|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
|
|
52-2154066
|
|
(State or other jurisdiction
of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
2910 Seventh Street, Berkeley,
California 94710
|
|
(510) 204-7200
|
|
(Address of principal executive offices,
including zip code)
|
|
(Telephone number)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $0.0075 par value
Preferred Stock Purchase Rights
|
The NASDAQ Global Market
|
| Large Accelerated Filer o | Accelerated Filer x | Non-Accelerated filer o | Smaller reporting company o |
|
Item 1.
|
1
|
|
|
Item 1A.
|
19
|
|
|
Item 1B.
|
38
|
|
|
Item 2.
|
38
|
|
|
Item 3.
|
38
|
|
|
Item 4.
|
39
|
|
|
39
|
||
|
PART II
|
||
|
Item 5.
|
40
|
|
|
Item 6.
|
41
|
|
|
Item 7.
|
43
|
|
|
Item 7A.
|
59
|
|
|
Item 8.
|
60
|
|
|
Item 9.
|
60
|
|
|
Item 9A.
|
61
|
|
|
Item 9B.
|
62
|
|
|
PART III
|
||
|
Item 10.
|
63
|
|
|
Item 11.
|
63
|
|
|
Item 12.
|
63
|
|
|
Item 13.
|
63
|
|
|
Item 14.
|
63
|
|
|
PART IV
|
||
|
Item 15.
|
64
|
|
|
65
|
||
|
F-1
|
||
|
i
|
||
|
Item 1.
|
Busi
ne
ss
|
|
|
·
|
Focus on advancing gevokizumab, our lead product candidate.
Using our proprietary antibody technologies, capabilities and expertise, we discovered gevokizumab, an antibody that inhibits IL-1 beta. Gevokizumab has the potential to address the underlying inflammatory causes of a wide range of unmet medical needs by targeting IL-1 beta, a cytokine that triggers inflammatory pathways in the body.
|
|
|
·
|
Advance our proprietary preclinical pipeline candidates and generate revenues from our proprietary technologies.
We will continue to develop our proprietary preclinical pipeline, primarily focusing on the development of allosteric modulating monoclonal antibodies. Our first program, which targets the insulin receptor, has generated two new classes of fully human monoclonal antibodies that activate (XMetA) or sensitize (XMetS) the insulin receptor in vivo. XMetA and XMetS represent the potential for distinct, new therapeutic approaches to the treatment of patients with diabetes. Separate studies of XMetA and XMetS demonstrated they reduced fasting blood glucose levels and improved glucose tolerance in mouse models of diabetes.
|
|
|
·
|
Complete current biodefense contracts.
To date, we have been awarded four contracts, totaling up to approximately $120 million, from NIAID to support development of XOMA 3AB and additional product candidates for the treatment of botulism poisoning. In addition, our biodefense programs included two subcontracts from SRI International totaling $4.3 million, funded through NIAID, for the development of antibodies to neutralize H1N1 and H5N1 influenza viruses and the virus that causes severe acute respiratory syndrome (“SARS”).
|
|
|
·
|
Gevokizumab
is a potent monoclonal antibody with the potential to improve the treatment of patients with a wide variety of inflammatory diseases. Gevokizumab binds strongly to IL-1 beta, a pro-inflammatory cytokine involved in the development of NIU and Behçet’s uveitis, moderate-to-severe inflammatory acne, erosive osteoarthritis of the hand, cardiovascular disease, rheumatoid arthritis, gout and other diseases. By binding to IL-1 beta, gevokizumab inhibits the activation of the IL-1 receptor, thereby preventing the cellular signaling events that produce inflammation. Gevokizumab is a humanized IgG2 antibody. Based on its binding properties, specificity for IL-1 beta and its half-life (the time it takes for the amount administered to be reduced by one-half) in the body, gevokizumab may provide convenient dosing of once per month or less frequently.
|
|
|
·
|
XOMA Metabolic Activating and Sensitizing Antibodies.
Insulin receptor-activating antibodies, such as XMetA, are designed to provide long-acting insulin-like activity to diabetic patients who cannot make sufficient insulin, potentially reducing the number of insulin injections needed to control their blood glucose levels. Insulin receptor-sensitizing antibodies, such as XMetS, are designed to reduce insulin resistance and could enable diabetic patients to use their own insulin more effectively to control blood glucose levels.
|
|
|
·
|
XOMA 3AB
is a multi-antibody product designed to neutralize the most potent of the botulinum toxins, Type A, which causes paralysis and is a bioterrorism threat. Our anti-botulism program also includes additional product candidates and is the first of its kind to combine multiple human antibodies in each product candidate to target a broad spectrum of the most toxic botulinum toxins, including the three most toxic serotypes, Types A, B and E. The antibodies are designed to bind to each toxin and enhance the clearance of the toxin from the body. The use of multiple antibodies increases the likelihood of clearing the harmful toxins by providing specific protection against each toxin type. In contrast to existing agents that treat botulism, XOMA uses advanced human monoclonal antibody technologies in an effort to achieve superior safety, potency and efficacy, and avoid life-threatening immune reactions associated with animal-derived products. XOMA 3AB is currently in a Phase 1 study funded and conducted by NIAID.
|
|
|
·
|
Preclinical Product Pipeline:
We are pursuing additional opportunities to further broaden our preclinical product pipeline. These include internal discovery programs, product development collaborations with other pharmaceutical and biotechnology companies and evaluation of product in-licensing, in-kind product trades and acquisition opportunities.
|
|
|
·
|
Therapeutic Antibodies with Takeda:
Since 2006, Takeda has been a collaboration partner for therapeutic monoclonal antibody discovery and development against multiple targets selected by them. In February 2009, we expanded our existing collaboration to provide Takeda with access to multiple antibody technologies, including a suite of research and development technologies and integrated information and data management systems. We may receive potential milestones and royalties on sales of antibody products in the future.
|
|
|
·
|
Therapeutic Antibodies with Novartis:
In November 2008, we restructured our product development collaboration with Novartis. Under the restructured agreement, Novartis received control over the two ongoing programs, HCD122 and LFA102, under the original product development collaboration entered into in 2004 with Novartis (then Chiron Corporation). We may, in the future, receive milestones and double-digit royalty rates for the programs and options to develop or receive royalties from four additional programs.
|
|
|
·
|
Therapeutic Antibodies with Merck/Schering- Plough
: Merck/Schering-Plough has been a collaboration partner since 2006 for therapeutic monoclonal antibody discovery and development against multiple targets selected by them. In January 2011, we successfully completed the services we had agreed to perform under the collaboration agreement with Merck/Schering-Plough.
|
|
|
·
|
ADAPT™ (Antibody Discovery Advanced Platform Technologies): proprietary phage display libraries integrated with yeast and mammalian display to enable antibody discovery;
|
|
|
·
|
ModulX™: technology that enables positive and negative modulation of biological pathways using a new class of monoclonal antibodies called allosterically modulating antibodies; and
|
|
|
·
|
OptimX™: technologies used for optimizing biophysical properties of antibodies, including affinity, immunogenicity, stability and manufacturability.
|
|
|
·
|
Antibody discovery technologies:
We use human antibody phage display libraries, integrated with yeast and mammalian display (“ADAPT™ Integrated Display”), in our discovery of therapeutic candidates, and we offer access to this platform, including novel phage libraries developed internally, as part of our collaboration business. We believe access to ADAPT™ Integrated Display offers a number of benefits to us and our collaboration partners, because it enables us to combine the diversity of phage libraries with accelerated discovery due to rapid IgG reformatting and FACS-based screening using yeast and mammalian display. This increases the probability of technical and business success in finding rare and unique functional antibodies directed to targets of interest.
|
|
|
·
|
ModulX™ technology:
ModulX™ technology allows modulation of biological pathways using monoclonal antibodies and offers insights into regulation of signaling pathways, homeostatic control, and disease biology. Using ModulX™, XOMA is generating a new class of product candidates with novel mechanisms of action that specifically alter the kinetics of interaction between molecular constituents (e.g. receptor-ligand). ModulX™ technology enables expanded target and therapeutic options, and offers a unique approach in the treatment of disease.
|
|
|
·
|
OptimX™ technologies:
|
|
|
·
|
Bacterial Cell Expression:
The production or expression of antibodies using bacteria is an enabling technology for the discovery and selection, as well as the development and manufacture, of recombinant protein pharmaceuticals, including diagnostic and therapeutic antibodies for commercial purposes. Genetically engineered bacteria are used in the recombinant expression of target proteins for biopharmaceutical research and development, primarily due to the relative simplicity of gene expression in bacteria, as well as many years of experience culturing such species as
E. coli
in laboratories and manufacturing facilities. Our scientists have developed bacterial expression technologies for producing antibodies and other recombinant protein products.
|
|
Active Biotech AB
|
Dompe, s.p.a.
|
MorphoSys AG
|
||
|
Affimed Therapeutics AG
|
Dyax Corp.
|
Novartis AG
|
||
|
Affitech AS
|
Eli Lilly and Company
|
Pfizer Inc.
|
||
|
Applied Molecular Evolution, Inc. (now a subsidiary of Eli Lilly and Company)
|
Genentech, Inc. (now a member of the Roche Group)
|
Takeda Pharmaceutical Company Ltd.
|
||
|
Bayer Healthcare AG
|
Invitrogen Corporation
|
The Medical Research Council
UCB S.A.
|
||
|
BioInvent International AB
|
MedImmune Ltd.
|
Verenium Corporation
|
||
|
Centocor Ortho Biotech (now a member of Johnson & Johnson)
|
Merck & Co., Inc.
|
Wyeth Pharmaceuticals Division (now a member of Pfizer Inc.)
|
||
|
Crucell Holland B.V. (now a member of Johnson & Johnson)
|
Mitsubishi Tanabe Pharma Corporation
|
ZymoGenetics, Inc. (now a member of Bristol-Myers Squibb Company)
|
|
Program
|
Description
|
Indication
|
Status
|
Developer
|
|
Gevokizumab
|
HE™ antibody to IL-1 beta
|
Non-infectious uveitis, Behçet’s uveitis, moderate to severe inflammatory acne, erosive osteoarthritis of the hand, and cardio-metabolic diseases
|
Planned Phase 3 for non-infectious uveitis in 2012, planned Phase 3 for Behçet’s uveitis, and planned Phase 2 cardiovascular study in 2012, ongoing Phase 2 for moderate to severe inflammatory acne, and planned initiation of erosive osteoarthritis of the hand and one additional proof-of-concept study in 2012
|
XOMA (in collaboration with Servier)
|
|
XMetA,
XMetS
|
Fully human monoclonal antibodies
|
Diabetes, metabolic disorders
|
Preclinical
|
XOMA
|
|
XOMA 3AB
|
Therapeutic antibodies to multiple Type A botulinum neurotoxins
|
Botulism poisoning
|
Phase 1
|
XOMA (NIAID-funded)
|
|
Multiple
preclinical
programs
|
Fully human monoclonal antibodies to multiple disease targets, including TGF-beta and FGFR-4.
|
Autoimmune, cardio-metabolic, infectious, inflammatory, and oncological diseases
|
Preclinical
|
XOMA
|
|
Program
|
Description
|
Indication
|
Status
|
Developer
|
|
FDC1
|
Perindopril arginine and amlodipine besylate
|
Hypertension
|
Phase 3
|
XOMA (partially funded by Servier)
|
|
HCD122 and
LFA102
|
Fully human antibody to CD40 and HE™ antibody to prolactin receptor
|
Hematologic tumors; certain breast and prostate cancers; other undisclosed diseases
|
Phase 1 and 2; Phase 1
|
Novartis (fully funded)
|
|
Therapeutic
antibodies
|
Fully human monoclonal antibodies to undisclosed disease targets
|
Undisclosed
|
Preclinical
|
Takeda (fully funded)
|
|
Therapeutic
antibodies
|
HE™ monoclonal antibody to HGF
|
Non-small cell lung cancer; solid tumors and multiple myeloma
|
Phase 2; Phase 1
|
AVEO (fully funded)
|
|
Product/Candidate
|
Competitors
|
|
Gevokizumab
|
Abbott
Biovitrum AB
Eli Lilly and Company
Lux Biosciences, Inc.
MedImmune
Novartis AG
Regeneron Pharmaceuticals, Inc.
Santen Pharmaceutical Co., Ltd.
|
|
ACEON
FDCs
|
Generic manufacturers
Novartis AG
Takeda Pharmaceutical Company Ltd.
