|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the fiscal year ended December 31, 2014
|
||
|
or
|
||
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from __________ to __________
|
||
|
Zoetis Inc.
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
46-0696167
|
|
|
(State or other jurisdiction of
|
(I.R.S. Employer Identification No.)
|
|
|
incorporation or organization)
|
||
|
100 Campus Drive, Florham Park, New Jersey
|
07932
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
(973) 822-7000
|
|
(Registrant’s telephone number, including area code)
|
|
Title of each class
|
Name of each exchange on which registered
|
||
|
Common Stock, $0.01 par value per share
|
New York Stock Exchange
|
||
|
Large accelerated filer
x
|
Accelerated filer
¨
|
Non-accelerated filer
¨
|
Smaller reporting company
¨
|
|||
|
Page
|
||||
|
Item 1.
|
||||
|
Item 1A.
|
||||
|
Item 1B.
|
||||
|
Item 2.
|
||||
|
Item 3.
|
||||
|
Item 4.
|
||||
|
Item 5.
|
||||
|
Item 6.
|
||||
|
Item 7.
|
||||
|
Item 7A.
|
||||
|
Item 8.
|
||||
|
Item 9.
|
||||
|
Item 9A.
|
||||
|
Item 9B.
|
||||
|
Item 10.
|
||||
|
Item 11.
|
||||
|
Item 12.
|
||||
|
Item 13.
|
||||
|
Item 14.
|
||||
|
Item 15.
|
||||
|
•
|
economic differences, such as standards of living in developed markets as compared to emerging markets;
|
|
•
|
cultural differences, such as dietary preferences for different animal proteins, pet ownership preferences and pet care standards;
|
|
•
|
epidemiological differences, such as the prevalence of certain bacterial and viral strains and disease dynamics;
|
|
•
|
treatment differences, such as utilization of different types of medicines and vaccines, as well as the pace of adoption of new technologies;
|
|
•
|
environmental differences, such as seasonality, climate and the availability of arable land and fresh water; and
|
|
•
|
regulatory differences, such as standards for product approval and manufacturing.
|
|
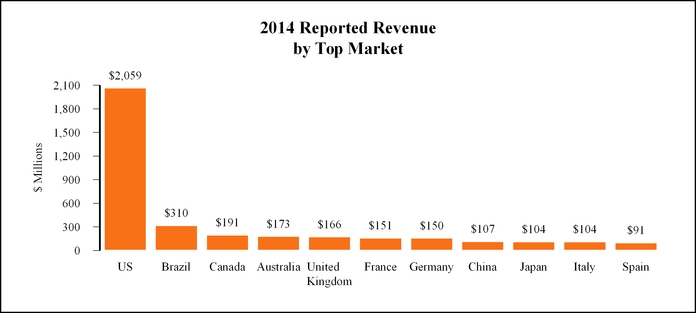
•
|
United States
with revenue of $
2,059 million
, or
43%
of total revenue for the year ended
December 31, 2014
.
|
|
•
|
Europe/Africa/Middle East
with revenue of $
1,141 million
, or
24%
of total revenue for the year ended
December 31, 2014
. Key developed markets in this segment include the United Kingdom, France and Germany. Key emerging markets in this segment include South Africa, Russia and Turkey.
|
|
•
|
Canada/Latin America
with revenue of $
815 million
, or
17%
of total revenue for the year ended
December 31, 2014
. The developed market in this segment is Canada. Key emerging markets in this segment include Brazil and Mexico.
|
|
•
|
Asia/Pacific
with revenue of $
720 million
, or
15%
of total revenue for the year ended
December 31, 2014
. Key developed markets in this segment include Australia, Japan and New Zealand. Key emerging markets in this segment include China, India and Thailand.
|
|
US
|
Brazil
|
Canada
|
Australia
|
UK
|
France
|
Germany
|
China
|
Japan
|
Italy
|
Spain
|
|
|
Livestock
|
56%
|
84%
|
63%
|
61%
|
55%
|
65%
|
58%
|
84%
|
56%
|
60%
|
79%
|
|
Companion Animal
|
44%
|
16%
|
37%
|
39%
|
45%
|
35%
|
42%
|
16%
|
44%
|
40%
|
21%
|



|
•
|
anti-infectives
: products that prevent, kill or slow the growth of bacteria, fungi or protozoa;
|
|
•
|
vaccines
: biological preparations that help prevent diseases of the respiratory, gastrointestinal and reproductive tracts or induce a specific immune response;
|
|
•
|
parasiticides
: products that prevent or eliminate external and internal parasites such as fleas, ticks and worms;
|
|
•
|
medicated feed additives
: products added to animal feed that provide medicines to livestock; and
|
|
•
|
other pharmaceutical products
: pain and sedation, oncology, antiemetic, allergy and dermatology; and reproductive products.
|
|
•
|
Improvac/Improvest/Vivax, a protein product that works like an immunization, is currently the only product that provides a safe and effective alternative to physical castration to manage unpleasant aromas that can occur when cooking pork; launched in Australia and New Zealand in 2004, in Brazil in 2007, in certain European countries beginning in 2008, and in the United States in 2011;
|
|
•
|
Convenia, the first single-injection anti-infective for common bacterial skin infections in cats and dogs, launched in 2006;
|
|
•
|
Cerenia®, the first and only product on the market to prevent vomiting due to motion sickness in dogs, was first launched in Europe in 2006, followed by the United States in 2007;
|
|
•
|
Palladia, the first drug to be approved by the FDA for treating cancer in dogs, launched in 2009;
|
|
•
|
Inforce
TM
3, the first and only respiratory vaccine for cattle that prevents respiratory disease caused by bovine respiratory syncytial virus (BRSV) while also aiding in the prevention of infectious bovine rhinotracheitis (IBR) and parainfluenza
3
(PI
3
), launched in 2010;
|
|
•
|
Fostera® PCV MH was introduced in November 2013 and developed to help protect pigs from PCVAD and enzootic pneumonia caused by M. hyo. Unlike other combination vaccines that require field mixing, the one-bottle formulation of Fostera PCV MH allows the convenience of a one-dose program or the flexibility of a two-dose program; and
|
|
•
|
Apoquel, the first Janus kinase inhibitor for use in veterinary medicine, was approved for the control of pruritus associated with allergic dermatitis and the control of atopic dermatitis in dogs at least 12 months of age. We launched Apoquel in the United States, United Kingdom, Austria and Germany in January 2014 and expect other market launches to follow.
|
|
Product line / product
|
Description
|
Primary species
|
||
|
Anti-infectives
|
||||
|
Ceftiofur injectable line
|
Broad-spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria, including ß-lactamase-producing strains, with some formulations producing a single course of therapy in one injection
|
Cattle, sheep, swine
|
||
|
Draxxin
|
Single-dose low-volume antibiotic for the treatment and prevention of bovine and swine respiratory disease, infectious bovine keratoconjunctivitis and bovine foot rot
|
Cattle, swine
|
||
|
Spectramast
|
Treatment of subclinical or clinical mastitis in dry or lactating dairy cattle, delivered via intramammary infusion; same active ingredient as the ceftiofur line
|
Cattle
|
||
|
Terramycin line
|
Antibiotic for the treatment of susceptible infections
|
Cattle, poultry, sheep, swine
|
||
|
Vaccines
|
||||
|
Bovi-shield
®
line
|
Aids in preventing diseases, including infectious bovine rhinotracheitis (IBR), bovine viral diarrhea (BVD) Types 1 and 2, parainfluenza
3
(PI
3
), bovine respiratory syncytial virus (BRSV), and leptospirosis caused by
Leptospira borgpetersenii
,
L.canicola, L grippotyphosa, L. hardjo,L. icterohaemorrhagiae, and L. pamona
, depending on formulation
|
Cattle
|
||
|
Improvac / Improvest / Vivax
|
Reduces boar taint, as an alternative to surgical castration
|
Swine
|
||
|
Rispoval
®
line
|
Aids in preventing three key viruses involved in cattle pneumonia-BRSV, PI
3
and BVD-as well as other respiratory diseases, depending on formulation
|
Cattle
|
||
|
Suvaxyn
®
PCV / Fostera™ PCV
|
Aids in preventing viremia and helps control lymphoid depletion caused by porcine circovirus
|
Swine
|
||
|
Parasiticides
|
||||
|
Cydectin
|
Injectable or pour-on endectocide to treat and control internal and external cattle parasites, including gastrointestinal roundworms, lungworms, cattle grubs, mites and lice
|
Cattle, sheep
|
||
|
Dectomax
|
Injectable or pour-on endectocide, characterized by extended duration of activity, for the treatment and control of internal and external parasite infections
|
Cattle, swine
|
||
|
Medicated Feed Additives
|
||||
|
Aureomycin
|
Provides livestock producers control, treatment and convenience against a wide range of respiratory, enteric and reproductive diseases
|
Cattle, poultry, sheep, swine
|
||
|
BMD
|
Aids in preventing and controlling enteritis; and increases rate of weight gain and improves feed efficiency in poultry and swine
|
Poultry, swine
|
||
|
Lasalocid line
|
Controls coccidiosis in poultry (Avatec) and cattle (Bovatec) and for increased rate of weight gain and improved feed efficiency in cattle
|
Poultry, cattle
|
||
|
Lincomycin line
|
Controls necrotic enteritis; treatment of dysentery (bloody scours), control of ileitis, treatment/reduction in severity of mycoplasmal pneumonia, increases weight gain in swine
|
Swine, poultry
|
||
|
Other
|
||||
|
Embrex
®
devices
|
Devices for enhancing hatchery operations' efficiency through
in ovo
detection and vaccination
|
Poultry
|
||
|
Lutalyse
|
For estrus control or in the induction of parturition or abortion
|
Cattle, swine
|
||
|
Orbeseal / Teatseal
|
Non-antibiotic intramammary infusion that prevents new intramammary infections in dairy cattle
|
Cattle
|
||
|
Product line / product
|
Description
|
Primary species
|
||
|
Anti-infectives
|
||||
|
Clavamox / Synulox
|
A broad-spectrum antibiotic and the first and only potentiated penicillin approved for use in dogs and cats
|
Cats, dogs
|
||
|
Convenia
|
Anti-infective for the treatment of common bacterial skin infections that provides a course of treatment in a single injection
|
Cats, dogs
|
||
|
Vaccines
|
||||
|
Vanguard
®
L4 (4-way Lepto)
|
Compatible with the Vanguard line and helps protect against leptospirosis caused by
Leptospira canicola
,
L. grippotyphosa
,
L. icterohaemorrhagiae
and
L. pomona
|
Dogs
|
||
|
Vanguard line
|
Aids in preventing canine distemper caused by canine distemper virus, infectious canine hepatitis caused by canine adenovirus type 1, respiratory disease caused by canine adenovirus type 2, canine parainfluenza caused by canine parainfluenza virus and canine parvoviral enteritis caused by canine parvovirus
|
Dogs
|
||
|
Parasiticides
|
||||
|
Revolution / Stronghold
|
An antiparasitic for protection against fleas, heartworm disease and ear mites in cats and dogs; canine sarcoptic mites and American dog tick and roundworms and hookworms for cats
|
Cats, dogs
|
||
|
ProHeart
|
Prevents heartworm infestation; also for treatment of existing larval and adult hookworm infections
|
Dogs
|
||
|
Other
|
||||
|
Cerenia
|
A medication that prevents and treats acute vomiting in dogs, treats acute vomiting in cats and prevents vomiting due to motion sickness in dogs
|
Cats, dogs
|
||
|
Rimadyl
|
For the relief of pain and inflammation associated with osteoarthritis and for the control of postoperative pain associated with soft tissue and orthopedic surgeries
|
Dogs
|
||
|
Anchor Sites
|
Satellite Sites
|
|||||
|
Site
|
Location
|
Site
|
Location
|
|||
|
Catania
|
Italy
|
Campinas
|
Brazil
|
|||
|
Charles City
|
Iowa, U.S.
|
Durham
|
North Carolina, U.S.
|
|||
|
Chicago Heights
|
Illinois, U.S.
|
Eagle Grove
|
Iowa, U.S.
|
|||
|
Guarulhos
(1)
|
Brazil
|
Hsinchu
(4)
|
Taiwan
|
|||
|
Haridwar
|
India
|
Laurinburg
|
North Carolina, U.S.
|
|||
|
Jilin
(2)
|
China
|
Longmont
|
Colorado, U.S.
|
|||
|
Kalamazoo
(3)
|
Michigan, U.S.
|
Medolla
|
Italy
|
|||
|
Lincoln
|
Nebraska, U.S.
|
Salisbury
|
Maryland, U.S.
|
|||
|
Louvain-la-Neuve
|
Belgium
|
San Diego
|
California, U.S.
|
|||
|
Melbourne
|
Australia
|
Shenzhou
|
China
|
|||
|
Olot
|
Spain
|
Van Buren
|
Arkansas, U.S.
|
|||
|
Suzhou
|
China
|
Wellington
|
New Zealand
|
|||
|
Willow Island
|
West Virginia, U.S.
|
White Hall
|
Illinois, U.S.
|
|||
|
Yantai
|
China
|
|||||
|
(1)
|
This site is owned by us and leased back to Pfizer, pursuant to an arrangement by which Pfizer operates the manufacturing operations at the site for a period of time. See
Item 13.
