|
ADDITIONAL
PAID IN
CAPITAL
|
ACCUMULATED OTHER COMPREHENSIVE LOSS
|
ACCUMULATED
DEFICIT
|
TOTAL
|
|||||||||||||||||||||||||||||||||||||
|
|
SHARES
|
AMOUNT
|
SHARES
|
AMOUNT
|
SHARES
|
AMOUNT
|
SHARES
|
AMOUNT
|
|||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2016
|
1,843,965
|
|
$
|
1,945
|
|
5,696,834
|
|
$
|
18,536
|
|
7,177,398
|
|
$
|
42,417
|
|
1,927,237
|
|
$
|
1
|
|
$
|
2,096
|
|
$
|
—
|
|
$
|
(34,031
|
)
|
$
|
(31,934
|
)
|
|||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(26,554
|
)
|
(26,554
|
)
|
|||||||||||||||||||||
|
Issuance of Series C Preferred Stock, net of
cost of $495 |
—
|
|
—
|
|
—
|
|
—
|
|
5,657,514
|
|
34,980
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||
|
Exercise and vesting of stock-based awards
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
231,831
|
|
—
|
|
159
|
|
—
|
|
—
|
|
159
|
|
|||||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
499
|
|
—
|
|
—
|
|
499
|
|
|||||||||||||||||||||
|
Balance, December 31, 2017
|
1,843,965
|
|
$
|
1,945
|
|
5,696,834
|
|
$
|
18,536
|
|
12,834,912
|
|
$
|
77,397
|
|
2,159,068
|
|
$
|
1
|
|
$
|
2,754
|
|
$
|
—
|
|
$
|
(60,585
|
)
|
$
|
(57,830
|
)
|
|||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(60,080
|
)
|
(60,080
|
)
|
|||||||||||||||||||||
|
Issuance of common stock upon Initial Public
Offering, net of cost of $9,422
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6,555,000
|
|
—
|
|
88,903
|
|
—
|
|
—
|
|
88,903
|
|
|||||||||||||||||||||
|
Issuance of Series C Preferred Stock, net of
cost of $24 |
—
|
|
—
|
|
—
|
|
—
|
|
707,680
|
|
4,414
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||
|
Conversion of convertible preferred stock into
common stock
|
(1,843,965
|
)
|
(1,945
|
)
|
(5,696,834
|
)
|
(18,536
|
)
|
(13,542,592
|
)
|
(81,811
|
)
|
11,837,073
|
|
1
|
|
102,292
|
|
—
|
|
—
|
|
102,293
|
|
|||||||||||||||||||||
|
Exercise and vesting of stock-based awards
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
248,978
|
|
—
|
|
395
|
|
—
|
|
—
|
|
395
|
|
|||||||||||||||||||||
|
Exercise of warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,247
|
|
—
|
|
49
|
|
—
|
|
—
|
|
49
|
|
|||||||||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,728
|
|
—
|
|
—
|
|
1,728
|
|
|||||||||||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(52
|
)
|
—
|
|
(52
|
)
|
|||||||||||||||||||||
|
Balance, December 31, 2018
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
20,808,366
|
|
$
|
2
|
|
$
|
196,121
|
|
$
|
(52
|
)
|
$
|
(120,665
|
)
|
$
|
75,406
|
|
|||||||||||||
|
Years Ended December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Cash flows from operating activities:
|
|||||||
|
Net loss
|
$
|
(60,080
|
)
|
$
|
(26,554
|
)
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||
|
Depreciation and amortization
|
320
|
|
225
|
|
|||
|
Amortization of short-term investments
|
(218
|
)
|
—
|
|
|||
|
Amortization of debt issuance costs
|
560
|
|
—
|
|
|||
|
Stock-based compensation
|