Daiichi Sankyo, Inc.
|
|
XOMA 3AB
|
Cangene Corporation
Emergent BioSolutions, Inc.
|
|
·
|
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports will be available as soon as reasonably practicable after such material is electronically filed with the SEC. All reports we file with the SEC can also be obtained free of charge via EDGAR through the SEC’s website at http://www.sec.gov.
|
|
·
|
Our policies related to corporate governance, including our Code of Ethics applying to our directors, officers and employees (including our principal executive officer and principal financial and accounting officer) that we have adopted to meet the requirements set forth in the rules and regulations of the SEC and its corporate governance principles are available.
|
|
·
|
The charters of the Audit, Compensation and Nominating & Governance Committees of our Board of Directors are available.
|
|
Item 1A.
|
Risk
Fa
ctors
|
|
·
|
terminate or delay clinical trials for one or more of our product candidates;
|
|
·
|
further reduce our headcount and capital or operating expenditures; or
|
|
·
|
curtail our spending on protecting our intellectual property.
|
|
·
|
operations will generate meaningful funds,
|
|
·
|
additional agreements for product development funding can be reached,
|
|
·
|
strategic alliances can be negotiated, or
|
|
·
|
adequate additional financing will be available for us to finance our own development on acceptable terms, or at all.
|
|
|
·
|
our future filings will be delayed,
|
|
|
·
|
our preclinical and clinical studies will be successful,
|
|
|
·
|
we will be successful in generating viable product candidates to targets,
|
|
|
·
|
we will be able to provide necessary additional data,
|
|
|
·
|
results of future clinical trials will justify further development, or
|
|
|
·
|
we will ultimately achieve regulatory approval for any of these product candidates.
|
|
|
·
|
testing,
|
|
|
·
|
manufacturing,
|
|
|
·
|
promotion and marketing, and
|
|
|
·
|
exporting.
|
|
·
|
results of preclinical studies and clinical trials,
|
|
·
|
information relating to the safety or efficacy of products or product candidates,
|
|
·
|
developments regarding regulatory filings,
|
|
·
|
announcements of new collaborations,
|
|
·
|
failure to enter into collaborations,
|
|
·
|
developments in existing collaborations,
|
|
·
|
our funding requirements and the terms of our financing arrangements,
|
|
·
|
technological innovations or new indications for our therapeutic products and product candidates,
|
|
·
|
introduction of new products or technologies by us or our competitors,
|
|
·
|
sales and estimated or forecasted sales of products for which we receive royalties, if any,
|
|
·
|
government regulations,
|
|
·
|
developments in patent or other proprietary rights,
|
|
·
|
the number of shares issued and outstanding,
|
|
·
|
the number of shares trading on an average trading day,
|
|
·
|
announcements regarding other participants in the biotechnology and pharmaceutical industries, and
|
|
·
|
market speculation regarding any of the foregoing.
|
|
·
|
In April 1996, we entered into an agreement with Genentech whereby we agreed to co-develop Genentech’s humanized monoclonal antibody product RAPTIVA®. In April 1999, March 2003, and January 2005, the companies amended the agreement. In October 2003, RAPTIVA® was approved by the FDA for the treatment of adults with chronic moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy and, in September 2004, Merck Serono announced the product’s approval in the European Union. In January 2005, we entered into a restructuring of our collaboration agreement with Genentech which ended our existing cost and profit sharing arrangement related to RAPTIVA® in the United States and entitled us to a royalty interest on worldwide net sales. In February 2009, the EMA announced that it had recommended suspension of the marketing authorization of RAPTIVA® in the European Union and EMD Serono announced that, in consultation with Health Canada, it would suspend marketing of RAPTIVA® in Canada. In March 2009, Merck Serono Australia, following a recommendation from the TGA, announced that it was withdrawing RAPTIVA® from the Australian market. In the second quarter of 2009, Genentech announced and carried out a phased voluntary withdrawal of RAPTIVA® from the U.S. market, based on the association of RAPTIVA® with an increased risk of PML. As a result, sales of RAPTIVA® ceased in the second quarter of 2009.
|
|
·
|
In March 2004, we announced we had agreed to collaborate with Chiron Corporation (now Novartis) for the development and commercialization of antibody products for the treatment of cancer. In April 2005, we announced the initiation of clinical testing of the first product candidate out of the collaboration, HCD122, an anti-CD40 antibody, in patients with advanced chronic lymphocytic leukemia. In October 2005, we announced the initiation of the second clinical trial of HCD122 in patients with multiple myeloma. In November 2008, we announced the restructuring of this product development collaboration, which involved six development programs including the ongoing HCD122 and LFA102 programs. In exchange for cash and debt reduction on our existing loan facility with Novartis, Novartis has control over the HCD122 and LFA102 programs and the additional ongoing program, as well as the right to expand the development of these programs into additional indications outside of oncology.
|
|
·
|
In March 2005, we entered into a contract with the National Institute of Allergy and Infectious Diseases (“NIAID”) to produce three monoclonal antibodies designed to protect United States citizens against the harmful effects of botulinum neurotoxin used in bioterrorism. In July 2006, we entered into an additional contract with NIAID for the development of an appropriate formulation for human administration of these three antibodies in a single injection. In September 2008, we announced that we were awarded an additional contract with NIAID to support our on-going development of drug candidates toward clinical trials in the treatment of botulism poisoning. In October 2011, we announced we had been awarded an additional contract with NIAID to develop broad-spectrum antitoxins for the treatment of human botulism poisoning.
|
|
·
|
In December 2010, we entered into a license and collaboration agreement with Servier, to jointly develop and commercialize gevokizumab in multiple indications. Under the terms of the agreement, Servier has worldwide rights to diabetes and cardiovascular disease indications and rights outside the U.S. and Japan to Behçet’s uveitis and other inflammatory and oncology indications. We retain development and commercialization rights for Behçet’s uveitis and other inflammatory disease and oncology indications in the U.S. and Japan, and have an option to reacquire rights to diabetes and cardiovascular disease indications from Servier in these territories. Should we exercise this option, we will be required to pay Servier an option fee and partially reimburse their incurred development expenses. The agreement contains customary termination rights relating to matters such as material breach by either party, safety issues and patents. Servier also has a unilateral right to terminate the agreement on a country-by-country basis or in its entirety on six months’ notice.
|
|
·
|
In December 2010, we also entered into a loan agreement with Servier, which provides for an advance of up to €15.0 million and was fully funded in January 2011 with the proceeds converting to approximately $19.5 million using the January 13, 2011 Euro to USD exchange rate. This loan is secured by an interest in our intellectual property rights to all gevokizumab indications worldwide, excluding the U.S. and Japan. The loan has a final maturity date in 2016; however, after a specified period prior to final maturity, the loan is required to be repaid (i) at Servier’s option, by applying up to a significant percentage of any milestone or royalty payments owed by Servier under our collaboration agreement and (ii) using a significant percentage of any upfront, milestone or royalty payments we receive from any third party collaboration or development partner for rights to gevokizumab in the U.S. and/or Japan. In addition, the loan becomes immediately due and payable upon certain customary events of default. At December 31, 2011, the €15.0 million outstanding principal balance under this loan agreement would have equaled approximately $19.4 million using the December 31, 2011 Euro to USD exchange rate.
|
|
·
|
In December 2011, we entered into a loan agreement with GECC, under which GECC agreed to make a term loan in an aggregate principal amount of $10 million to XOMA (US) LLC, our wholly owned subsidiary, and upon execution of the loan agreement, GECC funded the term loan. The term loan is guaranteed by us and our two other principal subsidiaries, XOMA Ireland Limited and XOMA Technology Ltd. As security for our obligations under the loan agreement, we, XOMA (US) LLC, XOMA Ireland Limited and XOMA Technology Ltd. each granted a security interest pursuant to a guaranty, pledge and security agreement in substantially all of our existing and after-acquired assets, excluding our intellectual property assets (such as those relating to our gevokizumab and anti-botulism products). We are required to repay the principal amount of the Term Loan over a period of 42 consecutive equal monthly installments of principal and accrued interest. The term loan matures on June 30, 2015, and at maturity, we will make an additional payment equal to 5% of the term loan (“Final Payment Fee”). The loan agreement contains customary representations and warranties and customary affirmative and negative covenants, including restrictions on the ability to incur indebtedness, grant liens, make investments, dispose of assets, enter into transactions with affiliates and amend existing material agreements, in each case subject to various exceptions. In addition, the loan agreement contains customary events of default that entitle GECC to cause any or all of the indebtedness under the loan agreement to become immediately due and payable. The events of default include any event of default under a material agreement or certain other indebtedness. We may voluntarily prepay the term loan in full, but not in part, and any voluntary and certain mandatory prepayments are subject to a prepayment premium of 3% in the first year of the loan, 2% in the second year and 1% thereafter, with certain exceptions. We will also be required to pay the Final Payment Fee in connection with any voluntary or mandatory prepayment. Pursuant to the loan agreement, we issued to GECC unregistered stock purchase warrants, which entitle GECC to purchase up to an aggregate of 263,158 unregistered shares of XOMA common stock at an exercise price equal to $1.14 per share, are immediately exercisable and expire on December 30, 2016.
|
|
·
|
Effective in January 2012, we entered into an amended and restated agreement with Servier for the U.S. commercialization rights to ACEON® and the development and commercialization in the U.S. of up to three products combining perindopril with other cardiovascular drugs in fixed-dose combinations, or FDCs. This agreement, together with a related trademark license agreement, provides us with exclusive U.S. rights to ACEON® and the first FDC product, and options on two additional FDCs. The arrangement also provides that Servier will supply to us, and we will purchase exclusively from Servier, the active ingredients in ACEON® and the FDCs, in some cases for a limited period. The agreement contains customary termination rights relating to matters such as material breach by either party, insolvency of either party or safety issues. Each party also has the right to terminate the arrangement if the first FDC product does not receive FDA approval by December 31, 2014. Servier also has the right to terminate the arrangement if certain aspects of our commercialization strategy are not successful and Servier does not consent to an alternative strategy or, as to the FDCs, if we breach our obligations to certain of our service providers.
|
|
·
|
We have licensed our bacterial cell expression technology, an enabling technology used to discover and screen, as well as develop and manufacture, recombinant antibodies and other proteins for commercial purposes, to over 60 companies. As of March 12, 2012, we were aware of two antibody products manufactured using this technology that have received FDA approval, Genentech’s LUCENTIS® (ranibizumab injection) for treatment of neovascular wet age-related macular degeneration and UCB’s CIMZIA® (certolizumab pegol) for treatment of Crohn’s disease and rheumatoid arthritis. In the third quarter of 2009, we sold our LUCENTIS® royalty interest to Genentech. In the third quarter of 2010, we sold our CIMZIA® royalty interest.
|
|
|
·
|
significantly greater financial resources,
|
|
|
·
|
larger research and development and marketing staffs,
|
|
|
·
|
larger production facilities,
|
|
|
·
|
entered into arrangements with, or acquired, biotechnology companies to enhance their capabilities, or
|
|
|
·
|
extensive experience in preclinical testing and human clinical trials.
|
|
·
|
Novartis markets and is developing Ilaris® (canakinumab, ACZ885), a fully human monoclonal antibody that selectively binds to and neutralizes IL-1 beta. Since 2009, canakinumab has been approved in over 50 countries for the treatment of children and adults suffering from Cryopyrin-Associated Periodic Syndrome (“CAPS”). Novartis has filed for regulatory approval of canakinumab in the U.S. and Europe for the treatment acute attacks in gouty arthritis. In August 2011, Novartis announced that the FDA had issued a Complete Response letter requesting additional information, including clinical data to evaluate the benefit risk profile of canakinumab in refractory gouty arthritis patients. In September 2011, Novartis announced positive results of a pivotal Phase 3 trial of canakinumab in patients with systemic juvenile idiopathic arthritis and that it plans to seek regulatory approval for this indication in 2012. Novartis is also pursuing other diseases in which IL-1 beta may play a prominent role, such as systemic secondary prevention of cardiovascular events and diabetes.
|
|
·
|
Eli Lilly and Company (“Lilly”) is developing a monoclonal antibody to IL-1 beta in Phase 1 development for the treatment of cardiovascular disease. In June 2011, Lilly reported results from a Phase 2 study of LY2189102 in 106 patients with Type 2 diabetes, showing a significant (p<0.05), early reduction in C reactive protein, moderate reduction in HbA1c and anti-inflammatory effects. We do not know whether LY2189102 remains in development.
|
|
·
|
In 2008, Swedish Orphan Biovitrum obtained from Amgen the global exclusive rights to Kineret® (anakinra) for rheumatoid arthritis as currently indicated in its label. In November 2009, the agreement regarding Swedish Orphan Biovitrum’s Kineret® license was expanded to include certain orphan indications. Kineret® is an IL-1 receptor antagonist (IL-1ra) which has been evaluated in multiple IL-1 mediated diseases, including indications we are considering for gevokizumab. In addition to other on-going studies, a proof-of-concept clinical trial in the United Kingdom investigating Kineret® in patients with a certain type of myocardial infarction, or heart attack, has been completed. In August 2010, Biovitrum announced that the FDA had granted orphan drug designation to Kineret® for the treatment of CAPS.