Certain Relationships and Related Transactions, and Director Independence—Relationship with Pfizer—Brazil lease agreements.
|
|
(2)
|
This site is operated by the China joint venture, Jilin Zoetis Guoyuan Animal Health Company, Ltd.
|
|
(3)
|
Prior to the Separation, Pfizer's manufacturing site in Kalamazoo manufactured both human health and animal health products. Since the Separation, we own the portions of this site that predominantly manufacture animal health products and Pfizer owns the portions of this site that predominantly manufacture human health products.
|
|
(4)
|
This site is operated by the Taiwan joint venture, Zoetis Biotech Manufacturing Limited.
|
|
•
|
livestock producers tend to be loyal to medicines and vaccines that have been demonstrated to be efficacious because medicines and vaccines are a small portion of a livestock producer's total production costs and ineffective medicines and vaccines could result in the loss of animals, causing disproportionate harm to such producer's investment;
|
|
•
|
livestock producers value the technical assistance provided through our veterinary operations' support of our products and field force; and
|
|
•
|
the importance of reliable supply.
|
|
•
|
Establish and implement harmonized technical requirements for the registration of veterinary medicinal products in the VICH regions, which meet high quality, safety and efficacy standards and minimize the use of test animals and costs of product development.
|
|
•
|
Provide a basis for wider international harmonization of registration requirements.
|
|
•
|
Monitor and maintain existing VICH guidelines, taking particular note of the ICH work program and, where necessary, update these VICH guidelines.
|
|
•
|
Ensure efficient processes for maintaining and monitoring consistent interpretation of data requirements following the implementation of VICH guidelines.
|
|
•
|
By means of a constructive dialogue between regulatory authorities and industry, provide technical guidance enabling response to significant emerging global issues and science that impact on regulatory requirements within the VICH regions.
|
|
•
|
environmental-related capital expenditures - $1 million; and
|
|
•
|
other environmental-related expenditures - $10 million.
|
|
•
|
our historical combined financial data does not reflect the Separation;
|
|
•
|
our historical combined financial data reflects expense allocations for certain support functions that are provided on a centralized basis within Pfizer, such as expenses for business technology, facilities, legal, finance, human resources, business development, public affairs and procurement, as well as certain manufacturing and supply costs incurred by manufacturing sites that are shared with other Pfizer business units that may be higher or lower than the comparable expenses we would have actually incurred, or will incur, as an independent company;
|
|
•
|
our cost of debt and our capital structure is different from that reflected in our historical combined financial statements;
|
|
•
|
significant increases may occur in our cost structure as a result of our being an independent public company, including costs related to public company reporting, investor relations and compliance with the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley Act); and
|
|
•
|
loss of economies of scale as a result of our no longer being a part of Pfizer.
|
|
•
|
the failure of us or any of our vendors or suppliers, including logistical service providers, to comply with applicable regulations and quality assurance guidelines;
|
|
•
|
construction delays;
|
|
•
|
equipment malfunctions;
|
|
•
|
shortages of materials;
|
|
•
|
labor problems;
|
|
•
|
natural disasters;
|
|
•
|
power outages;
|
|
•
|
criminal and terrorist activities;
|
|
•
|
changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, shipping distributions or physical limitations; and
|
|
•
|
the outbreak of any highly contagious diseases near our production sites.
|
|
•
|
volatility in the international financial markets;
|
|
•
|
compliance with governmental controls;
|
|
•
|
difficulties enforcing contractual and intellectual property rights;
|
|
•
|
parallel trade in our products (importation of our products from European Union countries where our products are sold at lower prices into European Union countries where the products are sold at higher prices);
|
|
•
|
compliance with a wide variety of laws and regulations, such as the FCPA and similar non-U.S. laws and regulations;
|
|
•
|
compliance with foreign labor laws;
|
|
•
|
burdens to comply with multiple and potentially conflicting foreign laws and regulations, including those relating to environmental, health and safety requirements;
|
|
•
|
changes in laws, regulations, government controls or enforcement practices with respect to our business and the businesses of our customers;
|
|
•
|
political and social instability, including crime, civil disturbance, terrorist activities and armed conflicts;
|
|
•
|
trade restrictions and restrictions on direct investments by foreign entities, including restrictions administered by the OFAC and the European Union, in relation to our products or the products of farmers and other customers (e.g., restrictions on the importation of agricultural products from the European Union to Russia)
;
|
|
•
|
changes in tax laws, challenges brought against our incentive tax rulings, and tariffs;
|
|
•
|
imposition of antidumping and countervailing duties or other trade-related sanctions;
|
|
•
|
costs and difficulties in staffing, managing and monitoring international operations; and
|
|
•
|
longer payment cycles and increased exposure to counterparty risk.
|
|
•
|
pay monetary damages;
|
|
•
|
obtain a license in order to continue manufacturing or marketing the affected products, which may not be available on commercially reasonable terms, or at all; or
|
|
•
|
stop activities, including any commercial activities, relating to the affected products, which could include a recall of the affected products and/or a cessation of sales in the future.
|
|
•
|
making it more difficult for us to satisfy our obligations with respect to our debt;
|
|
•
|
limiting our ability to obtain additional financing to fund future working capital, capital expenditures, business development or other general corporate requirements, including dividends;
|
|
•
|
increasing our vulnerability to general adverse economic and industry conditions;
|
|
•
|
exposing us to the risk of increased interest rates as certain of our borrowings are and may in the future be at variable rates of interest;
|
|
•
|
limiting our flexibility in planning for and reacting to changes in the animal health industry;
|
|
•
|
placing us at a competitive disadvantage to other, less leveraged competitors;
|
|
•
|
impacting our effective tax rate; and
|
|
•
|
increasing our cost of borrowing.
|
|
•
|
improving strategic and operational flexibility, increasing management focus and streamlining decision-making by providing the flexibility to implement our strategic plan and to respond more effectively to different customer needs and the changing economic environment;
|
|
•
|
allowing us to adopt the capital structure, investment policy and dividend policy best suited to our financial profile and business needs, without competing for capital with Pfizer's other businesses;
|
|
•
|
creating an independent equity structure that will facilitate our ability to effect future acquisitions utilizing our common stock; and
|
|
•
|
facilitating incentive compensation arrangements for employees more directly tied to the performance of our business, and enhancing employee hiring and retention by, among other things, improving the alignment of management and employee incentives with performance and growth objectives of our business.
|
|
•
|
Pfizer will retain ownership of, and license to us, the intellectual property that we develop under the R&D agreement. In many circumstances, the intellectual property we license from Pfizer will be non-exclusive as to Pfizer and third parties.
|
|
•
|
We are not assured access to Pfizer's newest programs.
|
|
•
|
Pfizer can prevent us from progressing pre-development compounds and, under certain circumstances, Pfizer may terminate our rights to a development stage compound by paying us the fair market value for such compound.
|
|
•
|
The R&D agreement may be terminated before the expiration of the seven year term in certain circumstances, including if we acquire an interest in, or assets of, a human pharmaceutical business, enter into a definitive agreement relating to, or undergo, a change of control or if Pfizer acquires, or is acquired by, an animal health business.
|
|
•
|
our operating performance and the performance of our competitors;
|
|
•
|
our or our competitors' press releases, other public announcements and filings with the SEC regarding new products or services, enhancements, significant contracts, acquisitions or strategic investments;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts, if any, who cover our common stock;
|
|
•
|
changes in our investor base;
|
|
•
|
failures to meet external expectations or management guidance;
|
|
•
|
fluctuations in our financial results or the financial results of companies perceived to be similar to us;
|
|
•
|
changes in our capital structure or dividend policy, including as a result of the Exchange Offer, future issuances of securities, sales of large blocks of common stock by our stockholders or the incurrence of additional debt;
|
|
•
|
reputational issues;
|
|
•
|
changes in general economic and market conditions in any of the regions in which we conduct our business;
|
|
•
|
the arrival or departure of key personnel;
|
|
•
|
the actions of speculators and financial arbitrageurs (such as hedge funds) during and after the Exchange Offer;
|
|
•
|
changes in applicable laws, rules or regulations and other dynamics; and
|
|
•
|
other developments or changes affecting us, our industry or our competitors.
|
|
•
|
a Board of Directors that is divided into three classes with staggered terms;
|
|
•
|
rules regarding how our stockholders may present proposals or nominate directors for election at stockholder meetings;
|
|
•
|
the right of our Board of Directors to issue preferred stock without stockholder approval; and
|
|
•
|
limitations on the right of stockholders to remove directors.
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
High
|
Low
|
|
|
2013
|
||
|
First Quarter (beginning February 1, 2013)
|
$35.42
|
$30.47
|
|
Second Quarter
|
$34.74
|
$29.40
|
|
Third Quarter
|
$32.90
|
$28.81
|
|
Fourth Quarter
|
$33.34
|
$30.76
|
|
2014
|
||
|
First Quarter
|
$32.73
|
$28.77
|
|
Second Quarter
|
$33.05
|
$28.14
|
|
Third Quarter
|
$37.31
|
$31.67
|
|
Fourth Quarter
|
$45.24
|
$34.16
|
|
Issuer Purchases of Equity Securities
|
||||
|
Total Number of Shares Purchased
(a)
|
Average Price Paid Per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Programs
|
Approximate Dollar Value of Shares that May Yet Be Purchased Under Plans or Programs
|
|
|
October 1 - October 31, 2014
|
296
|
$36.45
|
—
|
$500,000,000
|
|
November 1 - November 30, 2014
|
184
|
$38.60
|
—
|
500,000,000
|
|
December 1 - December 31, 2014
|
471
|
$38.28
|
—
|
500,000,000
|
|
Total
|
951
|
$37.77
|
—
|
$500,000,000
|
|
2014
|
2013
|
|
|
First Quarter
|
$0.072
|
$0.065
|
|
Second Quarter
|
$0.072
|
$0.065
|
|
Third Quarter
|
$0.072
|
$0.065
|
|
Fourth Quarter
|
$0.072
|
$0.065
|
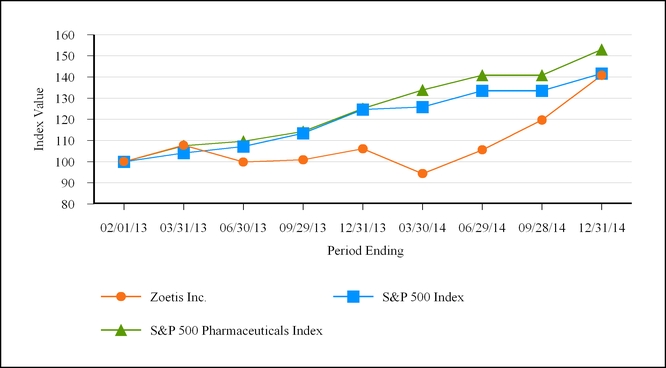
|
February 1, 2013
|
March 31, 2013
|
June 30, 2013
|
September 29, 2013
|
December 31, 2013
|
March 30, 2014
|
June 29, 2014
|
September 28, 2014
|
December 31, 2014
|
|
|
Zoetis Inc.
|
$100
|
$107.71
|
$99.81
|
$100.87
|
$106.07
|
$94.35
|
$105.56
|
$119.67
|
$140.84
|
|
S&P 500
|
$100
|
$104.11
|
$107.14
|
$113.44
|
$124.61
|
$125.86
|
$133.55
|
$135.72
|
$141.67
|
|
S&P 500 Pharmaceuticals Index
|
$100
|
$107.48
|
$109.67
|
$114.24
|
$125.16
|
$133.85
|
$140.83
|
$145.82
|
$152.97
|
|
Year Ended December 31,
(a)
|
||||||||||||||||||||
|
(MILLIONS, EXCEPT PER SHARE AMOUNTS)
|
2014
|
|
2013
|
|
2012
|
|
2011
|
|
2010
|
|
||||||||||
|
Statement of income data:
|
||||||||||||||||||||
|
Revenue
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
$
|
4,233
|
|
$
|
3,582
|
|
|||||
|
Net income attributable to Zoetis
|
583
|
|
504
|
|
436
|
|
245
|
|
110
|
|
||||||||||
|
Balance sheet data:
|
||||||||||||||||||||
|
Total assets
|
$
|
6,607
|
|
$
|
6,558
|
|
$
|
6,262
|
|
$
|
5,711
|
|
$
|
5,284
|
|
|||||
|
Long-term obligations
(b)
|
3,643
|
|
3,642
|
|
509
|
|
575
|
|
673
|
|
||||||||||
|
Other data (unaudited):
|
||||||||||||||||||||
|
Adjusted net income
(c)
|
$
|
790
|
|
$
|
709
|
|
$
|
539
|
|
$
|
503
|
|
$
|
275
|
|
|||||
|
Earnings per share attributable to Zoetis Inc. stockholders
(d)
:
|
||||||||||||||||||||
|
Basic
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
$
|
0.49
|
|
$
|
0.22
|
|
|||||
|
Diluted
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
$
|
0.49
|
|
$
|
0.22
|
|
|||||
|
Weighted average shares outstanding (in thousands):
|
||||||||||||||||||||
|
Basic
|
501,055
|
|
500,002
|
|
500,000
|
|
500,000
|
|
500,000
|
|
||||||||||
|
Diluted
|
502,025
|
|
500,317
|
|
500,000
|
|
500,000
|
|
500,000
|
|
||||||||||
|
(a)
|
Starting in 2011, includes the King Animal Health (KAH), business acquired as part of Pfizer's acquisition of King Pharmaceuticals, Inc., commencing on the acquisition date of January 31, 2011. See
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations—Comparability of historical results and our relationship with Pfizer—Recent significant acquisitions and government-mandated divestitures
.
|
|
(b)
|
In 2010 through 2012, primarily includes an allocation of Pfizer debt that was issued to partially finance the acquisition of Wyeth (including FDAH) in 2009. The debt has been allocated on a pro-rata basis using the deemed acquisition cost of FDAH as a percentage of the total acquisition cost of Wyeth.
|
|
(c)
|
Adjusted net income (a non-GAAP financial measure) is defined as reported net income attributable to Zoetis excluding purchase accounting adjustments, acquisition-related costs and certain significant items. Management uses adjusted net income, among other factors, to set performance goals and to measure the performance of the overall company, as described in
Item 7.