1,728
|
|
499
|
|
|||
|
Change in fair value of warrants
|
(196
|
)
|
46
|
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Accounts receivable
|
(1,670
|
)
|
(1,098
|
)
|
|||
|
Prepaid expenses and other current assets
|
(1,588
|
)
|
(5
|
)
|
|||
|
Other assets
|
62
|
|
(111
|
)
|
|||
|
Accounts payable
|
(1,110
|
)
|
661
|
|
|||
|
Accrued expenses
|
5,630
|
|
1,703
|
|
|||
|
Deferred grant awards
|
(2
|
)
|
(29
|
)
|
|||
|
Deferred rent
|
285
|
|
—
|
|
|||
|
Net cash used in operating activities
|
(56,279
|
)
|
(24,663
|
)
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Purchases of property and equipment
|
(1,510
|
)
|
(700
|
)
|
|||
|
Purchases of short-term investments
|
(68,851
|
)
|
—
|
|
|||
|
Sales and maturities of short-term investments
|
2,100
|
|
—
|
|
|||
|
Net cash used in investing activities
|
(68,261
|
)
|
(700
|
)
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Proceeds from Initial Public Offering
|
98,325
|
|
—
|
|
|||
|
Payments for Initial Public Offering costs
|
(9,422
|
)
|
—
|
|
|||
|
Proceeds from sale of Series C Preferred Stock
|
4,438
|
|
35,475
|
|
|||
|
Payments of Series C Preferred Stock offering costs
|
(24
|
)
|
(495
|
)
|
|||
|
Proceeds from issuance of long-term debt
|
35,000
|
|
—
|
|
|||
|
Payments of debt issuance costs
|
(333
|
)
|
—
|
|
|||
|
Proceeds from exercise of stock awards
|
227
|
|
159
|
|
|||
|
Net cash provided by financing activities
|
128,211
|
|
35,139
|
|
|||
|
Increase in cash and cash equivalents
|
3,671
|
|
9,776
|
|
|||
|
Cash and cash equivalents, beginning of period
|
42,045
|
|
32,269
|
|
|||
|
Cash and cash equivalents, end of period
|
$
|
45,716
|
|
$
|
42,045
|
|
|
|
Supplemental schedule of cash flow information:
|
|||||||
|
Cash paid for interest
|
$
|
1,711
|
|
$
|
2
|
|
|
|
Supplemental schedule of non-cash investing and financing activities:
|
|||||||
|
Allocation of debt costs to warrants
|
$
|
1,012
|
|
$
|
—
|
|
|
|
Accrued debt issuance costs
|
$
|
2,325
|
|
$
|
—
|
|
|
|
Vesting of early exercised awards
|
$
|
169
|
|
$
|
—
|
|
|
|
Lab equipment
|
5 years
|
|
|
Computer equipment
|
3 years
|
|
|
Leasehold improvements
|
Lesser of useful life or lease term
|
|
|
Software
|
3-5 years
|
|
|
Furniture and fixtures
|
5 years
|
|
|
Office equipment
|
5 years
|
|
|
December 31,
|
|||||
|
2018
|
2017
|
||||
|
Convertible preferred stock
|
—
|
|
11,439,752
|
|
|
|
Vested and unvested stock options
|
3,130,700
|
|
1,979,306
|
|
|
|
Warrants
|
102,647
|
|
19,931
|
|
|
|
3,233,347
|
|
13,438,989
|
|
||
|
(in thousands)
|
December 31, 2018
|
December 31, 2017
|
|||||
|
Lab equipment
|
$
|
1,658
|
|
$
|
860
|
|
|
|
Furniture and fixtures
|
541
|
|
128
|
|
|||
|
Computer equipment
|
87
|
|
100
|
|
|||
|
Office equipment
|
109
|
|
78
|
|
|||
|
Software
|
110
|
|
52
|
|
|||
|
Leasehold improvements
|
180
|
|
10
|
|
|||
|
2,685
|
|
1,228
|
|
||||
|
Less: accumulated depreciation and amortization
|
(651
|
)
|
(440
|
)
|
|||
|
Property and equipment, net
|
$
|