|
|
·
|
In February 2008, Regeneron Pharmaceuticals, Inc. (“Regeneron”) announced it had received marketing approval from the FDA for ARCALYST® (rilonacept) Injection for Subcutaneous Use, an interleukin-1 blocker or IL-1 Trap, for the treatment of CAPS, including Familial Cold Auto-inflammatory Syndrome and Muckle-Wells Syndrome in adults and children 12 and older. In September 2009, Regeneron announced that rilonacept was approved in the European Union for CAPS. In June 2010 and February 2011, Regeneron announced positive results of two Phase 3 clinical trials of rilonacept in gout. In November 2011, Regeneron announced that the FDA had accepted for review Regeneron’s supplemental BLA for ARCALYST® for the prevention and treatment of gout.
|
|
·
|
Amgen has been developing AMG 108, a fully-human monoclonal antibody that targets inhibition of the action of IL-1. In April 2008, Amgen discussed results from a Phase 2 study in rheumatoid arthritis. AMG 108 showed statistically significant improvement in the signs and symptoms of rheumatoid arthritis and was well tolerated. In January 2011, MedImmune, the worldwide biologics unit for AstraZeneca PLC, announced that Amgen granted it rights to develop AMG 108 worldwide except in Japan.
|
|
·
|
In June 2009, Cytos Biotechnology AG announced the initiation of an ascending dose Phase 1/2a study of CYT013-IL1bQb, a therapeutic vaccine targeting IL-1 beta, in Type 2 diabetes. In 2010, this study was extended to include two additional groups of patients.
|
|
·
|
We are aware that the following companies have completed or are conducting or planning Phase 3 clinical trials of the following products for the treatment of uveitis: Abbott - HUMIRA® (adalimumab); Lux Biosciences, Inc. - LUVENIQ (voclosporin); Novartis - Myfortic® (mycophenalate sodium) and Santen Pharmaceutical Co., Ltd. - Sirolimus (rapamycin).
|
|
·
|
The number one product (based on annual sales) within the ACE inhibitor category is lisinopril, formerly marketed by Astra-Zeneca Pharmaceuticals LP under the brands ZESTRIL® or Prinivil®.
|
|
·
|
There are multiple options in the fixed-dose combination market combining ACE inhibitors with diuretics, and some options combining an ACE inhibitor with a calcium channel blocker. Current options with a calcium channel blocker are benazepril/amlodipine, formerly marketed by Novartis Pharmaceuticals as Lotrel®, and trandolapril/verapamil, formerly marketed by Abbot Laboratories as Tarka®.
|
|
·
|
The most successful of the ARBs (in terms of annual sales) is valsartan, trade name Diovan®, which is marketed by Novartis. This compound, along with other ARBs, has been developed in multiple fixed-dose combination products: with a diuretic, a calcium channel blocker (amlodipine) and as a triple combining all three.
|
|
·
|
Cangene Corporation has a contract with the U.S. Department of Health & Human Services, expected to be for $423.0 million, to manufacture and supply an equine heptavalent botulism anti-toxin.
|
|
·
|
Emergent BioSolutions, Inc. is currently in development of a botulism immunoglobulin candidate that may compete with our anti-botulinum neurotoxin monoclonal antibodies.
|
|
|
·
|
imposition of government controls,
|
|
|
·
|
export license requirements,
|
|
|
·
|
political or economic instability,
|
|
|
·
|
trade restrictions,
|
|
|
·
|
changes in tariffs,
|
|
|
·
|
restrictions on repatriating profits,
|
|
|
·
|
exchange rate fluctuations,
|
|
|
·
|
withholding and other taxation, and
|
|
|
·
|
difficulties in staffing and managing international operations.
|
|
|
·
|
prevent our competitors from duplicating our products,
|
|
|
·
|
prevent our competitors from gaining access to our proprietary information and technology, or
|
|
|
·
|
permit us to gain or maintain a competitive advantage.
|
|
|
·
|
whether any pending or future patent applications held by us will result in an issued patent, or that if patents are issued to us, that such patents will provide meaningful protection against competitors or competitive technologies,
|
|
|
·
|
whether competitors will be able to design around our patents or develop and obtain patent protection for technologies, designs or methods that are more effective than those covered by our patents and patent applications, or
|
|
|
·
|
the extent to which our product candidates could infringe on the intellectual property rights of others, which may lead to costly litigation, result in the payment of substantial damages or royalties, and/or prevent us from using technology that is essential to our business.
|
|
|
·
|
require certain procedures to be followed and time periods to be met for any stockholder to propose matters to be considered at annual meetings of stockholders, including nominating directors for election at those meetings; and
|
|
|
·
|
authorize our Board of Directors to issue up to 1,000,000 shares of preferred stock without stockholder approval and to set the rights, preferences and other designations, including voting rights, of those shares as the Board of Directors may determine.
|
|
Item 1B.
|
Unresolv
ed
Staff Comments
|
|
Item 2.
|
Pro
pe
rties
|
|
Item 3.
|
Legal Pr
ocee
dings
|
|
Item 4.
|
Mine Safety
Disclosures
|
|
Name
|
Age
|
Title
|
||
|
John Varian
|
52
|
Chief Executive Officer
|
||
|
Patrick J. Scannon, M.D., Ph.D.
|
64
|
Executive Vice President and Chief Scientific Officer
|
||
|
Fred Kurland
|
61
|
Vice President, Finance and Chief Financial Officer
|
||
|
Christopher J. Margolin, Esq.
|
65
|
Vice President, General Counsel and Secretary
|
||
|
Paul D. Rubin, M.D.
|
58
|
Vice President, Clinical Development and Chief Medical Officer
|
|
Item 5.
|
Market
for Registrant’s
Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
Price Range
|
||||||||
|
High
|
Low
|
|||||||
|
2011
|
||||||||
|
First Quarter
|
$ | 7.71 | $ | 2.77 | ||||
|
Second Quarter
|
3.49 | 2.17 | ||||||
|
Third Quarter
|
2.45 | 1.38 | ||||||
|
Fourth Quarter
|
1.86 | 1.04 | ||||||
|
2010
|
||||||||
|
First Quarter
|
$ | 11.70 | $ | 6.00 | ||||
|
Second Quarter
|
12.60 | 6.15 | ||||||
|
Third Quarter
|
6.45 | 2.45 | ||||||
|
Fourth Quarter
|
7.48 | 2.24 | ||||||
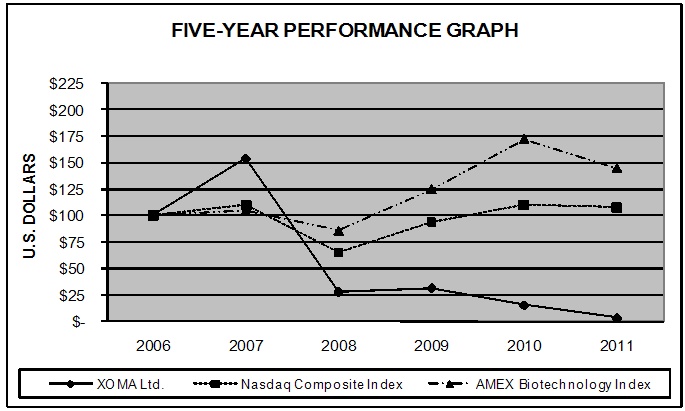
|
As of
December 31,
|
XOMA Ltd.
|
Nasdaq
Composite Index
|
AMEX
Biotechnology
Index
|
|||||||||
|
2006
|
$ | 100.00 | $ | 100.00 | $ | 100.00 | ||||||
|
2007
|
154.09 | 109.81 | 104.28 | |||||||||
|
2008
|
28.18 | 65.29 | 85.80 | |||||||||
|
2009
|
31.82 | 93.95 | 124.91 | |||||||||
|
2010
|
15.55 | 109.84 | 172.04 | |||||||||
|
2011
|
3.48 | 107.86 | 144.70 | |||||||||
|
Item 6.
|
Sel
ecte
d Financial Data
|
|
Year Ended December 31,
|
||||||||||||||||||||
|
2011
|
2010
|
2009
|
2008
|
2007
|
||||||||||||||||
|
(In thousands, except per share amounts)
|
||||||||||||||||||||
|
Consolidated Statement of Operations Data
|
||||||||||||||||||||
|
Total revenues
(1)
|
$ | 58,196 | $ | 33,641 | $ | 98,430 | $ | 67,987 | $ | 84,252 | ||||||||||
|
Total operating costs and expenses
|
92,151 | 100,663 | 81,867 | 106,721 | 86,796 | |||||||||||||||
|
Restructuring costs
|
- | 82 | 3,603 | - | - | |||||||||||||||
|
(Loss) income from operations
|
(33,955 | ) | (67,104 | ) | 12,960 | (38,734 | ) | (2,544 | ) | |||||||||||
|
Other income (expense), net
(2)
|
1,227 | (1,625 | ) | (6,683 | ) | (6,894 | ) | (9,782 | ) | |||||||||||
|
Net (loss) income before taxes
|
(32,728 | ) | (68,729 | ) | 6,277 | (45,628 | ) | (12,326 | ) | |||||||||||
|
Income tax expense (benefit), net
(3)
|
15 | 27 | 5,727 | (383 | ) | - | ||||||||||||||
|
Net (loss) income
|
$ | (32,743 | ) | $ | (68,756 | ) | $ | 550 | $ | (45,245 | ) | $ | (12,326 | ) | ||||||
|
Basic and diluted net (loss) income per share of common stock
|
$ | (1.04 | ) | $ | (3.69 | ) | $ | 0.05 | $ | (5.11 | ) | $ | (1.45 | ) | ||||||
|
December 31,
|
||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
|
(In thousands)
|
||||||||||||||||||||
|
Balance Sheet Data
|
||||||||||||||||||||
|
Cash and cash equivalents
|
$ | 48,344 | $ | 37,304 | $ | 23,909 | $ | 9,513 | $ | 22,500 | ||||||||||
|
Short-term investments
|
- | - | - | 1,299 | 16,067 | |||||||||||||||
|
Restricted cash
|
- | - | - | 9,545 | 6,019 | |||||||||||||||
|
Current assets
|
62,695 | 58,880 | 32,152 | 38,704 | 58,088 | |||||||||||||||
|
Working capital
|
41,685 | 23,352 | 13,474 | 11,712 | 34,488 | |||||||||||||||
|
Total assets
|
78,036 | 74,252 | 52,824 | 67,173 | 84,815 | |||||||||||||||
|
Current liabilities
|
21,010 | 35,528 | 18,678 | 26,992 | 23,600 | |||||||||||||||
|
Long-term liabilities
(4)
|
42,015 | 15,133 | 16,620 | 71,582 | 60,897 | |||||||||||||||
|
Redeemable convertible preferred stock, at par value
|
- | 1 | 1 | 1 | 1 | |||||||||||||||
|
Accumulated deficit
|
(886,053 | ) | (853,310 | ) | (784,554 | ) | (785,104 | ) | (739,859 | ) | ||||||||||
|
Total stockholders' equity (net capital deficiency)
|
15,011 | 23,591 | 17,526 | (31,401 | ) | 318 | ||||||||||||||
|
(1)
|
2010 includes a non-recurring fee of $4.0 million related to the sale of our CIMZIA
®
royalty interest to an undisclosed buyer. 2009 includes a non-recurring fee of $25 million related to the sale of our LUCENTIS
®
royalty interest to Genentech, Inc., a member of the Roche Group (“Genentech”). 2008 includes a non-recurring fee from Novartis AG (“Novartis”) of $13.7 million relating to a restructuring of the existing collaboration agreement.
|
|
(2)
|
2010 includes a loss associated with the $4.5 million paid in the first quarter of 2010 to the holders of warrants issued in June 2009, upon modification of the terms.
|
|
(3)
|
2009 includes foreign income tax expense of $5.8 million recognized in connection with the expansion of our existing collaboration with Takeda.
|
|
(4)
|
The balance in 2011 increased due to the execution of the €15.0 million loan from Servier, which has a principal balance equal to approximately $19.4 million using the December 31, 2011 Euro to USD exchange rate, and the $10.0 million Term Loan from GECC. The balance as of December 31, 2008 includes $50.4 million from our term loan with Goldman Sachs, which we repaid in 2009. In May 2008, the Company entered into a $55 million amended term loan facility with Goldman Sachs, paying off the remaining balance on the term loan completed in November 2006. In addition, the outstanding principal on our Novartis note was reduced by $7.5 million due to the restructure of our collaboration with Novartis. In 2007, we eliminated the remaining $44.5 million in convertible debt issued in 2006.
|
|
Item 7.
|
Management’s
Discussion and
Analysis of Financial Condition and Results of Operations
|
|
|
·
|
In December 2010, we entered into an agreement with Servier to jointly develop and commercialize gevokizumab in multiple indications, which provided for a non-refundable upfront payment of $15.0 million that we received in January 2011. In connection with this agreement, Servier will fully fund the first $50.0 million of future gevokizumab global clinical development and chemistry and manufacturing controls (“CMC”) expenses, and 50% of further expenses for the Behçet’s uveitis indication. Servier has agreed to include the NIU Phase 3 trial discussed below under the terms of the collaboration agreement for Behçet’s uveitis discussed above so long as the European Medicines Agency enables the results of the trial to be included in regulatory submissions in the EU. Based upon the timing of anticipated regulatory interactions, we anticipate initiating the NIU Phase 3 trial in the second quarter of 2012.