Management’s Discussion and Analysis of Financial Condition and Results of Operations—Adjusted net income
. We believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. Reconciliations of U.S. GAAP reported net income attributable to Zoetis to non-GAAP adjusted net income for the years ended December 31, 2014, 2013 and 2012 are provided in
Item 7.
Management’s Discussion and Analysis of Financial Condition and Results of Operations—Adjusted net income
. The adjusted net income measure is not, and should not be viewed as, a substitute for U.S. GAAP reported net income attributable to Zoetis.
|
|
(d)
|
The weighted average shares outstanding for both basic and diluted earnings per share for the years ended December 31, 2012, 2011 and 2010 was calculated using
500
million shares of common stock outstanding, which was the number of Zoetis Inc. shares outstanding at the time of the IPO, which was completed on February 6, 2013.
|
|
Section
|
Description
|
Page
|
|
Overview of our business
|
A general description of our business and the industry in which we operate. For more information regarding our business and the animal health industry, see
Item 1. Business.
|
|
|
Our operating environment
|
Information regarding the animal health industry and factors that affect our company.
|
|
|
Our growth strategies
|
An explanation of our growth strategies.
|
|
|
Components of revenue and costs and expenses
|
An explanation of the components of our consolidated and combined statements of income.
|
|
|
Comparability of historical results and our relationship with Pfizer
|
Information about the limitations of the predictive value of the consolidated and combined financial statements.
|
|
|
Significant accounting policies and application of critical accounting estimates
|
Accounting policies and estimates that we consider important to understanding our consolidated and combined financial statements.
|
|
|
Analysis of the consolidated and combined statements of income
|
Consists of the following for all periods presented:
|
|
|
•
Revenue
: An analysis of our revenue in total, by operating segment and by species.
|
||
|
•
Costs and expenses
: A discussion about the drivers of our costs and expenses.
|
||
|
•
Operating segment results
: A discussion of our revenue by operating segment and species and items impacting our earnings before income tax.
|
||
|
Adjusted net income
|
A discussion of adjusted net income, an alternative view of performance used by management. Adjusted net income is a non-GAAP financial measure.
|
|
|
Our financial guidance for 2015
|
A discussion of our 2015 financial guidance.
|
|
|
Analysis of the consolidated and combined statements of comprehensive income
|
An analysis of the components of comprehensive income for all periods presented.
|
|
|
Analysis of the consolidated balance sheets
|
A discussion of changes in certain balance sheet accounts for balance sheets presented.
|
|
|
Analysis of the consolidated and combined statements of cash flows
|
An analysis of the drivers of our operating, investing and financing cash flows for all periods presented.
|
|
|
Analysis of financial condition, liquidity and capital resources
|
An analysis of our ability to meet our short-term and long-term financing needs.
|
|
|
New accounting standards
|
Accounting standards that we have recently adopted.
|
|
|
Forward-looking statements and factors that may affect future results
|
A description of the risks and uncertainties that could cause actual results to differ materially from those discussed in forward-looking statements presented in this MD&A and elsewhere in this 2014 Annual Report. Such forward-looking statements are based on management's current expectations about future events, which are inherently susceptible to uncertainty and changes in circumstances.
|
|
|
Years Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
|
13/12
|
||||||||
|
Revenue
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
5
|
|
5
|
|||||
|
Net income attributable to Zoetis
|
583
|
|
504
|
|
436
|
|
16
|
|
16
|
||||||||
|
Adjusted net income
(a)
|
790
|
|
709
|
|
539
|
|
11
|
|
32
|
||||||||
|
(a)
|
Adjusted net income is a non-GAAP financial measure. See the
Adjusted net income
section of this MD&A for more information.
|
|
•
|
human population growth and increasing standards of living, particularly in many emerging markets;
|
|
•
|
increasing demand for improved nutrition, particularly animal protein;
|
|
•
|
natural resource constraints, such as scarcity of arable land, fresh water and increased competition for cultivated land, resulting in fewer resources that will be available to meet this increased demand for animal protein; and
|
|
•
|
increased focus on food safety.
|
|
•
|
economic development and related increases in disposable income, particularly in many emerging markets;
|
|
•
|
increasing pet ownership; and
|
|
•
|
companion animals living longer, increasing medical treatment of companion animals and advances in companion animal medicines and vaccines.
|
|
•
|
leverage our direct local presence and strong customer relationships
—Through our direct selling commercial model, we can deepen our understanding of our customers’ businesses and can encourage the adoption of more sophisticated animal health products;
|
|
•
|
further penetrate emerging markets
—We seek to maximize our presence where economic development is driving increased demand for animal protein and increased demand for and spending on companion animals;
|
|
•
|
pursue new product research and development and value-added product lifecycle development
to extend our product portfolio
—New product R&D and product lifecycle development enable us to deliver innovative products to address unmet needs and evolve our product lines so they remain relevant for our customers. We seek to leverage our strong direct presence in many regions and cost-effectively develop new products;
|
|
•
|
remain the partner of choice
for access to new products and technologies
—We seek to continue to support cutting-edge research and secure the right to develop and commercialize new products and technologies;
|
|
•
|
continue to provide high-quality products
and improve manufacturing production margins
—We believe our manufacturing and supply chain provides us with a global platform for continued expansion, including in emerging markets, and that our quality and reliability differentiate us from our competitors; and
|
|
•
|
expand into complementary businesses
to become a more complete, trusted partner in providing solutions
—We believe we have the potential to generate incremental and complementary revenue, in the areas of diagnostics, genetics, devices, dairy data management, e-learning and professional consulting, which could also enhance the loyalty of our customer base and may lead to increased product sales.
|
|
•
|
for sales returns, we perform calculations in each market that incorporate the following, as appropriate: local returns policies and practices; returns as a percentage of revenue; an understanding of the reasons for past returns; estimated shelf life by product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the estimate of future returns, product recalls, discontinuation of products or a changing competitive environment; and
|
|
•
|
for revenue incentives, we use our historical experience with similar incentives programs to estimate the impact of such programs on revenue.
|
|
•
|
a significant adverse change in the extent or manner in which an asset is used. For example, restrictions imposed by the regulatory authorities could affect our ability to manufacture or sell a product.
|
|
•
|
a projection or forecast that demonstrates losses or reduced profits associated with an asset. This could result, for example, from the introduction of a competitor’s product that results in a significant loss of market share or the inability to achieve the previously projected revenue growth, or from the lack of acceptance of a product by customers.
|
|
•
|
In 2014, the intangible asset impairment charges reflect (i) approximately $6 million of acquired in-process research and development (IPR&D) assets related to a pharmaceutical product for dogs acquired with the FDAH acquisition in 2009, as a result of the termination of the development program due to a re-assessment of economic viability; and (ii) approximately $1 million related to finite-lived developed technology rights and IPR&D due to negative market conditions and the re-assessment of economic viability.
|
|
•
|
In 2013, the intangible asset impairment charges reflect (i) approximately $2 million of finite-lived developed technology rights due to a re-assessment of economic viability; (ii) approximately $2 million of finite-lived developed technology rights and IPR&D as a result of exiting a combined manufacturing and R&D facility; and (iii) approximately $2 million related to acquired IPR&D as a result of the termination of certain development programs due to a re-assessment of their economic viability.
|
|
•
|
In 2012, the intangible asset impairment charges reflect: (i) approximately $2 million of finite-lived companion animal developed technology rights; (ii) approximately $1 million of finite-lived trademarks related to genetic testing services; and (iii) approximately $2 million of finite-lived patents related to poultry technology. The intangible asset impairment charges for 2012 reflect, among other things, loss of revenue as a result of negative market conditions and, with respect to the poultry technology, a re-assessment of economic viability.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
|
13/12
|
|
||||||||
|
Revenue
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
5
|
|
5
|
|
|||||
|
Costs and expenses:
|
||||||||||||||||||
|
Cost of sales
(a)
|
1,717
|
|
1,669
|
|
1,563
|
|
3
|
|
7
|
|
||||||||
|
% of revenue
|
36
|
%
|
37
|
%
|
36
|
%
|
||||||||||||
|
Selling, general and administrative expenses
(a)
|
1,643
|
|
1,613
|
|
1,470
|
|
2
|
|
10
|
|
||||||||
|
% of revenue
|
34
|
%
|
35
|
%
|
34
|
%
|
||||||||||||
|
Research and development expenses
(a)
|
396
|
|
399
|
|
409
|
|
(1
|
)
|
(2
|
)
|
||||||||
|
% of revenue
|
8
|
%
|
9
|
%
|
9
|
%
|
||||||||||||
|
Amortization of intangible assets
|
60
|
|
60
|
|
64
|
|
—
|
|
(6
|
)
|
||||||||
|
Restructuring charges and certain acquisition-related costs
|
25
|
|
26
|
|
135
|
|
(4
|
)
|
(81
|
)
|
||||||||
|
Interest expense, net of capitalized interest
|
117
|
|
113
|
|
31
|
|
4
|
|
*
|
|
||||||||
|
Other (income)/deductions—net
|
7
|
|
(9
|
)
|
(46
|
)
|
*
|
|
(80
|
)
|
||||||||
|
Income before provision for taxes on income
|
820
|
|
690
|
|
710
|
|
19
|
|
(3
|
)
|
||||||||
|
% of revenue
|
17
|
%
|
15
|
%
|
16
|
%
|
||||||||||||
|
Provision for taxes on income
|
233
|
|
187
|
|
274
|
|
25
|
|
(32
|
)
|
||||||||
|
Effective tax rate
|
28.4
|
%
|
27.1
|
%
|
38.6
|
%
|
||||||||||||
|
Net income before allocation to noncontrolling interests
|
587
|
|
503
|
|
436
|
|
17
|
|
15
|
|
||||||||
|
Less: Net income attributable to noncontrolling interests
|
4
|
|
(1
|
)
|
—
|
|
*
|
|
*
|
|
||||||||
|
Net income attributable to Zoetis
|
$
|
583
|
|
$
|
504
|
|
$
|
436
|
|
16
|
|
16
|
|
|||||
|
% of revenue
|
12
|
%
|
11
|
%
|
10
|
%
|
||||||||||||
|
(a)
|
Exclusive of amortization of intangible assets, except as disclosed in Notes to Consolidated and Combined Financial Statements—
Note 4. Significant Accounting Policies—Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
|
13/12
|
||||||||
|
U.S.
|
$
|
2,059
|
|
$
|
1,902
|
|
$
|
1,776
|
|
8
|
|
7
|
|||||
|
EuAfME
|
1,141
|
|
1,115
|
|
1,068
|
|
2
|
|
4
|
||||||||
|
CLAR
|
815
|
|
778
|
|
769
|
|
5
|
|
1
|
||||||||
|
APAC
|
720
|
|
713
|
|
695
|
|
1
|
|
3
|
||||||||
|
Contract Manufacturing
|
50
|
|
53
|
|
28
|
|
(6
|
)
|
89
|
||||||||
|
Total
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
5
|
|
5
|
|||||
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
|
13/12
|
||||||||
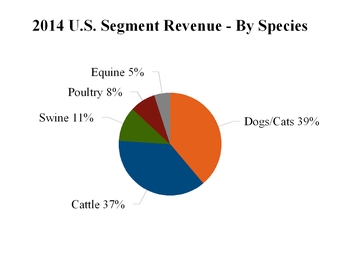
|
Livestock
|
$
|
3,103
|
|
$
|
2,916
|
|
$
|
2,795
|
|
6
|
|
4
|
|||||
|
Companion animal
|
1,632
|
|
1,592
|
|
1,513
|
|
3
|
|
5
|
||||||||
|
Contract Manufacturing
|
50
|
|
53
|
|
28
|
|
(6
|
)
|
89
|
||||||||
|
Total
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
5
|
|
5
|
|||||
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
13/12
|
||||||||
|
Cost of sales
(a)
|
$
|
1,717
|
|
$
|
1,669
|
|
$
|
1,563
|
|
3
|
7
|
|||||
|
% of revenue
|
36
|
%
|
37
|
%
|
36
|
%
|
||||||||||
|
(a)
|
Allocation of corporate enabling functions were: $3 million in 2013 and $1 million in 2012.
|
|
•
|
an increase in sales volume;
|
|
•
|
incremental global manufacturing and supply spending associated with the build-up of our operations in 2013, which is now impacting our 2014 cost of sales; and
|
|
•
|
an increase in inventory obsolescence, scrap and other charges,
|
|
•
|
favorable foreign exchange.