2,034
|
|
$
|
788
|
|
|
|
(in thousands)
|
December 31, 2018
|
December 31, 2017
|
|||||
|
Accrued employee costs
|
$
|
4,326
|
|
$
|
1,581
|
|
|
|
Accrued research and development costs
|
2,221
|
|
566
|
|
|||
|
Accrued other costs
|
1,667
|
|
410
|
|
|||
|
Accrued expenses
|
$
|
8,214
|
|
$
|
2,557
|
|
|
|
(in thousands)
|
December 31, 2018
|
December 31, 2017
|
|||||
|
Term A Loan
|
$
|
20,000
|
|
$
|
—
|
|
|
|
Term B Loan
|
15,000
|
|
—
|
|
|||
|
Less unamortized deferred costs
|
(3,110
|
)
|
—
|
|
|||
|
Long-term debt
|
$
|
31,890
|
|
$
|
—
|
|
|
|
2018
|
$
|
—
|
|
|
2019
|
—
|
|
|
|
2020
|
9,000
|
|
|
|
2021
|
12,000
|
|
|
|
2022
|
12,000
|
|
|
|
2023
|
2,000
|
|
|
|
$
|
35,000
|
|
|
|
Outstanding Warrants
|
Exercise Price per Warrant
|
Expiration
Date
|
|||
|
2014 Warrants
|
8,635
|
$5.912
|
August 2020
|
||
|
Term A Warrants
|
53,720
|
$11.169
|
February 2025
|
||
|
Term B Warrants
|
40,292
|
$11.169
|
September 2025
|
||
|
102,647
|
|||||
|
(in thousands)
|
|||
|
2019
|
$
|
891
|
|
|
2020
|
1,580
|
|
|
|
2021
|
2,218
|
|
|
|
2022
|
2,273
|
|
|
|
2023
|
1,756
|
|
|
|
Thereafter
|
9,848
|
|
|
|
Total minimum lease payments
|
$
|
18,566
|
|
|
Years Ended December 31,
|
|||||
|
2018
|
2017
|
||||
|
Expected term (years)
|
6.0
|
|
6.1
|
|
|
|
Risk-free interest rate
|
2.48
|
%
|
2.06
|
%
|
|
|
Expected volatility
|
56.84
|
%
|
61.10
|
%
|
|
|
Expected dividends
|
—
|
|
—
|
|
|
|
UNITS
|
WEIGHTED
AVERAGE
EXERCISE PRICE
|
WEIGHTED
AVERAGE CONTRACTUAL
LIFE (YEARS)
|
|||||
|
Outstanding - January 1, 2018
|
1,946,230
|
$
|
1.66
|
|
8.70
|
||
|
Issued
|
1,604,804
|
14.14
|
|
||||
|
Exercised
|
(225,986)
|
1.46
|
|
||||
|
Forfeited
|
(197,740)
|
1.89
|
|
||||
|
Outstanding - December 31, 2018
|
3,127,308
|
$
|
8.06
|
|
8.69
|
||
|
Exercisable - December 31, 2018
|
2,329,151
|
$
|
4.44
|
|
8.67
|
||
|
Vested and expected to vest at December 31, 2018
|
2,939,669
|
$
|
8.00
|
|
7.74
|
||
|
UNITS
|
WEIGHTED AVERAGE EXERCISE PRICE
|
WEIGHTED AVERAGE CONTRACTUAL LIFE (YEARS)
|
|||||
|
Outstanding - January 1, 2018
|
33,125
|
$
|
1.91
|
|
6.75
|
||
|
Issued
|
0
|
—
|
|
||||
|
Exercised
|
(22,996)
|
2.16
|
|
||||
|
Forfeited
|
(6,737)
|
0.82
|
|
||||
|
Outstanding - December 31, 2018
|
3,392
|
$
|
1.55
|
|
2.00
|
||
|
Exercisable - December 31, 2018
|
0
|
$
|
0.00
|
|
0.00
|
||
|
Vested and expected to vest at December 31, 2018
|
3,392
|
$
|
1.55
|
|
2.00
|
||
|
|
Years Ended December 31,
|
||||||
|
(in thousands)
|
2018
|
2017
|
|||||
|
Research and development
|
$
|
518
|
|
$
|
62
|
|
|
|
Selling, general and administrative
|
1,210
|
|
437
|
|
|||
|
Total stock-based compensation expense
|
$
|
1,728
|
|
$
|
499
|
|
|
|
(in thousands)
|
Total as of December 31, 2018
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
|
||||||||||||||||
|
Current Assets
|
||||||||||||||||
|
Cash and cash equivalents:
|
||||||||||||||||
|
Money market funds
|
$
|
45,716
|
|
$
|
45,716
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Short-term investments:
|
||||||||||||||||
|
U.S. government securities
|
$
|
38,737
|
|
$
|
38,737
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Corporate securities
|
15,066
|
|
—
|
|
15,066
|
|
—
|
|
||||||||
|
Agency securities
|
11,931
|
|
—
|
|
11,931
|
|
—
|
|
||||||||
|
Commercial paper
|
1,183
|
|
1,183
|
|
—
|
|
—
|
|
||||||||
|
Total short-term investments
|
$
|
66,917
|
|
$
|
39,920
|
|
$
|
26,997
|
|
$
|
—
|
|
||||
|
Other Current Liabilities
|
||||||||||||||||
|
Warrant liabilities
|
$
|
860
|
|
$
|
—
|
|
$
|
—
|
|
$
|
860
|
|
||||
|
(in thousands)
|
Total as of December 31, 2017
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Current Assets
|
||||||||||||||||
|
Money market funds
(a)
|
$
|
39,124
|
|
$
|
39,124
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Other current liabilities
|
||||||||||||||||
|
Warrant liabilities
|
$
|
93
|
|
$
|
—
|
|
$
|
—
|
|
$
|
93
|
|
||||
|
(in thousands)
|
|||
|
Balance at December 31, 2017
|
$
|
93
|
|
|
Fair value of Term A Warrants issued under the Loan and Security Agreement
|
326
|
|
|
|
Fair value of Term B Warrants issued under the Loan and Security Agreement
|
686
|
|
|
|
Exercise of warrants
|
(49
|
)
|
|
|
Change in fair value of warrants
|
(196
|
)
|
|
|
Balance at December 31, 2018
|
$
|
860
|
|
|
(in thousands)
|
Amortized
Cost
|
Gross Unrealized Losses
|
Total
Fair Value
|
|||||||||
|
Short-term investments:
|
||||||||||||
|
Agency securities
|
$
|
11,944
|
|
$
|
(13
|
)
|
$
|
11,931
|
|
|||
|
Commercial paper
|
1,183
|
|
—
|
|
1,183
|
|
||||||
|
Corporate securities
|
15,081
|
|
(15
|
)
|
15,066
|
|
||||||
|
U.S. government securities
|
38,761
|
|
(24
|
)
|
38,737
|
|
||||||
|
Total short-term investments
|
$
|
66,969
|
|
$
|
(52
|
)
|
$
|
66,917
|
|
|||
|
|
December 31,
|
|||||||
|
(in thousands)
|
2018
|
2017
|
||||||
|
Federal tax benefit at statutory rate
|
$
|
(12,617
|
)
|
$
|
(9,028
|
)
|
||
|
State tax benefit, net of federal benefit
|
(1,842
|
)
|
—
|
|
||||
|
Impact of rate change
|
—
|
|
7,478
|
|
||||
|
Research and development and orphan drug credits
|
(2,279
|
)
|
(517
|
)
|
||||
|
Uncertain tax positions
|
603
|
|
—
|
|
||||
|
Permanent adjustments to expenses
|
45
|
|
76
|
|
||||
|
Stock compensation
|
76
|
|
42
|
|
||||
|
Prior year adjustment
|
(2,470
|
)
|
(100
|
)
|
||||
|
Rate impact of deferred tax balance
|
(63
|
)
|
—
|
|
||||
|
Other
|
9
|
|
—
|
|
||||
|
Changes in valuation allowance
|
18,538
|
|
2,049
|
|
||||
|
Total income taxes
|
$
|
—
|
|
$
|
—
|
|
||
|
|
December 31,
|
|||||||
|
(in thousands)
|
2018
|
2017
|
||||||
|
Deferred tax assets:
|
||||||||
|
Net operating losses
|
$
|
25,372
|
|
$
|
11,715
|
|
||
|
Federal research and orphan drug credits
|
5,426
|
|
2,045
|
|
||||
|
Stock compensation
|
267
|
|
49
|
|
||||
|
Other temporary differences
|
1,679
|
|
349
|
|
||||
|
Valuation allowance
|
(32,662
|
)
|
(14,124
|
)
|
||||
|
Total assets
|
82
|
|
34
|
|
||||
|
Deferred tax liabilities:
|
||||||||
|
Fixed and intangible assets
|
(82