|
|
|
·
|
In January 2011, we received the full €15.0 million advance allowed under our loan agreement with Servier dated December 30, 2010, converting to U.S. dollar proceeds of approximately $19.5 million.
|
|
|
·
|
In March 2011, we announced that our Phase 2b trial of gevokizumab in Type 2 diabetes in 421 patients did not achieve the primary endpoint of reduction in hemoglobin A1c (“HbA1c”) after six monthly treatments with gevokizumab compared to placebo. Significant decreases were observed in C-reactive protein (“CRP”), a biomarker for the risk of heart attack, stroke and other cardiovascular diseases, in all dose groups versus placebo. In addition, significant improvements in high-density lipoprotein (“HDL”), or “good” cholesterol, were observed in two of four gevokizumab dose groups versus placebo. Gevokizumab was well-tolerated in this trial, with no significant differences in adverse events between gevokizumab and placebo and no serious drug-related adverse events.
|
|
|
·
|
In June 2011, we announced top line trial results from our six-month Phase 2a trial in 74 patients where gevokizumab was shown to be well-tolerated with no significant differences in adverse events between gevokizumab and placebo and no serious drug-related adverse events. Evidence of biological activity was observed including a reduction in CRP. There were no differences in glycemic control between the drug and placebo groups as measured by HbA1c levels.
|
|
|
·
|
In November 2011, we announced an expansion of our gevokizumab program together with our collaboration partner Servier. The expanded plan includes a global Phase 3 trial in NIU involving the intermediate and/or posterior segments of the eye, including Behçet’s uveitis and a Phase 3 trial outside the U.S. in Behçet’s uveitis.
|
|
|
·
|
In December 2011, we initiated a Phase 2 proof-of-concept study to evaluate the efficacy and safety of gevokizumab for the treatment of inflammatory lesions seen in moderate to severe inflammatory acne vulgaris. Approximately 170 patients will be randomized to receive one or two dose levels of gevokizumab or placebo over a three-month period. Dosing in patients began in December 2011.
|
|
|
·
|
In June 2011, we announced our discovery of two new classes of fully-human monoclonal antibodies, XMetA and XMetS, which activate or sensitize the insulin receptor in vivo, each representing a distinct new therapeutic approach to the treatment of patients with diabetes. Studies of XMetA demonstrated that it reduced fasting blood glucose levels and improved glucose tolerance in a mouse model of diabetes. After six weeks of treatment, there was a statistically significant reduction in HbA1c levels, a standard measure of average blood glucose levels over time, in mice treated with XMetA compared to a control group, and there was a statistically significant reduction in elevated non-HDL cholesterol levels. Studies of XMetS showed enhanced insulin sensitivity and statistically significant improvements in fasting blood glucose levels and glucose tolerance in mice treated with XMetS as compared to a control group, and there was a statistically significant reduction in elevated non-HDL cholesterol levels. These data were presented at the American Diabetes Association’s 71
st
Scientific Sessions.
|
|
|
·
|
In May 2011, the National Institute of Allergy and Infectious Diseases (“NIAID”), part of the National Institutes of Health (“NIH”), informed us that it is initiating a Phase 1 trial of XOMA 3AB, a novel formulation of three antibodies designed to prevent and treat botulism poisoning. This double-blind, dose-escalation study in approximately 24 healthy volunteers is designed to assess the safety and tolerability, and determine the pharmacokinetic profile, of XOMA 3AB.
|
|
|
·
|
In October 2011, we announced that NIAID had awarded us a new contract under Contract No. HHSN272201100031C for up to $28.0 million over 5 years to develop broad-spectrum antitoxins for the treatment of human botulism poisoning.
|
|
|
·
|
On August 31, 2011, we announced that Steven B. Engle resigned as Chief Executive Officer, President and Chairman of the Board of the Company. On January 4, 2012, the Company’s Board of Directors appointed John Varian, a current Board member, as Chief Executive Officer after serving as Interim Chief Executive Officer for five months. W. Denman Van Ness will continue to serve as Chairman of the Board.
|
|
|
·
|
In 2011, we sold 821,386 shares of our common stock through Wm Smith & Co. (“Wm Smith”) and McNicoll, Lewis & Vlak LLC (now known as MLV & Co. LLC, “MLV”) under our At Market Issuance Sales Agreement dated October 26, 2010 (the “2010 ATM Agreement”), for aggregate gross proceeds of $4.4 million, and 5,286,952 shares of our common stock through MLV under our At Market Issuance Sales Agreement dated February 4, 2011 (the “2011 ATM Agreement”), for aggregate gross proceeds of $11.3 million.
|
|
|
·
|
In April 2011, the 2,959 Series B convertible preference shares previously issued to Genentech, Inc. were converted by Genentech into 254,560 shares of our common stock, and the associated liquidation preference of $29.6 million was eliminated.
|
|
|
·
|
In May 2011, we entered into two foreign exchange options contracts in order to manage our foreign currency exposure relating to principal and interest payments on our €15.0 million loan from Servier. Upfront premiums paid on these contracts totaled $1.5 million.
|
|
|
·
|
In December 2011, we entered into a loan agreement (the “Loan Agreement”) with General Electric Capital Corporation (“GECC”) under which GECC agreed to make, and made, a term loan of $10 million. This loan accrues interest at a fixed rate of 11.71% per annum and is secured by substantially all of our existing and after-acquired assets, excluding our intellectual property assets. We are required to repay the principal amount over a period of 42 consecutive equal monthly installments of principal and accrued interest. The loan matures on June 30, 2015, at which time we will make an additional payment equal to 5% of the loan. We also issued to GECC unregistered stock purchase warrants, which entitle GECC to purchase up to an aggregate of 263,158 unregistered shares of XOMA common stock at an exercise price equal to $1.14 per share. These warrants are immediately exercisable and expire on December 30, 2016.
|
|
·
|
Effective December 31, 2011, we changed our jurisdiction of incorporation from Bermuda to Delaware and changed our name from XOMA Ltd. to XOMA Corporation. All outstanding common shares of the former XOMA Ltd. automatically converted into XOMA Corporation common stock on a one-for-one basis.
|
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
License and collaborative fees
|
$ | 17,991 | $ | 2,182 | $ | 43,822 | ||||||
|
Contract and other revenue
|
40,037 | 27,174 | 25,492 | |||||||||
|
Royalties
|
168 | 4,285 | 29,116 | |||||||||
|
Total revenues
|
$ | 58,196 | $ | 33,641 | $ | 98,430 | ||||||
|
Year ended December 31,
|
2010-2011
Increase
|
2009-2010
Increase
|
||||||||||||||||||
|
2011
|
2010
|
2009
|
(Decrease)
|
(Decrease)
|
||||||||||||||||
|
Servier
|
$ | 19,348 | $ | - | $ | - | $ | 19,348 | $ | - | ||||||||||
|
NIAID
|
18,781 | 21,414 | 6,632 | (2,633 | ) | 14,782 | ||||||||||||||
|
Takeda
|
1,217 | 3,568 | 7,549 | (2,351 | ) | (3,981 | ) | |||||||||||||
|
SRI International
|
546 | 1,594 | 331 | (1,048 | ) | 1,263 | ||||||||||||||
|
Merck/Schering-Plough
|
- | 468 | 7,586 | (468 | ) | (7,118 | ) | |||||||||||||
|
Novartis
|
- | - | 2,459 | - | (2,459 | ) | ||||||||||||||
|
Other
|
145 | 130 | 935 | 15 | (805 | ) | ||||||||||||||
|
Total revenues
|
$ | 40,037 | $ | 27,174 | $ | 25,492 | $ | 12,863 | $ | 1,682 | ||||||||||
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Beginning deferred revenue
|
$ | 18,130 | $ | 5,008 | $ | 17,213 | ||||||
|
Revenue deferred
|
12,673 | 15,949 | 16,220 | |||||||||
|
Revenue recognized
|
(17,569 | ) | (2,827 | ) | (28,425 | ) | ||||||
|
Ending deferred revenue
|
$ | 13,234 | $ | 18,130 | $ | 5,008 | ||||||
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Earlier stage programs
|
$ | 38,302 | $ | 44,251 | $ | 36,221 | ||||||
|
Later stage programs
|
29,835 | 33,162 | 21,910 | |||||||||
|
Total
|
$ | 68,137 | $ | 77,413 | $ | 58,131 | ||||||
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Internal projects
|
$ | 24,440 | $ | 52,031 | $ | 35,130 | ||||||
|
Collaborative and contract arrangements
|
43,697 | 25,382 | 23,001 | |||||||||
|
Total
|
$ | 68,137 | $ | 77,413 | $ | 58,131 | ||||||
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Interest expense
|
||||||||||||
|
Novartis note
|
$ | 341 | $ | 354 | $ | 455 | ||||||
|
Servier loan
|
2,087 | - | - | |||||||||
|
Goldman Sachs term loan
|
- | - | 3,932 | |||||||||
|
Other
|
34 | 31 | 14 | |||||||||
|
Total interest expense
|
$ | 2,462 | $ | 385 | $ | 4,401 | ||||||
|
Amortization of debt issuance costs
|
||||||||||||
|
Goldman Sachs term loan
|
$ | - | $ | - | $ | 487 | ||||||
|
Total interest expense
|
$ | 2,462 | $ | 385 | $ | 4,888 | ||||||
|
Year ended December 31,
|
2010-2011 | 2009-2010 | ||||||||||||||||||
|
2011
|
2010
|
2009
|
Increase
(Decrease)
|
Increase
(Decrease)
|
||||||||||||||||
|
Other income
|
||||||||||||||||||||
|
Gain on revaluation of warrant liabilities
|
$ | 3,866 | $ | 2,282 | $ | 1,782 | $ | 1,584 | $ | 500 | ||||||||||
|
Unrealized foreign exchange gain (loss)
(1)
|
(457 | ) | 6 | - | (463 | ) | 6 | |||||||||||||
|
Realized foreign exchange gain
(2)
|
554 | (7 | ) | (1 | ) | 561 | (6 | ) | ||||||||||||
|
Unrealized loss on foreign exchange options
|
(298 | ) | - | - | (298 | ) | - | |||||||||||||
|
Warrant modification expense
(3)
|
- | (4,500 | ) | - | 4,500 | (4,500 | ) | |||||||||||||
|
Loss on debt extinguishment
(4)
|
- | - | (3,645 | ) | - | 3,645 | ||||||||||||||
|
Other
|
24 | 979 | 69 | (955 | ) | 910 | ||||||||||||||
|
Total other income
|
$ | 3,689 | $ | (1,240 | ) | $ | (1,795 | ) | $ | 4,929 | $ | 555 | ||||||||
|
(1)
|
Unrealized foreign exchange gain (loss) for the year ended December 31, 2011 primarily relates to gains (losses) on the re-measurement of the €15 million Servier loan.
|
|
(2)
|
Realized foreign exchange gain for the year ended December 31, 2011 primarily relates to the conversion into U.S. dollars of the €15 million cash proceeds received from Servier in January of 2011.
|
|
(3)
|
Represents the 2010 loss associated with $4.5 million paid to the holders of warrants issued in June of 2009, upon modification of the terms.
|
|
(4)
|
Represents the loss associated with the 2009 repayment of our Goldman Sachs term loan.
|
|
Warrant
Liabilities
|
||||
|
Balance at December 31, 2009
|
$ | 4,760 | ||
|
Initial fair value of warrants
|
4,382 | |||
|
Reclassification of warrant liability to equity upon exercise of warrants
|
(2,615 | ) | ||
|
Change in fair value of warrant liabilities included in other income (expense)
|
(2,282 | ) | ||
|
Balance at December 31, 2010
|
4,245 | |||
|
Change in fair value of warrant liabilities included in other income (expense)
|
(3,866 | ) | ||
|
Balance at December 31, 2011
|
$ | 379 | ||
|
December 31,
|
2010-2011 | |||||||||||||||||||
|
2011
|
2010
|
Increase
|
||||||||||||||||||
|
Cash and cash equivalents
|
$ | 48,344 | $ | 37,304 | $ | 11,040 | ||||||||||||||
|
Working Capital
|
$ | 41,685 | $ | 23,352 | $ | 18,333 | ||||||||||||||
|
Year ended December 31,
|
2010-2011
Increase
|
2009-2010
Increase
|
||||||||||||||||||
| 2011 | 2010 | 2009 |
(Decrease)
|
(Decrease)
|
||||||||||||||||
|
Net cash (used in) provided by operating activities
|
$ | (29,062 | ) | $ | (52,537 | ) | $ | 7,435 | $ | 23,475 | $ | (59,972 | ) | |||||||
|
Net cash (used in) provided by investing activities
|
$ | (3,304 | ) | $ | (339 | ) | $ | 10,575 | $ | (2,965 | ) | $ | (10,914 | ) | ||||||
|
Net cash provided by (used in) financing activities
|
$ | 43,979 | $ | 66,271 | $ | (3,614 | ) | $ | (22,292 | ) | $ | 69,885 | ||||||||
|
Effect of exchange rate changes on cash
|
$ | (573 | ) | $ | - | $ | - | $ | (573 | ) | $ | - | ||||||||
|
Net increase in cash and cash equivalents
|
$ | 11,040 | $ | 13,395 | $ | 14,396 | ||||||||||||||
|
Contractual Obligations
|
Total
|
Less than 1 year
|
1 to 3 years
|
3 to 5 years
|
More than 5 years
|
|||||||||||||||
|
Operating leases
(a)
|
$ | 12,320 | $ | 8,084 | $ | 4,236 | $ | - | $ | - | ||||||||||
|
Debt Obligations
(b)
|
||||||||||||||||||||
|
Principal
|
43,457 | 2,857 | 5,714 | 34,886 | - | |||||||||||||||
|
Interest
|
6,953 | 2,155 | 3,286 | 1,512 | - | |||||||||||||||
|
Total
|
$ | 62,730 | $ | 13,096 | $ | 13,236 | $ | 36,398 | $ | - | ||||||||||
|
(a)
|
Operating leases are net of sublease income of $0.3 million.