|
|
•
|
revenue growth and product and geographic mix;
|
|
•
|
additional costs of $21 million related to becoming an independent public company, including expense of $2 million due to the accelerated vesting of certain Pfizer equity awards and associated cash payments, as a result of the Separation;
|
|
•
|
a $19 million charge associated with the write-offs of inventory and intercompany accounts that were transferred to us as part of the Separation from Pfizer;
|
|
•
|
higher costs associated with certain manufacturing agreements related to government-mandated divestitures from prior acquisitions; and
|
|
•
|
unfavorable foreign exchange,
|
|
•
|
operational efficiencies; and
|
|
•
|
lower employee benefit costs due to the termination of the defined benefit pension plan for U.S. employees.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
13/12
|
||||||||
|
Selling, general and administrative expenses
(a)
|
$
|
1,643
|
|
$
|
1,613
|
|
$
|
1,470
|
|
2
|
10
|
|||||
|
% of revenue
|
34
|
%
|
35
|
%
|
34
|
%
|
||||||||||
|
(a)
|
Allocation of corporate enabling functions were: $24 million in 2013 and $254 million in 2012.
|
|
•
|
increased field selling and distribution expenses in certain regions due to higher sales and increased costs associated with delivering our products to customers; and
|
|
•
|
additional costs due to the build-up of our supply chain and logistics organization and enabling functions and related costs post-separation from Pfizer,
|
|
•
|
a reduction in the amount of additional costs related to becoming an independent public company, including the nonrecurrence of additional costs in 2013 due to the accelerated vesting of stock options and associated expenses related to certain Pfizer equity awards as a result of the Separation;
|
|
•
|
favorable foreign exchange; and
|
|
•
|
lower direct marketing spending.
|
|
•
|
additional costs of $177 million related to becoming an independent public company, including expense of $25 million due to the accelerated vesting of certain Pfizer equity awards and associated cash payments, as a result of the Separation;
|
|
•
|
a $5 million charge associated with the write-offs of intercompany accounts that were transferred to us as part of the Separation from Pfizer; and
|
|
•
|
increased distribution expenses due to higher sales and increased temperature-controlled supply chain
costs in certain regions,
|
|
•
|
lower employee benefit costs due to the termination of the defined benefit pension plan for U.S. employees;
|
|
•
|
lower bad debt expense associated with improved accounts receivable collection experience; and
|
|
•
|
favorable foreign exchange.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
|
13/12
|
|
||||||||
|
Research and development expenses
(a)
|
$
|
396
|
|
$
|
399
|
|
$
|
409
|
|
(1
|
)
|
(2
|
)
|
|||||
|
% of revenue
|
8
|
%
|
9
|
%
|
9
|
%
|
||||||||||||
|
(a)
|
Allocation of corporate enabling functions were $55 million in 2012.
|
|
•
|
the nonrecurrence of additional costs in 2013 due to the accelerated vesting of stock options and associated expenses related to certain Pfizer equity awards as a result of the Separation;
|
|
•
|
savings associated with the closure of two R&D sites; and
|
|
•
|
favorable foreign exchange,
|
|
•
|
higher salary-related expenses.
|
|
•
|
the non-recurrence of depreciation expense incurred in 2012 related to the closing of an R&D facility in the UK; and
|
|
•
|
lower employee benefit costs due to the termination of the defined benefit pension plan for U.S. employees,
|
|
•
|
incremental costs of $7 million related to becoming an independent public company, including expense of $4 million due to the accelerated vesting of certain Pfizer equity awards and associated cash payments, as a result of the Separation; and
|
|
•
|
an increase in the volume of R&D activities.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
13/12
|
|
||||||||
|
Amortization of intangible assets
|
$
|
60
|
|
$
|
60
|
|
$
|
64
|
|
—
|
(6
|
)
|
|||||
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
|
13/12
|
|
||||||||
|
Restructuring charges and certain acquisition-related costs
(a)
|
$
|
25
|
|
$
|
26
|
|
$
|
135
|
|
(4
|
)
|
(81
|
)
|
|||||
|
(a)
|
Allocation of
Restructuring charges and certain acquisition-related costs
were $57 million in 2012.
|
|
•
|
a decrease in asset impairment charges due to the exiting of one of our manufacturing facilities in 2013; and
|
|
•
|
a decrease in integration costs related to the KAH and FDAH acquisitions,
|
|
•
|
an increase in employee termination costs primarily due to a reversal in 2013 related to a previously established termination reserve that was reversed in the second quarter of 2013 related to our operations in Europe.
|
|
•
|
a $27 million decrease in employee termination costs related to the reversal of a previously established termination reserve related to our operations in Europe;
|
|
•
|
a decrease in integration and restructuring costs related to the KAH and FDAH acquisitions; and
|
|
•
|
the non-recurrence of allocated charges from Pfizer,
|
|
•
|
asset impairment charges of approximately $17 million related to one of our manufacturing facilities in the United States; and
|
|
•
|
employee termination costs of $2 million, exit costs of $4 million, and accelerated depreciation of $5 million as a result of exiting certain manufacturing and research facilities.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
13/12
|
||||||||
|
Interest expense, net of capitalized interest
|
$
|
117
|
|
$
|
113
|
|
$
|
31
|
|
4
|
*
|
|||||
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
13/12
|
||||||||
|
Other (income)/deductions—net
|
$
|
7
|
|
$
|
(9
|
)
|
$
|
(46
|
)
|
*
|
*
|
|||||
|
•
|
a charge associated with a commercial settlement and recall in Mexico of $13 million, partially offset by the related insurance recovery of $1 million;
|
|
•
|
higher foreign currency losses of $8 million, primarily driven by costs related to hedging and exposures to certain emerging market currencies, as well as losses related to the depreciation of the Argentine peso in the first quarter of 2014;
|
|
•
|
an impairment charge related to IPR&D assets acquired with the FDAH acquisition in 2009, as a result of the termination of the development program due to a re-assessment of economic viability; and
|
|
•
|
a pension plan settlement charge related to the divestiture of a manufacturing facility,
|
|
•
|
an insurance recovery of litigation related charges.
|
|
•
|
the non-recurrence of income recognized in 2012 from a favorable legal settlement of $14 million and the non-recurrence of a favorable change in estimate for an environmental-related reserve of $7 million in 2012;
|
|
•
|
foreign currency loss of $9 million related to the Venezuela currency devaluation in February 2013; and
|
|
•
|
other foreign currency losses primarily related to Argentina,
|
|
•
|
a net gain on the government-mandated sale of certain product rights in Brazil that were acquired with the FDAH acquisition in 2009; and
|
|
•
|
lower asset impairment charges of identifiable intangible assets of approximately $4 million.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
13/12
|
|
||||||||
|
Provision for taxes on income
|
$
|
233
|
|
$
|
187
|
|
$
|
274
|
|
25
|
(32
|
)
|
|||||
|
Effective tax rate
|
28.4
|
%
|
27.1
|
%
|
38.6
|
%
|
|||||||||||
|
•
|
the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures;
|
|
•
|
changes in valuation allowances and resolution of other tax items;
|
|
•
|
the tax cost related to changes in uncertain tax positions, see Notes to Consolidated and Combined Financial Statements—
Note 8D. Tax Matters
—
Tax Contingencies
; and
|
|
•
|
an $8 million discrete tax expense during the first quarter of 2014 related to an intercompany inventory adjustment.
|
|
•
|
the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures;
|
|
•
|
incentive tax rulings in Belgium, effective December 1, 2012 through 2017, and Singapore, effective October 29, 2012 through 2016. These incentive tax rulings may be extended for another 5 and 6 years, respectively, if certain requirements are met; and
|
|
•
|
a $2 million discrete income tax benefit during the first quarter of 2013 related to the 2012 U.S. Research and Development Tax Credit which was retroactively extended on January 3, 2013,
|
|
•
|
the tax cost related to changes in uncertain tax positions, see Notes to Consolidated and Combined Financial Statements—
Note 8D. Tax Matters
—
Tax Contingencies
.
|
|
% Change
|
|||||||||||||||||||||||||
|
14/13
|
13/12
|
||||||||||||||||||||||||
|
Related to
|
Related to
|
||||||||||||||||||||||||
|
Year Ended December 31,
|
Foreign
|
|
Foreign
|
|
|||||||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
Total
|
|
Exchange
|
|
Operational
|
|
Total
|
Exchange
|
|
Operational
|
|||||||||
|
U.S.
|
|||||||||||||||||||||||||
|
Livestock
|
$
|
1,163
|
|
$
|
1,034
|
|
$
|
966
|
|
12
|
|
—
|
|
12
|
|
7
|
—
|
|
7
|
||||||
|
Companion animal
|
896
|
|
868
|
|
810
|
|
3
|
|
—
|
|
3
|
|
7
|
—
|
|
7
|
|||||||||
|
2,059
|
|
1,902
|
|
1,776
|
|
8
|
|
—
|
|
8
|
|
7
|
—
|
|
7
|
||||||||||
|
EuAfME
|
|||||||||||||||||||||||||
|
Livestock
|
772
|
|
762
|
|
729
|
|
1
|
|
(1
|
)
|
2
|
|
5
|
1
|
|
4
|
|||||||||
|
Companion animal
|
369
|
|
353
|
|
339
|
|
5
|
|
1
|
|
4
|
|
4
|
1
|
|
3
|
|||||||||
|
1,141
|
|
1,115
|
|
1,068
|
|
2
|
|
—
|
|
2
|
|
4
|
1
|
|
3
|
||||||||||
|
CLAR
|
|||||||||||||||||||||||||
|
Livestock
|
633
|
|
605
|
|
603
|
|
5
|
|
(8
|
)
|
13
|
|
—
|
(6
|
)
|
6
|
|||||||||
|
Companion animal
|
182
|
|
173
|
|
166
|
|
5
|
|
(8
|
)
|
13
|
|
4
|
(5
|
)
|
9
|
|||||||||
|
815
|
|
778
|
|
769
|
|
5
|
|
(8
|
)
|
13
|
|
1
|
(5
|
)
|
6
|
||||||||||
|
APAC
|
|||||||||||||||||||||||||
|
Livestock
|
535
|
|
515
|
|
497
|
|
4
|
|
(4
|
)
|
8
|
|
4
|
(4
|
)
|
8
|
|||||||||
|
Companion animal
|
185
|
|
198
|
|
198
|
|
(7
|
)
|
(5
|
)
|
(2
|
)
|
—
|
(7
|
)
|
7
|
|||||||||
|
720
|
|
713
|
|
695
|
|
1
|
|
(4
|
)
|
5
|
|
3
|
(4
|
)
|
7
|
||||||||||
|
Total
|
|||||||||||||||||||||||||
|
Livestock
|
3,103
|
|
2,916
|
|
2,795
|
|
6
|
|
(3
|
)
|
9
|
|
4
|
(2
|
)
|
6
|
|||||||||
|
Companion animal
|
1,632
|
|
1,592
|
|
1,513
|
|
3
|
|
(1
|
)
|
4
|
|
5
|
(1
|
)
|
6
|
|||||||||
|
Contract manufacturing
|
50
|
|
53
|
|
28
|
|
(6
|
)
|
(1
|
)
|
(5
|
)
|
89
|
3
|
|
86
|
|||||||||
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
5
|
|
(2
|
)
|
7
|
|
5
|
(2
|
)
|
7
|
|||||||
|
% Change
|
|||||||||||||||||||||
|
14/13
|
13/12
|
||||||||||||||||||||
|
Related to
|
Related to
|
||||||||||||||||||||
|
Year Ended December 31,
|
Foreign
|
|
Foreign
|
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
Total
|
|
Exchange
|
|
Operational
|
Total
|
|
Exchange
|
|
Operational
|
|||||
|
U.S.
|
$
|
1,176
|
|
$
|
1,045
|
|
$
|
921
|
|
13
|
|
—
|
|
13
|
13
|
|
—
|
|
13
|
||
|
EuAfME
|
437
|
|
412
|
|
376
|
|
6
|
|
(1
|
)
|
7
|
10
|
|
2
|
|
8
|
|||||
|
CLAR
|
310
|
|
266
|
|
253
|
|
17
|
|
—
|
|
17
|
5
|
|
(11
|
)
|
16
|
|||||
|
APAC
|
278
|
|
271
|
|
236
|
|
3
|
|
(6
|
)
|
9
|
15
|
|
(3
|
)
|
18
|
|||||
|
Total reportable segments
|
2,201
|
|
1,994
|
|
1,786
|
|
10
|
|
(2
|
)
|
12
|
12
|
|
(1
|
)
|
13
|
|||||
|
Other business activities
|
(314
|
)
|
(312
|
)
|
(276
|
)
|
1
|
|
13
|
|
|||||||||||
|
Reconciling Items:
|
|||||||||||||||||||||
|
Corporate
|
(571
|
)
|
(567
|
)
|
(506
|
)
|
1
|
|
12
|
|
|||||||||||
|
Purchase accounting adjustments
|
(51
|
)
|
(48
|
)
|
(52
|
)
|
6
|
|
(8
|
)
|
|||||||||||
|
Acquisition-related costs
|
(8
|
)
|
(22
|
)
|
(53
|
)
|
(64
|
)
|
(58
|
)
|
|||||||||||
|
Certain significant items
|
(205
|
)
|
(240
|
)
|
(96
|
)
|
(15
|
)
|
*
|
|
|||||||||||
|
Other unallocated
|
(232
|
)
|
(115
|
)
|
(93
|
)
|
*
|
|
24
|
|
|||||||||||
|
Income before income taxes
|
$
|
820
|
|
$
|
690
|
|
$
|
710
|
|
19
|
|
(3
|
)
|
||||||||
|
•
|
Livestock revenue growth was driven by increased sales across the cattle, swine, and poultry portfolios. Strong growth in sales of cattle products was primarily due to higher demand for our premium products as a result of improved market conditions, driven by higher cattle prices and lower costs of feed, compared with 2013. Growth in swine products was due to the successful launch of new products, tempered by the impact of PEDv on the number of treatable animals. Sales of poultry products benefited from new vaccines and growth in medicated feed additives.