|
)
|
(34
|
)
|
||||
|
Total liabilities
|
(82
|
)
|
(34
|
)
|
||||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
||
|
Valuation allowance at December 31, 2016
|
$
|
(12,075
|
)
|
|
|
Increase for 2017 activity
|
(2,049
|
)
|
||
|
Valuation allowance at December 31, 2017
|
(14,124
|
)
|
||
|
Increase for 2018 activity
|
(18,538
|
)
|
||
|
Valuation allowance at December 31, 2018
|
$
|
(32,662
|
)
|
|
|
December 31,
|
||||||||
|
(in thousands)
|
2018
|
2017
|
||||||
|
Beginning balance - uncertain tax positions
|
$
|
—
|
|
$
|
—
|
|
||
|
Increases related to tax positions taken during the current year
|
228
|
|
—
|
|
||||
|
Increases related to tax positions taken during the prior year
|
375
|
|
—
|
|
||||
|
Ending balance - uncertain tax positions
|
$
|
603
|
|
$
|
—
|
|
||
|
(a) The following documents are filed as part of this Form 10-K:
|
|||||
|
1. Financial Statements
|
|||||
|
See Index to Financial Statements at Item 8 herein.
|
|||||
|
2. Financial Statement Schedules
|
|||||
|
All schedules are omitted because they are not applicable or the required information is shown in the
financial statements or notes thereto.
|
|||||
|
3. Exhibits
|
|||||
|
Exhibit No.
|
Description
|
|
|
3.1
|
||
|
3.2
|
||
|
4.1
|
||
|
4.2
|
||
|
10.1#
|
||
|
10.2#
|
||
|
10.3#
|
||
|
10.4#
|
||
|
10.5#
|
||
|
10.6
|
||
|
10.7#
|
||
|
10.8#
|
||
|
10.9#
|
||
|
10.10#
|
||
|
10.11#
|
||
|
10.12#
|
||
|
10.13#
|
||
|
Exhibit No.
|
Description
|
|
|
10.14+
|
||
|
10.15+
|
||
|
10.16+
|
||
|
10.17+
|
||
|
10.18
|
||
|
10.19+
|
||
|
10.20#
|
||
|
10.21+
|
||
|
10.22#
|
||
|
10.23
|
||
|
23.1*
|
||
|
31.1*
|
||
|
31.2*
|
||
|
32.1*
|
||
|
32.2*
|
||
|
101
|
The following materials from Xeris Pharmaceuticals, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2018, formatted in XBRL (Extensible Business Reporting Language): (i) the Balance Sheets, (ii) the Statements of Operations and Comprehensive Loss, (iii) the Statements of Convertible Preferred Stock and Stockholders' Equity (Deficit), (iv) the Statements of Cash Flows and (v) Notes to Financial Statements
|
|
|
+
|
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to confidential treatment order, and this exhibit has been submitted separately to the U.S. Securities and Exchange Commission.
|
|
Xeris Pharmaceuticals, Inc.
|
||||
|
By
|
/s/ Paul R. Edick
|
|||
|
Paul R. Edick
|
||||
|
President, Chief Executive Officer and Chairman
|
||||
|
Date
|
March 6, 2019
|
|||
|
SIGNATURE
|
TITLE
|
|
|
/s/ Paul R. Edick
Paul R. Edick
|
President, Chief Executive Officer and Chairman
(Principal Executive Officer)
|
|
|
/s/ Barry M. Deutsch
Barry M. Deutsch
|
Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer)
|
|
|
/s/ John Schmid
John Schmid
|
Director
|
|
|
/s/ BJ Bormann
BJ Bormann
|
Director
|
|
|
/s/ Jeffrey Sherman
Jeffrey Sherman
|
Director
|
|
|
/s/ Jonathan Rigby
Jonathan Rigby
|
Director
|
|
|
/s/ Marla Persky
Marla Persky
|
Director
|
|
|
/s/ Dawn Halkuff
Dawn Halkuff
|
Director
|
|