|
|
(b)
|
See
|
|
Item 7A.
|
Quantita
tive
and Qualitative Disclosures about Market Risk
|
|
Maturity
|
Carrying
Amount
(in thousands)
|
Fair Value
(in thousands)
|
Weighted
Average
Interest Rate
|
||||||||||
|
December 31, 2011
|
|||||||||||||
|
Cash and cash equivalents
|
Daily to 90 days
|
$ | 48,344 | $ | 48,344 | 0.25 | % | ||||||
|
December 31, 2010
|
|||||||||||||
|
Cash and cash equivalents
|
Daily to 90 days
|
$ | 37,304 | $ | 37,304 | 0.09 | % | ||||||
|
Item 8.
|
Financial
Statements and
Supplementary Data
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
|
Consolidated Balance Sheets
|
F-3
|
|
|
Consolidated Statements of Operations
|
F-4
|
|
|
Consolidated Statements of Stockholders' Equity (Net Capital Deficiency)
|
F-5
|
|
|
Consolidated Statements of Cash Flows
|
F-6
|
|
|
Notes to the Consolidated Financial Statements
|
F-7
|
|
Item 9.
|
Changes in and Disa
gr
eements with Accountants on Accounting and Financial Disclosure
|
|
Item 9A.
|
Controls and
Procedures
|
|
Item 9B.
|
Other
Informa
tion
|
|
Item 10.
|
Directors
, Executive
Officers, Corporate Governance
|
|
Item 11.
|
Executive Comp
ensa
tion
|
|
Item 12.
|
Security
Ownership of
Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
Item 13.
|
Certain
Relation
ships and Related Transactions, and Director Independence
|
|
Item 14.
|
Principal Accountant
Fees and Services
|
|
Item 15.
|
Exhibits and
Fin
ancial Statement Schedules
|
|
(a)
|
The following documents are included as part of this Annual Report on Form 10-K:
|
|
(1)
|
Financial Statements:
|
|
All
financial statements of the registrant referred to in Item 8 of this Report on Form 10-K.
|
|
(2)
|
Financial Statement Schedules:
|
|
|
All financial statements schedules have been omitted because the required information is included in the consolidated financial statements or the notes thereto or is not applicable or required.
|
|
(3)
|
Exhibits:
|
|
|
See “Index to Exhibits” on page i of this report.
|
|
XOMA CORPORATION
|
||
|
By:
|
/s/ JOHN VARIAN
|
|
|
John Varian
|
||
|
Chief Executive Officer
|
||
| Signature | Title | Date | ||
| /s/ John Varian | Chief Executive Officer (Principal Executive | March 14, 2012 | ||
| (John Varian) | Officer) and Director | |||
| /s/ Fred Kurland | Vice President, Finance and Chief Financial Officer | March 14, 2012 | ||
| (Fred Kurland) | (Principal Financial and Principal Accounting Officer) | |||
| /s/ Patrick J. Scannon | Executive Vice President and Chief Scientific | March 14, 2012 | ||
| (Patrick J. Scannon) | Officer and Director | |||
| /s/ W. Denman Van Ness | Chairman of the Board | March 14, 2012 | ||
| (W. Denman Van Ness) |
|
/s/ William K. Bowes, Jr.
|
|
Director
|
March 14, 2012
|
|
|
(William K. Bowes, Jr.)
|
||||
|
/s/ Peter Barton Hutt
|
|
Director
|
March 14, 2012
|
|
|
(Peter Barton Hutt)
|
||||
|
/s/ Timothy P. Walbert
|
|
Director
|
March 14, 2012
|
|
|
(Timothy P. Walbert)
|
||||
|
/s/ Jack L. Wyszomierski
|
|
Director
|
March 14, 2012
|
|
|
(Jack L. Wyszomierski)
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
|
Consolidated Balance Sheets
|
F-3
|
|
|
Consolidated Statements of Operations
|
F-4
|
|
|
Consolidated Statements of Stockholders' Equity (Net Capital Deficiency)
|
F-5
|
|
|
Consolidated Statements of Cash Flows
|
F-6
|
|
|
Notes to the Consolidated Financial Statements
|
F-7
|
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
| $ | 48,344 | $ | 37,304 | |||||
|
Trade and other receivables, net
|
12,332 | 20,864 | ||||||
|
Prepaid expenses and other current assets
|
2,019 | 712 | ||||||
|
Total current assets
|
62,695 | 58,880 | ||||||
|
Property and equipment, net
|
12,709 | 14,869 | ||||||
|
Other assets
|
2,632 | 503 | ||||||
|
Total assets
|
$ | 78,036 | $ | 74,252 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 2,128 | $ | 3,581 | ||||
|
Accrued and other liabilities
|
10,012 | 10,658 | ||||||
|
Deferred revenue
|
5,695 | 17,044 | ||||||
|
Interest bearing obligation – current
|
2,796 | - | ||||||
|
Warrant liability
|
379 | 4,245 | ||||||
|
Total current liabilities
|
21,010 | 35,528 | ||||||
|
Deferred revenue – long-term
|
7,539 | 1,086 | ||||||
|
Interest bearing obligations – long-term
|
33,524 | 13,694 | ||||||
|
Other liabilities - long-term
|
952 | 353 | ||||||
|
Total liabilities
|
63,025 | 50,661 | ||||||
|
Commitments and contingencies (Note 11)
|
||||||||
|
Stockholders’ equity:
|
||||||||
|
Preferred stock, $0.05 par value, 1,000,000 shares authorized
|
||||||||
|
Series B, 8,000 designated, 0 and 2,959 shares issued and outstanding at December 31, 2011 and 2010, respectively
|
- | 1 | ||||||
|
Common stock, $0.0075 par value, 92,666,666 shares authorized, 35,107,007 and 28,491,318 shares outstanding at December 31, 2011 and 2010, respectively
|
263 | 214 | ||||||
|
Additional paid-in capital
|
900,801 | 876,686 | ||||||
|
Accumulated deficit
|
(886,053 | ) | (853,310 | ) | ||||
|
Total stockholders’ equity
|
15,011 | 23,591 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 78,036 | $ | 74,252 | ||||
|
Year Ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Revenues:
|
||||||||||||
|
License and collaborative fees
|
$ | 17,991 | $ | 2,182 | $ | 43,822 | ||||||
|
Contract and other revenue
|
40,037 | 27,174 | 25,492 | |||||||||
|
Royalties
|
168 | 4,285 | 29,116 | |||||||||
|
Total revenues
|
58,196 | 33,641 | 98,430 | |||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
68,137 | 77,413 | 58,131 | |||||||||
|
Selling, general and administrative
|
24,014 | 23,250 | 23,736 | |||||||||
|
Restructuring
|
- | 82 | 3,603 | |||||||||
|
Total operating expenses
|
92,151 | 100,745 | 85,470 | |||||||||
|
(Loss) income from operations
|
(33,955 | ) | (67,104 | ) | 12,960 | |||||||
|
Other income (expense):
|
||||||||||||
|
Interest (expense)
|
(2,462 | ) | (385 | ) | (4,888 | ) | ||||||
|
Loss on debt extinguishment
|
- | - | (3,645 | ) | ||||||||
|
Other income (expense)
|
3,689 | (1,240 | ) | 1,850 | ||||||||
|
Net (loss) income before taxes
|
(32,728 | ) | (68,729 | ) | 6,277 | |||||||
|
Income tax expense
|
(15 | ) | (27 | ) | (5,727 | ) | ||||||
|
Net (loss) income
|
$ | (32,743 | ) | $ | (68,756 | ) | $ | 550 | ||||
|
Basic and diluted net (loss) income per share of common stock
|
$ | (1.04 | ) | $ | (3.69 | ) | $ | 0.05 | ||||
|
Shares used in computing basic net (loss) income per share of common stock
|
31,590 | 18,613 | 10,993 | |||||||||
|
Shares used in computing diluted net (loss) income per share of common stock
|
31,590 | 18,613 | 11,313 | |||||||||
| Total | ||||||||||||||||||||||||||||||||
| Stockholders’ | ||||||||||||||||||||||||||||||||
| Accumulated | Equity | |||||||||||||||||||||||||||||||
|
Preferred Stock
|
Common Stock
|
Paid-In
|
Comprehensive
|
Accumulated
|
(Net Capital
|
|||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Income (Loss)
|
Deficit
|
Deficiency) | |||||||||||||||||||||||||
|
Balance, December 31, 2008
|
3 | $ | 1 | $ | 9,364 | $ | 70 | $ | 753,634 | $ | (2 | ) | $ | (785,104 | ) | $ | (31,401 | ) | ||||||||||||||
|
Exercise of stock options, contributions to 401(k) and incentive plans
|
─
|
─
|
135 | 1 | 1,358 |
─
|
─
|
1,359 | ||||||||||||||||||||||||
|
Stock-based compensation expense
|
─
|
─
|
─
|
─
|
4,395 |
─
|
─
|
4,395 | ||||||||||||||||||||||||
|
Sale of shares of common stock
|
─
|
─
|
4,036 | 30 | 42,591 |
─
|
─
|
42,621 | ||||||||||||||||||||||||
|
Comprehensive income (loss):
|
||||||||||||||||||||||||||||||||
|
Net change in unrealized loss on investments
|
─
|
─
|
─
|
─
|
─
|
2 |
─
|
2 | ||||||||||||||||||||||||
|
Net income
|
─
|
─
|
─
|
─
|
─
|
─
|
550 | 550 | ||||||||||||||||||||||||
|
Comprehensive loss
|
─
|
─
|
─
|
─
|
─
|
─
|
─
|
552 | ||||||||||||||||||||||||
|
Balance, December 31, 2009
|
3 | 1 | 13,536 | 101 | 801,978 | - | (784,554 | ) | 17,526 | |||||||||||||||||||||||
|
Exercise of stock options, contributions to 401(k) and incentive plans
|
─
|
─
|
94 | 1 | 945 |
─
|
─
|
946 | ||||||||||||||||||||||||
|
Stock-based compensation expense
|
─
|
─
|
─
|
─
|
4,913 |
─
|
─
|
4,913 | ||||||||||||||||||||||||
|
Sale of shares of common stock
|
─
|
─
|
14,469 | 109 | 66,232 |
─
|
─
|
66,341 | ||||||||||||||||||||||||
|
Exercise of warrants
|
─
|
─
|
392 | 3 | 2,618 |
─
|
─
|
2,621 | ||||||||||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||||||||||
|
Net loss
|
─
|
─
|
─
|
─
|
─
|
─
|
(68,756 | ) | (68,756 | ) | ||||||||||||||||||||||
|
Comprehensive income
|
─
|
─
|
─
|
─
|
─
|
─
|
─
|
(68,756 | ) | |||||||||||||||||||||||
|
Balance, December 31, 2010
|
3 | 1 | 28,491 | 214 | 876,686 | - | (853,310 | ) | 23,591 | |||||||||||||||||||||||
|
Exercise of stock options, contributions to 401(k) and incentive plans
|
─
|
─
|
253 | 2 | 1,099 |
─
|
─
|
1,101 | ||||||||||||||||||||||||
|
Stock-based compensation expense
|
─
|
─
|
─
|
─
|
7,759 |
─
|
─
|
7,759 | ||||||||||||||||||||||||
|
Sale of shares of common stock
|
─
|
─
|
6,108 | 45 | 15,043 |
─
|
─
|
15,088 | ||||||||||||||||||||||||
|
Conversion of Series B convertible preferred stock
|
(3 | ) | (1 | ) | 255 | 2 | (1 | ) |
─
|
─
|
- | |||||||||||||||||||||
|
Issuance of warrants
|
─
|
─
|
─
|
─
|
215 |
─
|
─
|
215 | ||||||||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||
|
Net loss
|
─
|
─
|
─
|
─
|
─
|
─
|
(32,743 | ) | (32,743 | ) | ||||||||||||||||||||||
|
Comprehensive loss
|
─
|
─
|
─
|
─
|
─
|
─
|
─
|
(32,743 | ) | |||||||||||||||||||||||
|
Balance, December 31, 2011
|
- | $ | - | $ | 35,107 | $ | 263 | $ | 900,801 | $ | - | $ | (886,053 | ) | $ | 15,011 | ||||||||||||||||
|
Year Ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
| $ | (32,743 | ) | $ | (68,756 | ) | $ | 550 | |||||
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities:
|
||||||||||||
|
Depreciation and amortization
|
5,357 | 5,721 | 6,831 | |||||||||
|
Common stock contribution to 401(k)
|
1,046 | 905 | 1,198 | |||||||||
|
Stock-based compensation expense
|
7,759 | 4,913 | 4,395 | |||||||||
|
Accrued interest on interest bearing obligations
|
1,023 | 353 | (1,116 | ) | ||||||||
|
Revaluation of warrant liability
|
(3,866 | ) | (2,283 | ) | (1,781 | ) | ||||||
|
Amortization of discount on debt and debt issuance costs
|
1,360 | - | 487 | |||||||||
|