|
|
•
|
Companion animal revenue growth was driven by the introduction of Apoquel
®
as well as sales growth in other key brands. Results were partially offset by competitive pressures in our vaccine and pain portfolios and were tempered by competition in our parasiticides portfolio.
|
|
•
|
Livestock revenue growth was primarily driven by higher sales in the cattle portfolio, particularly in emerging markets, driven by strong performance of our anti-infectives portfolio and the introduction of new products. Additionally, sales in the poultry portfolio increased due to strong performance in our vaccine portfolio and improved market conditions in several Middle Eastern markets.
|
|
•
|
Companion animal revenue growth was favorably impacted by the successful launch of Apoquel
®
in Germany and the UK.
|
|
•
|
Livestock revenue growth was primarily driven by increased sales in the cattle and swine portfolios, particularly in Venezuela, Brazil and Canada. Livestock sales were also favorably impacted by price increases in high inflationary markets such as Venezuela and Argentina.
|
|
•
|
Companion animal growth was favorably impacted by increased sales in Venezuela and Brazil, as well as higher prices in Argentina and Canada.
|
|
•
|
Livestock revenue growth was primarily driven by increased sales in the swine portfolio in China. Additionally, there was growth in sales of cattle products in China and Australia.
|
|
•
|
The decrease in companion animal revenue was primarily due to a decrease in sales in Japan due to an inventory buyback related to the termination of a distributor agreement and unfavorable market conditions. Results were partially offset by an increase in equine product sales in Australia and an increase in small animal product sales in China.
|
|
•
|
Livestock revenue growth was achieved in all species. The growth in swine products was due to continued customer acceptance of new products and the successful execution of marketing programs developed for and focused on specific brands, therapeutic categories or customer segments. Growth in sales of poultry products was due to growth in medicated feed additives, and growth in sales of cattle products was driven by improved market conditions in the second half of 2013.
|
|
•
|
Companion animal revenue growth was driven by solid growth in small animal products reflecting the benefit of realigning our field force in late 2012 to more effectively cover our customer base, the positive outcomes of new cross-portfolio pricing programs, and price increases. Growth was slightly offset by a decline in the sales of equine products reflecting a continuing contraction of the market.
|
|
•
|
Livestock revenue growth was primarily driven by emerging markets, particularly Russia. Additionally, growth in swine products was favorably impacted by the launch of a new swine vaccine (that prevents porcine circovirus type 2) across many markets in the region, particularly in Germany and Russia. This growth was partially offset by continuing challenging market conditions throughout Western Europe affecting the cattle portfolio.
|
|
•
|
Companion animal revenue growth was favorably impacted by increased sales of products that are related to certain third-party manufacturing agreements. Additionally, sales in the UK and France increased due to the benefit of increased promotional programs. Results were partially offset by continuing adverse macroeconomic conditions throughout Western Europe.
|
|
•
|
Livestock revenue growth was primarily driven by increased sales in the poultry and cattle portfolios. Growth in sales of poultry products was primarily driven by higher sales of medicated feed additives in Brazil. Increased cattle product sales were primarily due to growth in Mexico, Canada and Venezuela. This growth was partially offset by challenging market conditions affecting the cattle market in Brazil, where sales were relatively flat primarily due to increased local competition and drought conditions in certain areas of the country.
|
|
•
|
Companion animal growth was favorably impacted by an increasing companion animal market in Brazil and marketing programs in Brazil and Mexico. Gains were partially offset by lower sales of equine products as a result of a reduced number of horses in Canada.
|
|
•
|
Livestock revenue growth was driven primarily by increased sales in emerging markets across swine, poultry and cattle. Growth in sales of swine products was driven by higher demand and market penetration in China, as well as good performance in Japan which benefited from recently launched vaccines. Growth in the poultry and cattle portfolios was primarily driven by increased sales in India. Results were tempered by flat growth in Australia and New Zealand due to the impact of prolonged drought conditions on cattle and sheep herd sizes.
|
|
•
|
Companion animal revenue growth was primarily due to the successful launch of new products in Japan. Results were partially offset by declines in equine product sales in Australia due to increased competition.
|
|
•
|
Corporate,
which includes costs associated with business technology, facilities, legal, finance, human resources, business development, public affairs and procurement, among others. These costs also include compensation costs and other miscellaneous operating expenses not charged to our operating segments, as well as interest income and expense;
|
|
•
|
Certain transactions and events such as (i)
Purchase accounting adjustments
, which includes expenses associated with the amortization of fair value adjustments to inventory, intangible assets and property, plant and equipment; (ii)
Acquisition-related activities
, which includes costs for restructuring and integration; and (iii)
Certain significant items
, which includes non-acquisition-related restructuring charges, certain asset impairment charges and costs associated with cost reduction/productivity initiatives; and
|
|
•
|
Other unallocated
, which includes certain overhead expenses associated with our global manufacturing operations not charged to our operating segments. Effective January 1, 2014,
Other unallocated
also includes certain costs associated with business technology and finance that specifically support our global manufacturing operations. These costs were previously reported in
Corporate
. Also, beginning in the first quarter of 2014, certain supply chain and global logistics costs that were previously reported in the four reportable segments are reported in
Other unallocated
. This presentation better reflects how we measure the performance of the global manufacturing organization.
|
|
•
|
senior management receives a monthly analysis of our operating results that is prepared on an adjusted net income basis;
|
|
•
|
our annual budgets are prepared on an adjusted net income basis; and
|
|
•
|
other goal setting and performance measurements.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
2013
|
2012
|
14/13
|
|
13/12
|
|
|||||||||||
|
GAAP Reported net income attributable to Zoetis
|
$
|
583
|
|
$
|
504
|
|
$
|
436
|
|
16
|
|
16
|
|
|||||
|
Purchase accounting adjustments—net of tax
|
34
|
|
32
|
|
35
|
|
6
|
|
(9
|
)
|
||||||||
|
Acquisition-related costs—net of tax
|
5
|
|
14
|
|
34
|
|
(64
|
)
|
(59
|
)
|
||||||||
|
Certain significant items—net of tax
|
168
|
|
159
|
|
34
|
|
6
|
|
*
|
|
||||||||
|
Non-GAAP adjusted net income
(a)(b)
|
$
|
790
|
|
$
|
709
|
|
$
|
539
|
|
11
|
|
32
|
|
|||||
|
(a)
|
The effective tax rate on adjusted pretax income is
26.8%
,
29.2%
and
40.8%
for full year 2014, 2013 and 2012, respectively. The lower effective tax rate in 2014 compared to 2013 is primarily due to changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs, changes in valuation allowances and resolution of other tax items. In addition, we recognized an $8 million discrete tax expense during the first quarter of 2014 related to an intercompany inventory adjustment. The
lower
effective tax rate in 2013 compared to 2012 is primarily due to incentive tax rulings in Belgium, effective December 1, 2012, and Singapore, effective October 29, 2012, as well as changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs. In addition, we recognized a $2 million discrete income tax benefit during the first quarter of 2013 related to the 2012 U.S. Research and Development Tax Credit which was retroactively extended on January 3, 2013.
|
|
(b)
|
The impact of the incentive tax rulings in Belgium, effective December 1, 2012 through 2017, and Singapore, effective October 29, 2012 through 2016, continue to be a component of the 2014 effective tax in rate, as well as the 2014 U.S. Research and Development Tax Credit which was extended on December 19, 2014.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
2014
|
2013
|
2012
|
14/13
|
|
13/12
|
|
||||||||||||
|
Earnings per share—diluted
(a)(b)
:
|
||||||||||||||||||
|
GAAP Reported net income attributable to Zoetis
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
15
|
|
16
|
|
|||||
|
Purchase accounting adjustments—net of tax
|
0.07
|
|
0.06
|
|
0.07
|
|
17
|
|
(14
|
)
|
||||||||
|
Acquisition-related costs—net of tax
|
0.01
|
|
0.03
|
|
0.07
|
|
(67
|
)
|
(57
|
)
|
||||||||
|
Certain significant items—net of tax
|
0.33
|
|
0.32
|
|
0.07
|
|
3
|
|
*
|
|
||||||||
|
Non-GAAP adjusted net income
|
$
|
1.57
|
|
$
|
1.42
|
|
$
|
1.08
|
|
11
|
|
31
|
|
|||||
|
(a)
|
The weighted-average shares outstanding for diluted earnings per share for the period prior to the IPO was calculated using an aggregate of 500 million shares of common stock outstanding, which was the number of Zoetis Inc. shares outstanding immediately prior to the IPO. For the years ended
December 31, 2014
and 2013, diluted earnings per share was computed using the weighted-average common shares outstanding during the period plus the common stock equivalents related to stock options, RSUs and DSUs.
|
|
(b)
|
EPS amounts may not add due to rounding.
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Interest expense, net of capitalized interest
|
$
|
117
|
|
$
|
113
|
|
$
|
31
|
|
|||
|
Interest income
|
6
|
|
3
|
|
1
|
|
||||||
|
Taxes
|
290
|
|
292
|
|
372
|
|
||||||
|
Depreciation
|
131
|
|
138
|
|
119
|
|
||||||
|
Amortization
|
17
|
|
17
|
|
18
|
|
||||||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Purchase accounting adjustments:
|
||||||||||||
|
Amortization and depreciation
(a)
|
$
|
47
|
|
$
|
46
|
|
$
|
48
|
|
|||
|
Cost of sales
(b)
|
4
|
|
2
|
|
4
|
|
||||||
|
Total purchase accounting adjustments—pretax
|
51
|
|
48
|
|
52
|
|
||||||
|
Income taxes
(c)
|
17
|
|
16
|
|
17
|
|
||||||
|
Total purchase accounting adjustments—net of tax
|
34
|
|
32
|
|
35
|
|
||||||
|
Acquisition-related costs
(d)
:
|
||||||||||||
|
Integration costs
(e)
|
8
|
|
22
|
|
47
|
|
||||||
|
Restructuring charges
(e)
|
—
|
|
—
|
|
(4
|
)
|
||||||
|
Additional depreciation—asset restructuring
(f)
|
—
|
|
—
|
|
10
|
|
||||||
|
Total acquisition-related costs—pretax
|
8
|
|
22
|
|
53
|
|
||||||
|
Income taxes
(c)
|
3
|
|
8
|
|
19
|
|
||||||
|
Total acquisition-related costs—net of tax
|
5
|
|
14
|
|
34
|
|
||||||
|
Certain significant items
(g)
:
|
||||||||||||
|
Restructuring charges (benefits)
(h)
|
17
|
|
(20
|
)
|
92
|
|
||||||
|
Implementation costs and additional depreciation--asset restructuring
(f)
|
1
|
|
8
|
|
23
|
|
||||||
|
Certain asset impairment charges
(i)
|
6
|
|
20
|
|
—
|
|
||||||
|
Net gains on sale of assets
(j)
|
(5
|
)
|
(6
|
)
|
—
|
|
||||||
|
Stand-up costs
(k)
|
168
|
|
206
|
|
—
|
|
||||||
|
Inventory and intercompany account write-offs
(l)
|
—
|
|
24
|
|
—
|
|
||||||
|
Other
(m)
|
18
|
|
8
|
|
(19
|
)
|
||||||
|
Total certain significant items—pretax
|
205
|
|
240
|
|
96
|
|
||||||
|
Income taxes
(c)
|
37
|
|
81
|
|
62
|
|
||||||
|
Total certain significant items—net of tax
|
168
|
|
159
|
|
34
|
|
||||||
|
Total purchase accounting adjustments, acquisition-related costs, and certain significant items—net of tax
|
$
|
207
|
|
$
|
205
|
|
$
|
103
|
|
|||
|
(a)
|
Amortization and depreciation expense related to purchase accounting adjustments with respect to identifiable intangible assets and property, plant and equipment were distributed as follows in 2014, 2013 and 2012, respectively: $45 million, $46 million and $49 million included in
Amortization of intangible assets;
$0 million, $1 million income and $1 million income included in
Selling, general and administrative expenses;
and
$2 million, $1 million and $0 million included in
Research and development expenses.
|
|
(b)
|
Depreciation expense included in
Cost of sales.
|
|
(c)
|
Included in
Provision for taxes on income
.
|
|
(d)
|
Acquisition-related costs were distributed as follows in 2014, 2013 and 2012, respectively: $0 million, $0 million and $9 million included in
Cost of sales
; $0 million, $0 million and $1 million included in
Selling, general and administrative expenses;
and
$8 million, $22 million and $43 million included in
Restructuring charges and certain acquisition-related costs.
|
|
(e)
|
Included in
Restructuring charges and certain acquisition-related costs.
See Notes to Consolidated and Combined Financial Statements
—Note 6. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
for more information.
|
|
(f)
|
Amounts primarily relate to our cost-reduction/productivity initiatives and other asset restructuring. See Notes to Consolidated and Combined Financial Statements—
Note 6.