Unrealized loss on foreign currency exchange
|
513 | - | - | |||||||||
|
Unrealized loss on foreign exchange options
|
298 | - | - | |||||||||
|
Warrant modification expense
|
- | 4,500 | - | |||||||||
|
Loss on debt extinguishment
|
- | - | 3,645 | |||||||||
|
Other non-cash adjustments
|
107 | 19 | 12 | |||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Trade and other receivables, net
|
8,532 | (13,633 | ) | 9,455 | ||||||||
|
Prepaid expenses and other assets
|
(2,469 | ) | 199 | 284 | ||||||||
|
Accounts payable and accrued liabilities
|
(2,144 | ) | 2,650 | (2,844 | ) | |||||||
|
Deferred revenue
|
(13,794 | ) | 13,122 | (12,205 | ) | |||||||
|
Other liabilities
|
(41 | ) | (247 | ) | (1,476 | ) | ||||||
|
Net cash (used in) provided by operating activities
|
(29,062 | ) | (52,537 | ) | 7,435 | |||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Proceeds from maturities of investments
|
- | - | 1,300 | |||||||||
|
Transfer of restricted cash
|
- | - | 9,545 | |||||||||
|
Purchase of property and equipment
|
(3,304 | ) | (339 | ) | (270 | ) | ||||||
|
Net cash (used in) provided by investing activities
|
(3,304 | ) | (339 | ) | 10,575 | |||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Proceeds from issuance of long-term debt, net of issuance costs
|
28,836 | - | - | |||||||||
|
Principal payments of debt
|
- | - | (50,394 | ) | ||||||||
|
Payment of prepayment premium on repayment of
short-term debt
|
- | - | (2,543 | ) | ||||||||
|
Proceeds from issuance of common stock
|
15,143 | 70,771 | 49,323 | |||||||||
|
Payment for modification of warrants
|
- | (4,500 | ) | - | ||||||||
|
Net cash provided by (used in) financing activities
|
43,979 | 66,271 | (3,614 | ) | ||||||||
|
Effect of exchange rate changes on cash
|
(573 | ) | - | - | ||||||||
|
Net increase in cash and cash equivalents
|
11,040 | 13,395 | 14,396 | |||||||||
|
Cash and cash equivalents at the beginning of the period
|
37,304 | 23,909 | 9,513 | |||||||||
|
Cash and cash equivalents at the end of the period
|
$ | 48,344 | $ | 37,304 | $ | 23,909 | ||||||
|
Supplemental Cash Flow Information:
|
||||||||||||
|
Cash paid during the year for:
|
||||||||||||
|
Interest
|
$ | 7 | $ | - | $ | 5,510 | ||||||
|
Income taxes
|
15 | 16 | 5,800 | |||||||||
|
Non-cash investing and financing activities:
|
||||||||||||
|
Discount on long-term debt
|
$ | (9,114 | ) | $ | - | $ | - | |||||
|
Issuance and Extinguishment of warrants
|
$ | 215 | $ | 1,767 | $ | 6,541 | ||||||
|
Interest added to principal balances on long-term debt
|
$ | 669 | $ | 353 | $ | 462 | ||||||
|
1.
|
Description of Business
|
|
2.
|
Basis of Presentation and Significant Accounting Policies
|
|
December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Options for common stock
|
3,890 | 2,180 | 1,156 | |||||||||
|
Convertible preferred stock
|
67 | 254 | - | |||||||||
|
Warrants for common stock
(1)
|
1,609 | 1,535 | 740 | |||||||||
|
Total
|
5,566 | 3,969 | 1,896 | |||||||||
|
(1)
|
263 warrants issued in December of 2011
|
|
Year ended
December 31,
|
||||
|
2009
|
||||
|
Numerator
|
||||
|
Net income used for basic and diluted net income per share
|
$ | 550 | ||
|
Denominator
|
||||
|
Weighted average shares outstanding used for basic net income per share
|
10,993 | |||
|
Effect of dilutive stock options
|
66 | |||
|
Effect of convertible preferred stock
|
254 | |||
|
Weighted average shares outstanding and dilutive securities used for diluted net income per share
|
11,313 | |||
|
3.
|
Consolidated Financial Statement Detail
|
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Trade receivables, net
|
$ | 11,820 | $ | 20,309 | ||||
|
Other receivables
|
512 | 555 | ||||||
|
Total
|
$ | 12,332 | $ | 20,864 | ||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Furniture and equipment
|
$ | 33,483 | $ | 31,700 | ||||
|
Buildings, leasehold and building improvements
|
21,490 | 21,463 | ||||||
|
Construction-in-progress
|
973 | 203 | ||||||
|
Land
|
310 | 310 | ||||||
| 56,256 | 53,676 | |||||||
|
Less: Accumulated depreciation and amortization
|
(43,547 | ) | (38,807 | ) | ||||
|
Property and equipment, net
|
$ | 12,709 | $ | 14,869 | ||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Accrued management incentive compensation
|
$ | 4,096 | $ | 4,982 | ||||
|
Accrued payroll and other benefits
|
3,007 | 2,752 | ||||||
|
Accrued severance payments
|
1,207 | - | ||||||
|
Accrued professional fees
|
917 | 1,020 | ||||||
|
Accrued clinical trial costs
|
140 | 1,020 | ||||||
|
Other
|
645 | 884 | ||||||
|
Total
|
$ | 10,012 | $ | 10,658 | ||||
|
Year ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Beginning deferred revenue
|
$ | 18,130 | $ | 5,008 | ||||
|
Revenue deferred
|
12,673 | 15,949 | ||||||
|
Revenue recognized
|
(17,569 | ) | (2,827 | ) | ||||
|
Ending deferred revenue
|
$ | 13,234 | $ | 18,130 | ||||
|
4.
|
Collaborative, Licensing and Other Arrangements
|
|
5.
|
Restructuring Charges
|
|
6.
|
Fair Value Measurements
|
|
Fair Value Measurements at December 31, 2011 Using
|
||||||||||||||||
|
Quoted Prices in
Active Markets
for Identical Assets
|
Significant
Other
Observable
Inputs
|
Significant
Unobservable
Inputs
|
||||||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Money market funds
(1)
|
27,222 | - | - | 27,222 | ||||||||||||
|
Foreign exchange options
|
- | 1,202 | - | 1,202 | ||||||||||||
|
Total
|
$ | 27,222 | $ | 1,202 | $ | - | $ | 28,424 | ||||||||
|
Liabilities:
|
||||||||||||||||
|
Warrant liabilities
|
$ | - | $ | - | $ | 379 | $ | 379 | ||||||||
|
Fair Value Measurements at December 31, 2010 Using
|
||||||||||||||||
|
Quoted Prices in
Active Markets
for Identical
Assets
|
Significant
Other
Observable
Inputs
|
Significant
Unobservable
Inputs
|
||||||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Repurchase agreements
(1)
|
$ | 1,428 | $ | - | $ | - | $ | 1,428 | ||||||||
|
Money market funds
(1)
|
6,340 | - | - | 6,340 | ||||||||||||
|
Total
|
$ | 7,768 | $ | - | $ | - | $ | 7,768 | ||||||||
|
Liabilities:
|
||||||||||||||||
|
Warrant liabilities
|
$ | - | $ | - | $ | 4,245 | $ | 4,245 | ||||||||
|
(1)
|
Included in cash and cash equivalents
|
|
December 31,
2011
|
December 31,
2010
|
|||||||
|
Expected volatility
|
102.1 - 103.2 | % | 93.5 - 94.9 | % | ||||
|
Risk-free interest rate
|
0.4 | % | 2.0 | % | ||||
|
Expected term
|
2.9 - 3.1 years
|
3.9 - 4.1 years
|
||||||
|
Warrant
Liabilities
|
||||
|
Balance at December 31, 2009
|
$ | 4,760 | ||
|
Initial fair value of warrants
|
4,382 | |||
|
Reclassification of warrant liability to equity upon exercise of warrants
|
(2,615 | ) | ||
|
Change in fair value of warrant liabilities included in other income (expense)
|
(2,282 | ) | ||
|
Balance at December 31, 2010
|
4,245 | |||
|
Change in fair value of warrant liabilities included in other income (expense)
|
(3,866 | ) | ||
|
Balance at December 31, 2011
|
$ | 379 | ||
|
7.
|
Long-Term Debt and Other Arrangements
|
|
Year Ending December 31,
|
Total
|
|||
|
2012
|
$ | 2,857 | ||
|
2013
|
2,857 | |||
|
2014
|
2,857 | |||
|
2015
|
35,386 | |||
| 43,957 | ||||
|
Less current portion
|
(2,857 | ) | ||
|
Total
|
$ | 41,100 | ||
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Interest expense
|
||||||||||||
|
Novartis note
|
$ | 341 | $ | 354 | $ | 455 | ||||||
|
Servier loan
|
2,087 | - | - | |||||||||
|
Goldman Sachs term loan
|
- | - | 3,932 | |||||||||
|
Other
|
34 | 31 | 14 | |||||||||
|
Total interest expense
|
$ | 2,462 | $ | 385 | $ | 4,401 | ||||||
|
Amortization of debt issuance costs
|
||||||||||||
|
Goldman Sachs term loan
|
$ | - | $ | - | $ | 487 | ||||||
|
Total interest expense
|
$ | 2,462 | $ | 385 | $ | 4,888 | ||||||
|
8.
|
Income Taxes
|
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Federal income tax provision
|
$ | 15 | $ | 27 | $ | (113 | ) | |||||
|
State income tax provision
|
- | - | 6 | |||||||||
|
Foreign income tax provision
|
- | - | 5,834 | |||||||||
|
Total
|
$ | 15 | $ | 27 | $ | 5,727 | ||||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Capitalized research and development expenses
|
$ | 68.7 | $ | 65.4 | ||||
|
Net operating loss carryforwards
|
135.7 | 117.4 | ||||||
|
Research and development and other credit carryforwards
|
21.6 | 20.4 | ||||||
|
Other
|
14.1 | 11.1 | ||||||
|
Total deferred tax assets
|
240.1 | 214.3 | ||||||
|
Valuation allowance
|
(240.1 | ) | (214.3 | ) | ||||
|
Net deferred tax assets
|
$ | - | $ | - | ||||
|
9.
|
Compensation and Other Benefit Plans
|
|
2011
|
2010
|
2009
|
||||||||||||||||||||||
|
Options:
|
Shares
|
Price*
|
Shares
|
Price*
|
Shares
|
Price*
|
||||||||||||||||||
|
Outstanding at beginning of year
|
2,331,450 | $ | 25.36 | 1,520,102 | $ | 38.40 | 1,320,679 | $ | 48.60 | |||||||||||||||
|
Granted
|
2,920,166 | 2.81 | 978,264 | 7.07 | 432,400 | 9.00 | ||||||||||||||||||
|
Exercised
|
- | - | (19 | ) | 8.40 | (2,056 | ) | 8.40 | ||||||||||||||||
|
Forfeited, expired or cancelled
|
(198,181 | ) | 35.56 | (166,897 | ) | 37.26 | (230,921 | ) | 41.40 | |||||||||||||||
|
Outstanding at end of year
|
5,053,435 | 12.55 | 2,331,450 | 25.36 | 1,520,102 | 38.40 | ||||||||||||||||||
|
Exercisable at end of year
|
3,366,807 | $ | 16.33 | 1,259,272 | $ | 36.51 | 823,096 | $ | 49.05 | |||||||||||||||
|
*
|
Weighted-average exercise price
|
|
Weighted-
|
||||||||
| Number of |
Average Grant-
|
|||||||
|
Shares
|
Date Fair Value
|
|||||||
|
Unvested balance at December 31, 2010
|
- | $ | - | |||||
|
Granted
|
1,177,082 | 1.69 | ||||||
|
Vested
|
(272,439 | ) | 1.69 | |||||
|
Forfeited
|
(769 | ) | 1.69 | |||||
|
Unvested balance at December 31, 2011
|
903,874 | $ | 1.69 | |||||
|
Year Ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Dividend yield
|
0 | % | 0 | % | 0 | % | ||||||
|
Expected volatility
|
88 | % | 79 | % | 75 | % | ||||||
|
Risk-free interest rate
|
1.48 | % | 1.67 | % | 2.00 | % | ||||||
|
Expected term
|
5.4 years
|
5.3 years
|
5.6 years
|
|||||||||
|
Year Ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
Research and development
|
$ | 3,672 | $ | 2,302 | $ | 2,182 | ||||||
|
Selling, general and administrative
|
4,087 | 2,611 | 2,213 | |||||||||
|
Total stock-based compensation expense
|
$ | 7,759 | $ | 4,913 | $ | 4,395 | ||||||
|
10.
|
Capital Stock
|
|
11.
|
Commitments and Contingencies
|
|
Operating
Leases
|
||||
|
2012
|
8,190 | |||
|
2013
|
3,522 | |||
|
2014
|
872 | |||
|
Minimum lease payments
|
$ | 12,584 | ||
|
12.
|
Concentration of Risk, Segment and Geographic Information
|
|
Year ended December 31,
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
United States
|
$ | 20,447 | $ | 25,306 | $ | 47,656 | ||||||
|
Europe
|
35,718 | 4,728 | 613 | |||||||||
|
Asia Pacific
|
2,031 | 3,607 | 50,161 | |||||||||
|
Total
|
$ | 58,196 | $ | 33,641 | $ | 98,430 | ||||||
|
13.
|
Subsequent Events
|
|
14.
|
Quarterly Financial Information (unaudited)
|
|
Consolidated Statements of Operations
|
||||||||||||||||
|
Quarter Ended
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
(In thousands, except per share amounts)
|
||||||||||||||||
|
2011
|
||||||||||||||||
|
Total revenues
(1)
|
$ | 15,595 | $ | 16,525 | $ | 16,229 | $ | 9,847 | ||||||||
|
Total operating costs and expenses
|
22,716 | 24,394 | 23,147 | 21,894 | ||||||||||||
|
Other income (expense), net
|
801 | (261 | ) | 375 | 312 | |||||||||||
|
Net loss
|
(6,335 | ) | (8,130 | ) | (6,543 | ) | (11,735 | ) | ||||||||
|
Basic and diluted net loss per share of common stock
|
$ | (0.22 | ) | $ | (0.27 | ) | $ | (0.20 | ) | $ | (0.34 | ) | ||||
|
2010
|
||||||||||||||||
|
Total revenues
(1)
|
$ | 7,202 | $ | 5,942 | $ | 10,897 | $ | 9,601 | ||||||||
|
Total operating costs and expenses
|
23,140 | 24,372 | 27,542 | 25,691 | ||||||||||||
|
Other (expense) income, net
(2)
|
(5,847 | ) | 2,866 | 3,013 | (1,657 | ) | ||||||||||
|
Net loss
|
(21,785 | ) | (15,580 | ) | (13,633 | ) | (17,758 | ) | ||||||||
|
Basic and diluted net loss per share of common stock
|
$ | (1.36 | ) | $ | (0.93 | ) | $ | (0.69 | ) | $ | (0.84 | ) | ||||
|
(1)
|
Revenue in the first three quarters of 2011 includes the recognition of $14.9 million of the non-recurring license fee received as consideration for the collaboration with Servier entered into in December 2010. Revenue in the third quarter of 2010 includes a non-recurring fee of $4.0 million related to the sale of the Company’s CIMZIA
®
royalty interest to an undisclosed buyer.
|
|
(2)
|
Other expense of $5.8 million and $1.7 million in the first and fourth quarters of 2010, respectively, and other income of $2.9 million and $3.0 million in the second and third quarters of 2010, respectively primarily relates to a loss on the revaluation of the warrant liabilities.
|
|
Exhibit
Number
|
Inde
x to Exhibits
|
|
|
3.1
|
Certificate of Incorporation of XOMA Corporation (Exhibit 3.1)
1
|
|
|
3.2
|
By-laws of XOMA Corporation (Exhibit 3.2)
1
|
|
|
4.1
|
Form of Stock Certificate (Exhibit 4.1)
1
|
|
|
4.2
|
Shareholder Rights Agreement dated as of February 26, 2003 by and between XOMA Ltd. and Mellon Investor Services LLC as Rights Agent (Exhibit 4.1)
2
|
|
|
4.2A
|
Amendment to Shareholder Rights Agreement dated December 21, 2010 between XOMA Ltd. and Wells Fargo Bank, N.A. as Rights Agent (Exhibit 4.1A)
3
|
|
|
4.2B
|
Amendment No. 2 to Shareholder Rights Agreement dated December 31, 2011 between XOMA Corporation and Wells Fargo Bank, N.A. as Rights Agent (Exhibit 4.2)
1
|
|
|
4.2C
|
Amendment No. 3 to Shareholder Rights Agreement dated March 5, 2012 between XOMA Corporation and Wells Fargo Bank, N.A. as Rights Agent (Exhibit 4.2)
44
|
|
|
4.3
|
Form of Certificate of Designations of Series A Preferred Stock (Annex A to Exhibit 3.1)
1
|
|
|
4.4
|
Resolution Regarding Preferences and Rights of Series B Preference Shares (Exhibit B to Exhibit 3)
4
|
|
|
4.5
|
Indenture between XOMA Ltd. and Wells Fargo Bank, National Association, as trustee, relating to the Company’s 6.50% Convertible SNAPs
SM
due February 1, 2012 (Exhibit 2)
5
|
|
|
4.6
|
Form of Warrant (May 2009 Warrants) (Exhibit 10.2)
6
|
|
|
4.6A
|
Form of Amended and Restated Warrant (May 2009 Warrants) (Exhibit 10.5)
7
|
|
|
4.7
|
Form of Warrant (June 2009 Warrants) (Exhibit 10.2)
8
|
|
|
4.7A
|
Form of Amended and Restated Warrant (June 2009 Warrants) (Exhibit 10.6)
7
|
|
|
4.8
|
Form of Warrant (February 2010 Warrants) (Exhibit 10.2)
7
|
|
|
Form of Warrant (December 2011 Warrants)*
|
||
|
4.10
|
Form of Warrant (March 2012 Warrants) (Exhibit 4.1)
44
|
|
|
10.1
|
1981 Share Option Plan as amended and restated (Exhibit 10.1)
9
|
|
|
10.1A
|
Form of Share Option Agreement for 1981 Share Option Plan (Exhibit 10.1A)
10
|
|
|
10.2
|
Restricted Share Plan as amended and restated (Exhibit 10.2)
9
|
|
|
10.2A
|
Form of Share Option Agreement for Restricted Share Plan (Exhibit 10.2A)
10
|
|
|
10.3
|
2007 CEO Share Option Plan (Exhibit 10.7)
11
|
|
|
10.4
|
1992 Directors Share Option Plan as amended and restated (Exhibit 10.3)
9
|
|
|
10.4A
|
Form of Share Option Agreement for 1992 Directors Share Option Plan (initial grants) (Exhibit 10.3A)
10
|
|
10.4B
|
Form of Share Option Agreement for 1992 Directors Share Option Plan (subsequent grants) (Exhibit 10.3B)
10
|
|
|
10.5
|
2002 Director Share Option Plan (Exhibit 10.10)
12
|
|
|
10.6
|
Amended and Restated 2010 Long Term Incentive and Stock Award Plan (Exhibit 10.1)
13
|
|
|
Form of Stock Option Agreement for Amended and Restated 2010 Long Term Incentive and Stock Award Plan*
|
||
|
Form of Restricted Stock Unit Agreement for Amended and Restated 2010 Long Term Incentive and Stock Award Plan*
|
||
|
10.7
|
Management Incentive Compensation Plan as amended and restated (Exhibit 10.3)
14
|
|
|
10.7A
|
CEO Incentive Compensation Plan (Exhibit 10.4A)
10
|
|
|
Amendment No. 1 to CEO Incentive Compensation Plan*
|
||
|
10.7C
|
Bonus Compensation Plan (Exhibit 10.4B)
10
|
|
|
10.8
|
Amended and Restated 1998 Employee Stock Purchase Plan (Exhibit 10.2)
13
|
|
|
10.9
|
Form of Amended and Restated Indemnification Agreement for Officers (Exhibit 10.6)
15
|
|
|
10.9A
|
Form of Amended and Restated Indemnification Agreement for Employee Directors (Exhibit 10.7)
15
|
|
|
10.9B
|
Form of Amended and Restated Indemnification Agreement for Non-employee Directors (Exhibit 10.8)
15
|
|
|
10.10
|
Amended and Restated Employment Agreement entered into between XOMA (US) LLC and Steven B. Engle, dated as of December 30, 2008 (Exhibit 10.7)
16
|
|
|
10.10A
|
Amended and Restated Employment Agreement entered into between XOMA (US) LLC and Patrick J. Scannon, dated as of December 30, 2008 (Exhibit 10.7A)
16
|
|
|
10.10B
|
Employment Agreement entered into between XOMA (US) LLC and Fred Kurland, dated as of December 29, 2008 (Exhibit 10.7B)
16
|
|
|
10.10C
|
Amended and Restated Employment Agreement entered into between XOMA (US) LLC and Christopher J. Margolin, dated as of December 30, 2008 (Exhibit 10.7C)
16
|
|
|
10.10D
|
Amended and Restated Employment Agreement entered into between XOMA (US) LLC and Charles C. Wells, dated as of December 30, 2008 (Exhibit 10.7D)
16
|
|
|
10.10E
|
Employment Agreement effective as of May 31, 2011 between XOMA (US) LLC and Paul Rubin (Exhibit 10.1)
17
|
|
|
10.10F
|
Employment Agreement effective as of August 31, 2011 between XOMA (US) LLC and John Varian (Exhibit 10.2)
18
|
|
|
Employment Agreement effective as of January 4, 2012 between XOMA (US) LLC and John Varian*
|
||
|
10.11
|
Consulting Agreement effective as of August 3, 2007 between XOMA (US) LLC and John L. Castello (Exhibit 10.8)
11
|
|
10.11A
|
Consulting Agreement effective as of August 31, 2011 between XOMA (US) LLC and Steven B. Engle (Exhibit 10.1)
18
|
|
|
10.12
|
Form of Change of Control Severance Agreement entered into between XOMA Ltd. and certain of its executives, with reference schedule (Exhibit 10.12)
3
|
|
|
Change of Control Agreement entered into between XOMA Ltd. and John Varian, dated January 4, 2012*
|
||
|
10.13
|
Lease of premises at 890 Heinz Street, Berkeley, California dated as of July 22, 1987 (Exhibit 10.12)
19
|
|
|
10.14
|
Lease of premises at Building E at Aquatic Park Center, Berkeley, California dated as of July 22, 1987 and amendment thereto dated as of April 21, 1988 (Exhibit 10.13)
19
|
|
|
10.15
|
Lease of premises at Building C at Aquatic Park Center, Berkeley, California dated as of July 22, 1987 and amendment thereto dated as of August 26, 1987 (Exhibit 10.14)
19
|
|
|
10.16
|
Letter of Agreement regarding CPI adjustment dates for leases of premises at Buildings C, E and F at Aquatic Park Center, Berkeley, California dated as of July 22, 1987 (Exhibit 10.15)
19
|
|
|
10.17
|
Lease of premises at 2910 Seventh Street, Berkeley, California dated March 25, 1992 (Exhibit 10.16)
19
|
|
|
10.17A
|
Fifth amendment to lease of premises at 2910 Seventh Street, Berkeley, California dated June 1, 2006 (Exhibit 10.58)
20
|
|
|
10.18
|
Lease of premises at 5860 and 5864 Hollis Street, Emeryville, California dated as of November 2, 2001 (with addendum) (Exhibit 10.19)
21
|
|
|
10.19
|
Lease of premises at 2850 Seventh Street, Second Floor, Berkeley, California dated as of December 28, 2001 (with addendum and guaranty) (Exhibit 10.20)
21
|
|
|
10.20
|
Second Amended and Restated Collaboration Agreement dated January 12, 2005, by and between XOMA (US) LLC and Genentech, Inc. (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)
22
|
|
|
10.20A
|
Agreement related to LUCENTIS® License Agreement and RAPTIVA® Collaboration Agreement dated September 9, 2009, by and between XOMA (Bermuda) Ltd., XOMA (US) LLC and Genentech, Inc. (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.18A)
23
|
|
|
10.21
|
License Agreement by and between XOMA Ireland Limited and MorphoSys AG, dated as of February 1, 2002 (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.43)
24
|
|
|
10.22
|
Amended and Restated License Agreement by and between XOMA Ireland Limited and DYAX Corp., dated as of October 27, 2006 (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.32)
15
|
|
|
10.23
|
License Agreement by and between XOMA Ireland Limited and Cambridge Antibody Technology Limited, dated as of December 22, 2002 (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.46)
2
|
|
10.24
|
License Agreement, dated as of December 29, 2003, by and between Diversa Corporation and XOMA Ireland Limited (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 2)
25
|
|
|
10.24A
|
GSSM License Agreement, effective as of May 2, 2008, by and between Verenium Corporation and XOMA Ireland Limited (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.25A)
3
|
|
|
10.25
|
Secured Note Agreement, dated as of May 26, 2005, by and between Chiron Corporation and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.3)
26
|
|
|
10.25A
|
Amended and Restated Research, Development and Commercialization Agreement, executed November 7, 2008, by and between Novartis Vaccines and Diagnostics, Inc. (formerly Chiron Corporation) and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)(Exhibit 10.24c)
27
|
|
|
Amendment No. 1 to Amended and Restated Research, Development and Commercialization Agreement, effective as of April 30, 2010, by and between Novartis Vaccines and Diagnostics, Inc. and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
10.25C
|
Manufacturing and Technology Transfer Agreement, executed December 16, 2008, by and between Novartis Vaccines and Diagnostics, Inc. (formerly Chiron Corporation) and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)(Exhibit 10.24D)
27
|
|
|
10.26
|
Collaboration Agreement, dated as of September 23, 2004, by and between Aphton Corporation and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 2)
28
|
|
|
10.27
|
Agreement dated March 8, 2005, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.53)
22
|
|
|
10.27A
|
Agreement dated July 28, 2006, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases (Exhibit 10.60)
20
|
|
|
10.27B
|
Agreement dated September 15, 2008, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.39)
29
|
|
|
10.27C
|
Second Amendment to Agreement dated September 15, 2008, between XOMA (US) LLC and the National Institute of Allergy and Infectious Diseases (Exhibit 10.24C)
30
|
|
|
10.28
|