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
.
|
|
(g)
|
Certain significant items were distributed as follows in 2014, 2013 and 2012, respectively: $33 million, $42 million and $1 million included in
Cost of sales
; $136 million, $188 million and $18 million included in
Selling, general and administrative expenses
; $1 million, $7 million and $10 million included in
Research and development expenses
; $17 million, $4 million and $92 million, included in
Restructuring charges and certain acquisition-related costs
; and $18 million, $1 million income and $25 million income included in
Other (income)/deductions—net.
|
|
(h)
|
Represents restructuring charges incurred for our cost-reduction/productivity initiatives. The restructuring charges in 2014 primarily represent employee severance costs in Europe and our global manufacturing operations. The restructuring benefit in the year ended December 31, 2013, is primarily due to a $27 million decrease in employee termination expenses related to the reversal of a previously established termination reserve related to our operations in Europe. Included in
Restructuring charges and certain acquisition-related costs
. See Notes to Consolidated and Combined Financial Statements—
Note 6.
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
for more information.
|
|
(i)
|
In 2014, amounts primarily represent an impairment charge related to an IPR&D project acquired with the FDAH acquisition in 2009 and were included in
Other (income)/deductions—net
.
In 2013, amounts primarily relate to restructuring initiatives in 2013 and were included in
Restructuring charges and certain acquisition-related costs
. See Notes to Consolidated and Combined Financial Statements—
Note 6.
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
and
Note 7. Other (Income)/Deductions—Net
for more information
.
|
|
(j)
|
In 2014, primarily represents the Zoetis portion of a net gain on the sale of land by our Taiwan joint venture and the net gain on the government-mandated sale of certain product rights in Argentina that were acquired with the FDAH acquisition in 2009. In 2013, represents the net gain on the government-mandated sale of certain product rights in Brazil in 2013 that were acquired with the FDAH acquisition in 2009. Included in
Other (income)/deductions—net
. See Notes to Consolidated and Combined Financial Statements—
Note 7. Other (Income)/Deductions—Net
for more information.
|
|
(k)
|
Certain non-recurring costs related to becoming an independent public company, such as new branding (including changes to the manufacturing process for required new packaging), the creation of standalone systems and infrastructure, site separation, accelerated vesting and associated cash payment related to certain Pfizer equity awards, and certain legal registration and patent assignment costs which were distributed as follows in 2014 and 2013, respectively: $32 million and $21 million included in
Cost of sales
; $131 million and $177 million included in
Selling, general and administrative expenses
, $0 million and $7 million included in
Research and development expenses,
and $5 million and $1 million included in
Other (income)/deductions—net.
|
|
(l)
|
Amounts relate to write-offs of inventory and intercompany accounts that were transferred to us as part of the Separation from Pfizer and were distributed as follows: $19 million included in
Cost of sales
and $5 million included in
Selling, general and administrative expenses
. Because these expenses relate primarily to the periods prior to our initial public offering, we do not consider them to be reflective of our current operations and we have therefore, excluded them from our Adjusted earnings non-GAAP measure. Although fully written off in the current period, all of the adjustments relate back several years.
|
|
(m)
|
For 2014, primarily includes a charge associated with a commercial settlement in Mexico ($13 million), partially offset by the insurance recovery ($1 million income), charges due to unusual investor-related activities ($5 million), a pension plan settlement charge related to the divestiture of a manufacturing plant ($4 million), and an insurance recovery of other litigation related charge ($2 million income). For 2013, primarily relates to litigation-related charges ($5 million) and charges related to transitional manufacturing purchase agreements associated with divestitures ($1 million). For 2012, primarily relates to income related to a favorable legal settlement for an intellectual property matter ($14 million) and income due to a change in estimate related to transitional manufacturing purchase agreements associated with divestitures ($4 million). See Notes to Consolidated and Combined Financial Statements—
Note 7. Other (Income)/Deductions—Net
for more information
.
|
|
Selected Line Items
|
||
|
Revenue
|
$4,800 to $4,900 million
|
|
|
Operational growth
(a)
|
6.5% to 8.5%
|
|
|
Adjusted cost of sales as a percentage of revenue
(b)
|
35.5% to 36.0%
|
|
|
Adjusted SG&A expenses
(b)
|
$1,420 to $1,470 million
|
|
|
Adjusted R&D expenses
(b)
|
$385 to $405 million
|
|
|
Adjusted interest expense and other (income)/deductions
(b)
|
Approximately $110 million
|
|
|
Effective tax rate on adjusted income
(b)
|
Approximately 29%
|
|
|
Adjusted diluted EPS
(b)
|
$1.61 to $1.68
|
|
|
Adjusted net income
(b)
|
$810 to $845 million
|
|
|
Operational growth
(a)
|
11% to 16%
|
|
|
Certain significant items
(c)
and acquisition-related costs
|
$140 to $160 million
|
|
|
Reported diluted EPS
|
$1.32 to $1.39
|
|
|
(a)
|
Growth excluding the impact of foreign exchange.
|
|
(b)
|
For an understanding of adjusted net income and its components, see the
Adjusted net income
section of this MD&A.
|
|
(c)
|
Includes certain nonrecurring costs related to becoming an independent public company, such as new branding (including changes to the manufacturing process for required new packaging), the creation of standalone systems and infrastructure, site separation and certain legal registration and patent assignment costs.
|
|
Full Year 2015 Guidance
|
||||
|
(MILLION OF DOLLARS, EXCEPT PER SHARE AMOUNTS)
|
Net Income
|
Diluted EPS
|
||
|
Adjusted net income/diluted EPS
(a)
guidance
|
~$810 - $845
|
~$1.61 - $1.68
|
||
|
Purchase accounting adjustments
|
~(35)
|
~(0.07)
|
||
|
Certain significant items
(b)
and acquisition-related costs
|
~(105 - 120)
|
~(0.21 - 0.24)
|
||
|
Reported net income attributable to Zoetis Inc./diluted EPS guidance
|
~$665 - $700
|
~$1.32 - $1.39
|
||
|
(a)
|
For an understanding of adjusted net income, see the
Adjusted net income
section of this MD&A.
|
|
(b)
|
Includes certain nonrecurring costs related to becoming an independent public company, such as new branding (including changes to the manufacturing process for required new packaging), the creation of standalone systems and infrastructure, site separation and certain legal registration and patent assignment costs.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
14/13
|
|
13/12
|
||||||||
|
Cash provided by/(used in):
|
|||||||||||||||||
|
Operating activities
|
$
|
626
|
|
$
|
681
|
|
$
|
454
|
|
(8
|
)
|
50
|
|||||
|
Investing activities
|
(187
|
)
|
(179
|
)
|
(135
|
)
|
4
|
|
33
|
||||||||
|
Financing activities
|
(154
|
)
|
(200
|
)
|
(78
|
)
|
(23
|
)
|
*
|
||||||||
|
Effect of exchange-rate changes on cash and cash equivalents
|
(13
|
)
|
(9
|
)
|
(3
|
)
|
44
|
|
*
|
||||||||
|
Net increase in cash and cash equivalents
|
$
|
272
|
|
$
|
293
|
|
$
|
238
|
|
(7
|
)
|
23
|
|||||
|
December 31,
|
|
December 31,
|
|
||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
|||
|
Cash and cash equivalents
(a)
|
$
|
882
|
|
$
|
610
|
|
|
|
Accounts receivable, net
(b)
|
980
|
|
1,138
|
|
|||
|
Short-term borrowings
|
7
|
|
15
|
|
|||
|
Long-term debt
(c)
|
3,643
|
|
3,642
|
|
|||
|
Working capital
|
2,379
|
|
1,942
|
|
|||
|
Ratio of current assets to current liabilities
|
3.19:1
|
|
2.37:1
|
|
|||
|
(a)
|
Prior to our IPO, we participated in Pfizer's centralized cash management system, and generally all of our excess cash was transferred to Pfizer on a daily basis. Cash disbursements for operations and/or investing activities were funded, as needed, by Pfizer.
|
|
(b)
|
Accounts receivable are usually collected over a period of 60 to 90 days
.
For the year ended
December 31, 2014
, compared to the year ended December 31, 2013, the number of days that accounts receivables are outstanding remained approximately the same. We regularly monitor our accounts receivable for collectability, particularly in markets where economic conditions remain uncertain. We believe that our allowance for doubtful accounts is appropriate. Our assessment is based on such factors as past due aging, historical and expected collection patterns, the financial condition of our customers, the robust nature of our credit and collection practices and the economic environment.
|
|
(c)
|
Primarily consists of
$3.65 billion
aggregate principal amount of our senior notes, with an original issue discount of
$10 million
. The senior notes are comprised of
$400 million
aggregate principal amount of our
1.150%
senior notes due 2016,
$750 million
aggregate principal amount of our
1.875%
senior notes due 2018,
$1.35 billion
aggregate principal amount of our
3.250%
Senior Notes due 2023 and
$1.15 billion
aggregate principal amount of our
4.700%
senior notes due 2043.
|
|
2016-
|
|
2018-
|
|
There-
|
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
Total
|
|
2015
|
|
2017
|
|
2019
|
|
after
|
|
||||||||||
|
Long-term debt, including current portion and interest obligations
(a)
|
$
|
5,574
|
|
$
|
117
|
|
$
|
627
|
|
$
|
947
|
|
$
|
3,883
|
|
|||||
|
Other long-term liabilities reflected on our consolidated and combined
|
||||||||||||||||||||
|
balance sheets under U.S. GAAP
(b)
|
47
|
|
4
|
|
8
|
|
9
|
|
26
|
|
||||||||||
|
Operating lease commitments
|
104
|
|
27
|
|
40
|
|
20
|
|
17
|
|
||||||||||
|
Purchase obligations and other
(c)
|
81
|
|
34
|
|
25
|
|
10
|
|
12
|
|
||||||||||
|
Benefit plans - continuing service credit obligations
(d)
|
30
|
|
4
|
|
8
|
|
8
|
|
10
|
|
||||||||||
|
Uncertain tax positions
(e)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
(a)
|
Long-term debt consists of senior notes and other notes. Our calculations of expected interest payments incorporate only current period assumptions for interest rates, foreign currency translation rates and Zoetis hedging strategies. See Notes to Consolidated and Combined Financial Statements—
|
|
(b)
|
Includes expected payments to Pfizer related to the transfer of certain product registration and application rights associated with our operations in Indonesia, expected payments related to our unfunded U.S. supplemental (non-qualified) savings plans, deferred compensation and expected payments relating to our future benefit payments net of plan assets (included in the determination of the projected benefit obligation) for pension plans that are dedicated to Zoetis employees and those transferred to us from Pfizer in 2014 and 2013. Excludes the pension obligation associated with a defined benefit plan in the Philippines that Pfizer will transfer to us in 2015 as described in the applicable local separation agreement or employee matters agreement. See Notes to Consolidated and Combined Financial Statements—
Note 13. Benefit Plans
and
Note 19B. Transactions and Agreements with Pfizer—Agreements with Pfizer—Employee matters agreement.
Excludes approximately $160 million of noncurrent liabilities related to legal and environmental accruals, employee termination and exit costs, deferred income and other accruals, most of which do not represent contractual obligations. See Notes to Consolidated and Combined Financial Statements—
Note 6
.
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
and
Note 17
.
Commitments and Contingencies
.
|
|
(c)
|
Includes agreements to purchase goods and services that are enforceable and legally binding and includes amounts relating to advertising, information technology services and employee benefit administration services.
|
|
(d)
|
Includes the cost of service credit continuation for certain Zoetis employees in the Pfizer U.S. qualified defined benefit pension and U.S. retiree medical plans, in accordance with the employee matters agreement. See Notes to Consolidated and Combined Financial Statements—
Note 13. Benefit Plans.
|
|
(e)
|
Except for amounts reflected in
Income taxes payable
, we are unable to predict the timing of tax settlements, as tax audits can involve complex issues and the resolution of those issues may span multiple years, particularly if subject to negotiation or litigation.
|
|
Description
|
Principal Amount
|
Interest Rate
|
Terms
|
|
Lines of credit
|
$3 million
|
6.400%
|
Due 2016-2018
|
|
2016 Senior Note
|
$400 million
|
1.150%
|
Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2016
|
|
2018 Senior Note
|
$750 million
|
1.875%
|
Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2018
|
|
2023 Senior Note
|
$1,350 million
|
3.250%
|
Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2023
|
|
2043 Senior Note
|
$1,150 million
|
4.700%
|
Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2043
|
|
Commercial
|
||||||||
|
Paper
|
Long-term Debt
|
Date of
|
||||||
|
Name of Rating Agency
|
Rating
|
Rating
|
Outlook
|
Last Action
|
||||
|
Moody’s
|
P-2
|
Baa2
|
Stable
|
January 2013
|
||||
|
S&P
|
A-3
|
BBB-
|
Stable
|
January 2013
|
||||
|
•
|
emerging restrictions and bans on the use of antibacterials in food-producing animals;
|
|
•
|
perceived adverse effects on human health linked to the consumption of food derived from animals that utilize our products;
|
|
•
|
increased regulation or decreased governmental support relating to the raising, processing or consumption of food-producing animals;
|
|
•
|
fluctuations in foreign exchange rates and potential currency controls;
|
|
•
|
changes in tax laws, regulations, and challenges brought against our incentive tax rulings;
|
|
•
|
legal factors, including product liability claims, antitrust litigation and governmental investigations, including tax disputes, environmental concerns, commercial disputes and patent disputes with branded and generic competitors, any of which could preclude commercialization of products or negatively affect the profitability of existing products;
|
|
•
|
an outbreak of infectious disease carried by animals;
|
|
•
|
adverse weather conditions and the availability of natural resources;
|
|
•
|
adverse global economic conditions;
|
|
•
|
failure of our R&D, acquisition and licensing efforts to generate new products;
|
|
•
|
quarterly fluctuations in demand and costs; and
|
|
•
|
governmental laws and regulations affecting domestic and foreign operations, including without limitation, tax obligations and changes affecting the tax treatment by the United States of income earned outside the United States that may result from pending and possible future proposals.