License Agreement, effective as of June 20, 2005, by and between Merck & Co., Inc. and XOMA Ireland Limited (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.4)
26
|
|
10.29
|
Collaboration Agreement dated as of May 22, 2006, by and between Schering Corporation, acting through its Schering-Plough Research Institute division, and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.59)
20
|
|
|
10.30
|
Collaboration Agreement, dated as of November 1, 2006, between Takeda Pharmaceutical Company Limited and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.46)
15
|
|
|
10.30A
|
First Amendment to Collaboration Agreement, effective as of February 28, 2007, between Takeda Pharmaceutical Company Limited and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.48)
31
|
|
|
10.30B
|
Second Amendment to Collaboration Agreement, effective as of February 9, 2009, among Takeda Pharmaceutical Company Limited and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)
27
|
|
|
10.31
|
Amended & Restated Loan Agreement, dated as of May 9, 2008 between Goldman Sachs Specialty Lending Holdings, Inc., XOMA Ltd. and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.37)
32
|
|
|
10.32
|
License Agreement, effective as of August 27, 2007, by and between Pfizer Inc. and XOMA Ireland Limited (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 2)
33
|
|
|
10.33
|
Common Stock Purchase Agreement, dated as of October 21, 2008, by and between XOMA Ltd. and Azimuth Opportunity Ltd. (Exhibit 10.1)
34
|
|
|
10.33A
|
Common Stock Purchase Agreement, dated as of July 23, 2010, by and between XOMA Ltd. and Azimuth Opportunity Ltd. (Exhibit 10.1)
35
|
|
|
10.34
|
Securities Purchase Agreement dated May 15, 2009, between XOMA Ltd. and the investors named therein (Exhibit 10.1)
6
|
|
|
10.34A
|
Engagement Letter dated May 15, 2009 (Exhibit 10.3)
6
|
|
|
10.34B
|
Securities Purchase Agreement dated June 5, 2009, between XOMA Ltd. and the investors named therein (Exhibit 10.1)
8
|
|
|
10.34C
|
Engagement Letter dated June 4, 2009 (Exhibit 10.3)
8
|
|
|
10.35
|
Discovery Collaboration Agreement dated September 9, 2009, by and between XOMA Development Corporation and Arana Therapeutics Limited (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.35)
36
|
|
|
10.36
|
At Market Issuance Sales Agreement dated July 14, 2009, between XOMA Ltd. and Wm Smith & Co. (Exhibit 10.36)
23
|
|
|
10.36A
|
At Market Issuance Sales Agreement dated October 26, 2010, between XOMA Ltd. and Wm Smith & Co. and McNicoll, Lewis & Vlak LLC (Exhibit 10.1)
37
|
|
10.36B
|
At Market Issuance Sales Agreement dated February 4, 2011, between XOMA Ltd. and McNicoll, Lewis & Vlak LLC (Exhibit 1.2)
38
|
|
|
10.36C
|
Amendment to At Market Issuance Sales Agreement dated December 31, 2011, between XOMA Corporation and MLV & Co. LLC (Exhibit 1.2A)
39
|
|
|
10.37
|
Discovery Collaboration Agreement dated October 29, 2009, by and between XOMA Development Corporation and The Chemo-Sero-Therapeutic Research Institute (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)
40
|
|
|
10.38
|
Underwriting Agreement dated February 2, 2010 (Exhibit 10.1)
7
|
|
|
10.39
|
Warrant Amendment Agreement dated February 2, 2010 (May 2009 Warrants) (Exhibit 10.3)
7
|
|
|
10.39A
|
Form of Warrant Amendment Agreement dated February 2, 2010 (June 2009 Warrants) (Exhibit 10.4)
7
|
|
|
10.40
|
Royalty Purchase Agreement, dated as of August 12, 2010, by and among XOMA CDRA LLC, XOMA (US) LLC, XOMA Ltd. and the buyer named therein (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.38)
41
|
|
|
10.41
|
Collaboration and License Agreement dated as of December 30, 2010, by and between XOMA Ireland Limited, Les Laboratoires Servier and Institut de Recherches Servier (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.42)
3
|
|
|
Amended and Restated Collaboration and License Agreement dated as of February 14, 2012, by and between XOMA Ireland Limited, Les Laboratoires Servier and Institut de Recherches Servier (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
10.41B
|
Loan Agreement dated as of December 30, 2010, by and between XOMA Ireland Limited and Les Laboratoires Servier (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission) (Exhibit 10.42A)
43
|
|
|
10.42
|
Foreign Exchange and Options Master Agreement (FEOMA) dated as of May 16, 2011, between Royal Bank of Canada and XOMA Ltd., with letter agreement dated May 17, 2011 (Exhibit 10.1)
43
|
|
|
Loan Agreement dated as of December 30, 2011, among XOMA (US) LLC, as Borrower, XOMA Ltd., as Parent, each other loan party from time to time party thereto, General Electric Capital Corporation, as Agent, and each other lender from time to time party thereto (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
Guaranty, Pledge and Security Agreement dated as of December 30, 2011, among XOMA (US) LLC, each other guarantor from time to time party thereto and General Electric Capital Corporation, as Agent (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
Amended and Restated License and Commercialization Agreement effective as of January 11, 2012, by and between Les Laboratoires Servier and XOMA Ireland Limited (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
Amended and Restated Trademark License Agreement entered into as of January 11, 2012, between Biofarma and XOMA Ireland Limited (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
Master Services Agreement dated as of November 9, 2009, between Medpace, Inc. and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
Amendment No. 1 to Master Services Agreement dated as of October 4, 2011, between Medpace, Inc. and XOMA (US) LLC (with certain confidential information omitted, which omitted information is the subject of a confidential treatment request and has been filed separately with the Securities and Exchange Commission)*
|
||
|
Subsidiaries of the Company*
|
||
|
Consent of Independent Registered Public Accounting Firm*
|
||
|
Certification of John Varian, filed pursuant to Section 302 of the Sarbanes-Oxley Act of 2002*
|
||
|
Certification of Fred Kurland, filed pursuant to Section 302 of the Sarbanes-Oxley Act of 2002*
|
||
|
Certification of John Varian, furnished pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
||
|
Certification of Fred Kurland, furnished pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
||
|
Press Release dated March 14, 2012 furnished herewith
|
||
| 101. INS | XBRL Instance Document | |
| 101. SCH | XBRL Schema Document | |
| 101. CAL | XBRL Caluculation Linkbase Document | |
| 101. DEF | XBRL Definition Linkbase Document | |
| 101. LAB | XBRL Label Linkbase Document | |
| 101. PRE | XBRL Presentation Linkbase Document |
|
*
|
Filed herewith
|
|
1
|
Incorporated by reference to the referenced appendix to the Company’s Current Report on Form 8-K filed January 3, 2012.
|
|
2
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2002.
|
|
3
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2010.
|
|
4
|
Incorporated by reference to the referenced exhibit to the Company’s Amendment No. 1 to Form 8-K/A filed April 18, 2003.
|
|
5
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed February 13, 2006.
|
|
6
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed May 19, 2009.
|
|
7
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed February 2, 2010.
|
|
8
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed June 10, 2009.
|
|
9
|
Incorporated by reference to the referenced exhibit to the Company’s Registration Statement on Form S-8 (File No. 333-171429) filed December 27, 2010.
|
|
10
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2007.
|
|
11
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed August 7, 2007.
|
|
12
|
Incorporated by reference to the referenced exhibit to the Company’s Registration Statement on Form S-8 (File No. 333-151416) filed June 4, 2008.
|
|
13
|
Incorporated by reference to the referenced exhibit to the Company’s Post-Effective Amendment No. 1 to Registration Statements on Form S-8 (File Nos. 333-108306, 333-151416, 333-171429 and 333-174730) filed January 3, 2012.
|
|
14
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed November 6, 2007.
|
|
15
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2006.
|
|
16
|
Incorporated by reference to the referenced exhibit to the Company’s Amendment No. 2 to Annual Report on Form 10-K/A for the fiscal year ended December 31, 2009 filed December 27, 2010.
|
|
17
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed June 16, 2011.
|
|
18
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed September 1, 2011.
|
|
19
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 1997, as amended.
|
|
20
|
Incorporated by reference to the referenced exhibit to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2006.
|
|
21
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2001.
|
|
22
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2004.
|
|
23
|
Incorporated by reference to the referenced exhibit to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2009.
|
|
24
|
Incorporated by reference to the referenced exhibit to Amendment No. 2 to the Company’s Quarterly Report on Form 10Q/A for the quarterly period ended March 31, 2002 filed December 12, 2002.
|
|
25
|
Incorporated by reference to the referenced exhibit to the Company’s Amendment No. 2 on Form 8-K/A filed March 19, 2004.
|
|
26
|
Incorporated by reference to the referenced exhibit to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2005.
|
|
27
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2008.
|
|
28
|
Incorporated by reference to the referenced exhibit to the Company’s Amendment No. 1 on Form 8-K/A filed October 26, 2004.
|
|
29
|
Incorporated by reference to the referenced exhibit to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2008.
|
|
30
|
Incorporated by reference to the referenced exhibit to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2010.
|
|
31
|
Incorporated by reference to the referenced exhibit to Amendment No. 1 to the Company’s Quarterly Report on Form 10Q/A for the quarterly period ended March 31, 2007 filed on March 5, 2010.
|
|
32
|
Incorporated by reference to the referenced exhibit to Amendment No. 2 to the Company’s Quarterly Report on Form 10Q/A for the quarterly period ended June 30, 2008 filed on March 5, 2010.
|
|
33
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed September 13, 2007.
|
|
34
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed October 22, 2008.
|
|
35
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed July 23, 2010.
|
|
36
|
Incorporated by reference to the referenced exhibit to Amendment No. 1 to the Company’s Quarterly Report on Form 10Q/A for the quarterly period ended September 30, 2009 filed on March 5, 2010.
|
|
37
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed October 26, 2010.
|
|
38
|
Incorporated by reference to the referenced exhibit to the Company’s Registration Statement on Form S-3 (File No. 333-172197) filed February 11, 2011.
|
|
39
|
Incorporated by reference to the referenced exhibit to the Company’s Post-Effective Amendment No. 1 to Registration Statement on Form S-3 (File No. 333-172197) filed January 3, 2012.
|
|
40
|
Incorporated by reference to the referenced exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2009.
|
|
41
|
Incorporated by reference to the referenced exhibit to Amendment No. 2 to the Company’s Quarterly Report on Form 10Q/A for the quarterly period ended September 30, 2010 filed on April 4, 2011.
|
|
42
|
Incorporated by reference to the referenced exhibit to Amendment No. 1 to the Company’s Annual Report on Form 10-K/A for the fiscal year period ended December 31, 2010 filed on May 26, 2011.
|
|
43
|
Incorporated by reference to the referenced exhibit to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2011.
|
|
44
|
Incorporated by reference to the referenced exhibit to the Company’s Current Report on Form 8-K filed March 7, 2012.
|