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
|
Page
|
|
|
Audited Consolidated and Combined Financial Statements of Zoetis Inc. and Subsidiaries:
|
|
|
Consolidated and Combined Statements of Income for the Years Ended December 31, 2014, 2013 and 2012
|
|
|
Consolidated and Combined Statements of Comprehensive Income for the Years Ended December 31, 2014, 2013 and 2012
|
|
|
Consolidated Balance Sheets as of December 31, 2014 and 2013
|
|
|
Consolidated and Combined Statements of Equity for the Years Ended December 31, 2014, 2013 and 2012
|
|
|
Consolidated and Combined Statements of Cash Flows for the Years Ended December 31, 2014, 2013 and 2012
|
|
|
Schedule II—Valuation and Qualifying Accounts
|
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS, EXCEPT PER SHARE DATA)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Revenue
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
|||
|
Costs and expenses:
|
||||||||||||
|
Cost of sales
(a)
|
1,717
|
|
1,669
|
|
1,563
|
|
||||||
|
Selling, general and administrative expenses
(a)
|
1,643
|
|
1,613
|
|
1,470
|
|
||||||
|
Research and development expenses
(a)
|
396
|
|
399
|
|
409
|
|
||||||
|
Amortization of intangible assets
|
60
|
|
60
|
|
64
|
|
||||||
|
Restructuring charges and certain acquisition-related costs
|
25
|
|
26
|
|
135
|
|
||||||
|
Interest expense, net of capitalized interest
|
117
|
|
113
|
|
31
|
|
||||||
|
Other (income)/deductions––net
|
7
|
|
(9
|
)
|
(46
|
)
|
||||||
|
Income before provision for taxes on income
|
820
|
|
690
|
|
710
|
|
||||||
|
Provision for taxes on income
|
233
|
|
187
|
|
274
|
|
||||||
|
Net income before allocation to noncontrolling interests
|
587
|
|
503
|
|
436
|
|
||||||
|
Net income/(loss) attributable to noncontrolling interests
|
4
|
|
(1
|
)
|
—
|
|
||||||
|
Net income attributable to Zoetis
|
$
|
583
|
|
$
|
504
|
|
$
|
436
|
|
|||
|
Earnings per share attributable to Zoetis Inc. stockholders:
|
||||||||||||
|
Basic
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
|||
|
Diluted
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
|||
|
Weighted-average common shares outstanding
(b)
:
|
||||||||||||
|
Basic
|
501.055
|
|
500.002
|
|
500.000
|
|
||||||
|
Diluted
|
502.025
|
|
500.317
|
|
500.000
|
|
||||||
|
Dividends declared per common share
|
$
|
0.299
|
|
$
|
0.267
|
|
$
|
—
|
|
|||
|
(a)
|
Exclusive of amortization of intangible assets, except as disclosed in
|
|
(b)
|
The weighted average shares outstanding for both basic and diluted earnings per share for the year ended December 31, 2012 was calculated using
500
million shares of common stock outstanding, which was the number of Zoetis Inc. shares outstanding at the time of the initial public offering, which was completed on February 6, 2013. There were no Zoetis restricted stock units, deferred stock units, stock options or performance shares outstanding prior to the initial public offering.
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Net income before allocation to noncontrolling interests
|
$
|
587
|
|
$
|
503
|
|
$
|
436
|
|
|||
|
Other comprehensive loss, net of tax and reclassification adjustments:
|
||||||||||||
|
Foreign currency translation adjustments, net
|
(123
|
)
|
(54
|
)
|
(93
|
)
|
||||||
|
Benefit plans: Actuarial gains/(losses), net
(a)
|
(10
|
)
|
(2
|
)
|
1
|
|
||||||
|
Plan settlement, net
(b)
|
3
|
|
—
|
|
—
|
|
||||||
|
Total other comprehensive loss, net of tax
|
(130
|
)
|
(56
|
)
|
(92
|
)
|
||||||
|
Comprehensive income before allocation to noncontrolling interests
|
457
|
|
447
|
|
344
|
|
||||||
|
Comprehensive income/(loss) attributable to noncontrolling interests
|
5
|
|
(1
|
)
|
—
|
|
||||||
|
Comprehensive income attributable to Zoetis
|
$
|
452
|
|
$
|
448
|
|
$
|
344
|
|
|||
|
(a)
|
Presented net of reclassification adjustments and tax impacts, which are not significant in any period presented. Reclassification adjustments related to benefit plans are generally reclassified, as part of net periodic pension cost, into
Cost of sales, Selling, general and administrative expenses,
and/or
Research and development expenses,
as appropriate, in the consolidated and combined statements of income.
|
|
(b)
|
Reflects the 2014 settlement charge associated with the 2012 sale of our Netherlands manufacturing facility which was recorded to
Other (income)/deductions—net
. See
Note 13. Benefit Plans
for additional information.
|
|
December 31,
|
|
December 31,
|
|
|||||
|
(MILLIONS OF DOLLARS, EXCEPT PER SHARE DATA)
|
2014
|
|
2013
|
|
||||
|
Assets
|
||||||||
|
Cash and cash equivalents
|
$
|
882
|
|
$
|
610
|
|
||
|
Accounts receivable, less allowance for doubtful accounts of $32 in 2014 and $31 in 2013
|
980
|
|
1,138
|
|
||||
|
Inventories
|
1,289
|
|
1,293
|
|
||||
|
Current deferred tax assets
|
109
|
|
97
|
|
||||
|
Other current assets
|
205
|
|
219
|
|
||||
|
Total current assets
|
3,465
|
|
3,357
|
|
||||
|
Property, plant and equipment, less accumulated depreciation of $1,145 in 2014 and $1,028 in 2013
|
1,318
|
|
1,295
|
|
||||
|
Goodwill
|
976
|
|
982
|
|
||||
|
Identifiable intangible assets, less accumulated amortization
|
727
|
|
803
|
|
||||
|
Noncurrent deferred tax assets
|
54
|
|
63
|
|
||||
|
Other noncurrent assets
|
67
|
|
58
|
|
||||
|
Total assets
|
$
|
6,607
|
|
$
|
6,558
|
|
||
|
Liabilities and Equity
|
||||||||
|
Short-term borrowings
|
$
|
7
|
|
$
|
15
|
|
||
|
Accounts payable
|
290
|
|
506
|
|
||||
|
Dividends payable
|
42
|
|
36
|
|
||||
|
Accrued expenses
|
475
|
|
570
|
|
||||
|
Accrued compensation and related items
|
238
|
|
229
|
|
||||
|
Income taxes payable
|
26
|
|
40
|
|
||||
|
Other current liabilities
|
8
|
|
19
|
|
||||
|
Total current liabilities
|
1,086
|
|
1,415
|
|
||||
|
Long-term debt, net of discount
|
3,643
|
|
3,642
|
|
||||
|
Noncurrent deferred tax liabilities
|
277
|
|
322
|
|
||||
|
Other taxes payable
|
57
|
|
49
|
|
||||
|
Other noncurrent liabilities
|
207
|
|
168
|
|
||||
|
Total liabilities
|
5,270
|
|
5,596
|
|
||||
|
Commitments and contingencies
|
|
|
||||||
|
Stockholders' equity:
|
||||||||
|
Preferred stock, $0.01 par value; 1,000,000,000 authorized, none issued
|
—
|
|
—
|
|
||||
|
Common stock, $0.01 par value: 6,000,000,000 authorized, 501,342,267 and 500,007,735 shares issued;
501,327,524 and 500,007,428 shares outstanding at December 31, 2014 and 2013, respectively
|
5
|
|
5
|
|
||||
|
Treasury stock, at cost, 14,743 and 307 shares of common stock at December 31, 2014 and 2013, respectively
|
—
|
|
—
|
|
||||
|
Additional paid-in capital
|
958
|
|
878
|
|
||||
|
Retained earnings
|
709
|
|
276
|
|
||||
|
Accumulated other comprehensive loss
|
(361
|
)
|
(219
|
)
|
||||
|
Total Zoetis Inc. equity
|
1,311
|
|
940
|
|
||||
|
Equity attributable to noncontrolling interests
|
26
|
|
22
|
|
||||
|
Total equity
|
1,337
|
|
962
|
|
||||
|
Total liabilities and equity
|
$
|
6,607
|
|
$
|
6,558
|
|
||
|
Zoetis
|
||||||||||||||||||||||||||||||||
|
Accumulated
|
|
Equity
|
|
|||||||||||||||||||||||||||||
|
Business
|
|
Additional
|
|
Other
|
|
Attributable to
|
|
|||||||||||||||||||||||||
|
Common
|
|
Treasury
|
|
Unit
|
|
Paid-in
|
|
Retained
|
|
Comprehensive
|
|
Noncontrolling
|
|
Total
|
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
Stock
(a)
|
|
Stock
(a)
|
|
Equity
(b)
|
|
Capital
|
|
Earnings
|
|
Loss
|
|
Interests
|
|
Equity
|
|
||||||||||||||||
|
Balance, December 31, 2011
|
$
|
—
|
|
$
|
—
|
|
$
|
3,785
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(65
|
)
|
$
|
16
|
|
$
|
3,736
|
|
||||||||
|
Net income
|
—
|
|
—
|
|
436
|
|
—
|
|
—
|
|
—
|
|
—
|
|
436
|
|
||||||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(92
|
)
|
—
|
|
(92
|
)
|
||||||||||||||||
|
Share-based compensation awards
|
—
|
|
—
|
|
28
|
|
—
|
|
—
|
|
—
|
|
—
|
|
28
|
|
||||||||||||||||
|
Net transfers between Pfizer and noncontrolling interests
|
—
|
|
—
|
|
1
|
|
—
|
|
—
|
|
—
|
|
(1
|
)
|
—
|
|
||||||||||||||||
|
Net transfers—Pfizer
(b)
|
—
|
|
—
|
|
(4
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(4
|
)
|
||||||||||||||||
|
Dividends declared
|
—
|
|
—
|
|
(63
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(63
|
)
|
||||||||||||||||
|
Balance, December 31, 2012
|
$
|
—
|
|
$
|
—
|
|
$
|
4,183
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(157
|
)
|
$
|
15
|
|
$
|
4,041
|
|
||||||||
|
Net income (loss)
|
—
|
|
—
|
|
94
|
|
—
|
|
410
|
|
—
|
|
(1
|
)
|
503
|
|
||||||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(56
|
)
|
—
|
|
(56
|
)
|
||||||||||||||||
|
Share-based compensation awards
(c)
|
—
|
|
—
|
|
3
|
|
40
|
|
—
|
|
—
|
|
—
|
|
43
|
|
||||||||||||||||
|
Net transfers—Pfizer Inc.
(b)
|
—
|
|
—
|
|
(271
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(271
|
)
|
||||||||||||||||
|
Separation adjustments
(d)
|
—
|
|
—
|
|
414
|
|
29
|
|
—
|
|
(6
|
)
|
8
|
|
445
|
|
||||||||||||||||
|
Employee benefit plan contribution from Pfizer Inc.
(e)
|
—
|
|
—
|
|
—
|
|
2
|
|
—
|
|
—
|
|
—
|
|
2
|
|
||||||||||||||||
|
Reclassification of net liability due to Pfizer Inc.
(f)
|
—
|
|
—
|
|
(60
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(60
|
)
|
||||||||||||||||
|
Consideration paid to Pfizer Inc. in connection with the Separation
(g)
|
—
|
|
—
|
|
—
|
|
(3,551
|
)
|
—
|
|
—
|
|
—
|
|
(3,551
|
)
|
||||||||||||||||
|
Issuance of common stock to Pfizer Inc. in connection with the Separation and reclassification of Business Unit Equity
(g)
|
5
|
|
—
|
|
(4,363
|
)
|
4,358
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Dividends declared
|
—
|
|
—
|
|
—
|
|
—
|
|
(134
|
)
|
—
|
|
—
|
|
(134
|
)
|
||||||||||||||||
|
Balance, December 31, 2013
|
$
|
5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
878
|
|
$
|
276
|
|
$
|
(219
|
)
|
$
|
22
|
|
$
|
962
|
|
||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
583
|
|
—
|
|
4
|
|
587
|
|
||||||||||||||||
|
Other comprehensive income (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(131
|
)
|
1
|
|
(130
|
)
|
||||||||||||||||
|
Share-based compensation awards
(c)
|
—
|
|
—
|
|
—
|
|
31
|
|
—
|
|
—
|
|
—
|
|
31
|
|
||||||||||||||||
|
Defined contribution plan transactions
(h)
|
—
|
|
—
|
|
—
|
|
36
|
|
—
|
|
—
|
|
—
|
|
36
|
|
||||||||||||||||
|
Pension plan transfer from Pfizer Inc.
(i)
|
—
|
|
—
|
|
—
|
|
11
|
|
—
|
|
(11
|
)
|
—
|
|
—
|
|
||||||||||||||||
|
Employee benefit plan contribution from Pfizer Inc.
(e)
|
—
|
|
—
|
|
—
|
|
2
|
|
—
|
|
—
|
|
—
|
|
2
|
|
||||||||||||||||
|
Dividends declared
|
—
|
|
—
|
|
—
|
|
—
|
|
(150
|
)
|
—
|
|
(1
|
)
|
(151
|
)
|
||||||||||||||||
|
Balance, December 31, 2014
|
$
|
5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
958
|
|
$
|
709
|
|
$
|
(361
|
)
|
$
|
26
|
|
$
|
1,337
|
|
||||||||
|
(a)
|
As of
December 31, 2014
, there were
501,327,524
outstanding shares of common stock and
14,743
shares or treasury stock. Treasury stock is recognized at the cost to reacquire the shares, which totaled approximately
$0.5
million for the year ended December 31, 2014. The cost to reacquire treasury shares for the year ended December 31, 2013 was insignificant.
|
|
(b)
|
All amounts associated with
Business Unit Equity
relate to periods prior to the Separation. See
Note 2A. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
The Separation.
|
|
(c)
|
Includes the issuance of shares of Zoetis Inc. common stock and the reacquisition of shares of treasury stock associated with exercises of employee share-based awards. Treasury shares are reacquired from employees for withholding tax purposes in connection with the vesting and exercise of awards under our equity compensation plan. For additional information, see
Note 14. Share-Based Payments
and
Note 15. Stockholders' Equity.
|
|
(d)
|
For additional information, see
Note 2B. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
Adjustments Associated with the Separation.
|
|
(e)
|
Represents contributed capital from Pfizer Inc. associated with service credit continuation for certain Zoetis Inc. employees in Pfizer Inc.'s U.S. qualified defined benefit and U.S. retiree medical plans.
See Note 13. Benefit Plans.
|
|
(f)
|
Represents the reclassification of the Receivable from Pfizer Inc. and the Payable to Pfizer Inc. from
Business Unit Equity
as of the Separation date. See
Note 2A. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
The Separation.
|
|
(g)
|
Reflects the Separation transaction. See
Note 2A. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
The Separation.
|
|
(h)
|
Reflects company matching and profit-sharing contributions funded through the issuance of shares of Zoetis Inc. common stock for the year ended December 31, 2014. For additional information, see
|
|
(i)
|
Reflects the 2014 transfers of defined benefit pension plans from Pfizer Inc. and the associated reclassification from
Additional Paid in Capital
to
Accumulated Other Comprehensive Loss.
See
Note 13. Benefit Plans.
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Operating Activities
|
||||||||||||
|
Net income before allocation to noncontrolling interests
|
$
|
587
|
|
$
|
503
|
|
$
|
436
|
|
|||
|
Adjustments to reconcile net income before noncontrolling interests to net cash
|
||||||||||||
|
provided by operating activities:
|
||||||||||||
|
Depreciation and amortization expense
|
204
|
|
209
|
|
200
|
|
||||||
|
Share-based compensation expense
|
32
|
|
43
|
|
28
|
|
||||||
|
Asset write-offs and asset impairments
|
10
|
|
15
|
|
10
|
|
||||||
|
Deferred taxes
|
(49
|
)
|
23
|
|
(74
|
)
|
||||||
|
Employee benefit plan contribution from Pfizer Inc.
|
2
|
|
2
|
|
—
|
|
||||||
|
Other non-cash adjustments
|
(3
|
)
|
(10
|
)
|
3
|
|
||||||
|
Other changes in assets and liabilities, net of acquisitions and divestitures and transfers with Pfizer Inc.
|
||||||||||||
|
Accounts receivable
|
69
|
|
(99
|
)
|
(65
|
)
|
||||||
|
Inventories
|
(16
|
)
|
(104
|
)
|
(318
|
)
|
||||||
|
Other assets
|
(2
|
)
|
(24
|
)
|
(5
|
)
|
||||||
|
Accounts payable
|
(210
|
)
|
(82
|
)
|
96
|
|
||||||
|
Other liabilities
|
11
|
|
196
|
|
62
|
|
||||||
|
Other tax accounts, net
|
(9
|
)
|
9
|
|
81
|
|
||||||
|
Net cash provided by operating activities
|
626
|
|
681
|
|
454
|
|
||||||
|
Investing Activities
|
||||||||||||
|
Purchases of property, plant and equipment
|
(180
|
)
|
(184
|
)
|
(126
|
)
|
||||||
|
Milestone payment related to previously acquired intangibles
|
(15
|
)
|
—
|
|
—
|
|
||||||
|
Net proceeds from sales of assets
|
9
|
|
9
|
|
3
|
|
||||||
|
Other investing activities
|
(1
|
)
|
(4
|
)
|
(12
|
)
|
||||||
|
Net cash used in investing activities
|
(187
|
)
|
(179
|
)
|
(135
|
)
|
||||||
|
Financing Activities
|
||||||||||||
|
Increase/(decrease) in short-term borrowings, net
|
(8
|
)
|
16
|
|
—
|
|
||||||
|
Proceeds from issuance of long-term debt—senior notes, net of discount and fees
|
—
|
|
2,625
|
|
—
|
|
||||||
|
Stock-based compensation-related proceeds and excess tax benefits
|
2
|
|
—
|
|
—
|
|
||||||
|
Consideration paid to Pfizer Inc. in connection with the Separation
(a)
|
—
|
|
(2,559
|
)
|
—
|
|
||||||
|
Cash dividends paid
(b)
|
(146
|
)
|
(98
|
)
|
(63
|
)
|
||||||
|
Other net financing activities with Pfizer Inc.
|
(2
|
)
|
(184
|
)
|
(15
|
)
|
||||||
|
Net cash used in financing activities
|
(154
|
)
|
(200
|
)
|
(78
|
)
|
||||||
|
Effect of exchange-rate changes on cash and cash equivalents
|
(13
|
)
|
(9
|
)
|
(3
|
)
|
||||||
|
Net increase in cash and cash equivalents
|
272
|
|
293
|
|
238
|
|
||||||
|
Cash and cash equivalents at beginning of period
|
610
|
|
317
|
|
79
|
|
||||||
|
Cash and cash equivalents at end of period
|
$
|
882
|
|
$
|
610
|
|
$
|
317
|
|
|||
|
Supplemental cash flow information
|
||||||||||||
|
Cash paid during the period for:
|
||||||||||||
|
Income taxes
|
$
|
278
|
|
$
|
134
|
|
$
|
276
|
|
|||
|
Interest, net of capitalized interest
|
118
|
|
60
|
|
31
|
|
||||||
|
Non-cash transactions:
|
||||||||||||
|
Intangible asset acquisition
(c)
|
$
|
8
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Purchases of property, plant and equipment
|
9
|
|
16
|
|
14
|
|
||||||
|
Contingent purchase price consideration
|
—
|
|
3
|
|
—
|
|
||||||
|
Dividends declared, not paid
|
42
|
|
36
|
|
—
|
|
||||||
|
Zoetis Inc. senior notes transferred to Pfizer Inc. in connection with the Separation
(d)
|
—
|
|
992
|
|
—
|
|
||||||
|
(a)
|
Reflects the Separation transaction. Amount is net of the non-cash portion. See
Note 2A. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
The Separation.
|
|
(b)
|
For the twelve months ended December 31, 2012, reflects payments to other non-Zoetis Pfizer Inc. entities.
|
|
(c)
|
Reflects the non-cash portion of the acquisition of product registration and application rights from Pfizer. See
Note 19. Transactions and Agreements with Pfizer.
|
|
(d)
|
Reflects the non-cash portion of the Separation transaction. See
Note 2A. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
The Separation.
|
|
2.
|
The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
|
|
A.
|
The Separation
|
|
B.
|
Adjustments Associated with the Separation
|
|
•
|
The removal of inventories (approximately $
74 million
), property, plant and equipment (approximately $
28 million
) and miscellaneous other net liabilities (approximately $
21 million
) associated with certain non-dedicated manufacturing sites that were retained by Pfizer;
|
|
•
|
The addition of property, plant and equipment (approximately $
56 million
) associated with a non-dedicated manufacturing site that was transferred to us by Pfizer (and then leased back to Pfizer under operating leases), and the removal of the inventory (approximately $
46 million
) and net other assets (approximately $
4 million
) at that site as these assets were retained by Pfizer;
|
|
•
|
The addition of net defined benefit plan liabilities (approximately $
21 million
) and deferred compensation liabilities (approximately $
4 million
);
|
|
•
|
The elimination of (i) noncurrent deferred tax assets (some of which were included within noncurrent deferred tax liabilities due to jurisdictional netting) related to net operating loss and tax credit carryforwards; (ii) net tax liabilities associated with uncertain tax positions; (iii) noncurrent deferred tax liabilities related to deferred income taxes on unremitted earnings; and (iv) other allocated net tax assets, all of which (approximately $
49 million
in net tax asset accounts) were retained by Pfizer;
|
|
•
|
The addition of (i) noncurrent deferred tax assets (approximately $
8 million
, some of which were included within noncurrent deferred tax liabilities due to jurisdictional netting) related to net benefit plan liabilities transferred to us by Pfizer; (ii) noncurrent deferred tax assets (approximately $
2 million
) related to net operating loss and tax credit carryforwards; and (iii) noncurrent deferred tax liabilities (approximately $
2 million
) related to property, plant and equipment transferred to us by Pfizer;
|
|
•
|
The elimination of allocated long-term debt (approximately $
582 million
), allocated accrued interest payable (approximately $
16 million
) and allocated unamortized deferred debt issuance costs (approximately $
2 million
) that were retained by Pfizer;
|
|
•
|
Certain net financial assets retained by Pfizer (approximately $
45 million
);
|
|
•
|
The removal of cash (approximately $
7 million
), inventories (approximately $
5 million
), property, plant and equipment (approximately $
8 million
), miscellaneous other assets (approximately $
3 million
) and other miscellaneous liabilities (approximately $
2 million
) associated with non-U.S. Pfizer businesses that did not transfer to us from Pfizer;
|
|
•
|
The addition of net receivables from Pfizer (approximately $
5 million
) associated with certain foreign taxes directly resulting from certain aspects of the Separation that were the responsibility of Pfizer under the terms of the tax matters agreement, see
|
|
•
|
The addition of (i) inventory (approximately $
15 million
); (ii) net deferred tax assets (approximately $
1 million
); and (iii) miscellaneous other assets (approximately $
5 million
) transferred to us by Pfizer, and the removal of (i) property, plant and equipment (approximately $
2
|
|
•
|
The addition of net benefit plan liabilities (approximately $
21 million
) associated with certain international plans that transferred from Pfizer to Zoetis in 2014. See
|
|
C.
|
Senior Notes Offering
|
|
D.
|
Initial Public Offering (IPO)
|
|
E.
|
Exchange Offer
|
|
3.
|
Basis of Presentation
|
|
A.
|
Basis of Presentation Prior to the Separation
|
|
•
|
The combined statement of income for the year ended December 31, 2012, and the pre-Separation period included in the consolidated statement of income for the year ended December 31, 2013, include allocations from certain support functions (Enabling Functions) that are provided on a centralized basis within Pfizer, such as expenses for business technology, facilities, legal, finance, human resources, and, to a lesser extent, business development, public affairs and procurement, among others, as Pfizer did not routinely allocate these costs to any of its business units. These allocations were based on either a specific identification basis or, when specific identification is not practicable, proportional allocation methods (e.g., using third-party sales, headcount, etc.), depending on the nature of the services.
|
|
•
|
The combined statement of income for the year ended December 31, 2012, and the pre-Separation period included in the consolidated statement of income for the year ended December 31, 2013, includes allocations of certain manufacturing and supply costs incurred by manufacturing plants that were shared with other Pfizer business units, Pfizer's global external supply group and Pfizer's global logistics and support group (collectively, Pfizer Global Supply, or PGS). These costs may include manufacturing variances and changes in the standard costs of inventory, among others, as Pfizer did not routinely allocate these costs to any of its business units. These allocations were based on either a specific identification basis or, when specific identification is not practicable, proportional allocation methods, such as animal health identified manufacturing costs, depending on the nature of the costs.
|
|
•
|
The combined statement of income for the year ended December 31, 2012, and the pre-Separation period included in the consolidated statement of income for the year ended December 31, 2013, also includes allocations from the Enabling Functions and PGS for restructuring charges, integration costs, additional depreciation associated with asset restructuring and implementation costs, as Pfizer did not routinely allocate these costs to any of its business units. For additional information about allocations of restructuring charges and other costs associated with acquisitions and cost-reduction/productivity initiatives, see
Note 6. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
.
|
|
•
|
The combined statement of income for the year ended December 31, 2012, and the pre-Separation period included in the consolidated statement of income for the year ended December 31, 2013, includes an allocation of share-based compensation expense and certain other compensation expense items, such as certain fringe benefit expenses, maintained on a centralized basis within Pfizer, as Pfizer does not routinely allocate these costs to any of its business units. For additional information about allocations of share-based payments, see
|
|
•
|
Enabling Functions operating expenses––
$11 million
and
$310 million
in 2013 and 2012, respectively (
$1 million
in 2012 in
Cost of sales
;
$11 million
and
$254 million
in 2013 and 2012, respectively, in
Selling, general and administrative expenses
; and
$55 million
in 2012, in
Research and development expenses
).
|
|
•
|
PGS manufacturing costs—
$25 million
in 2012 (in
Cost of sales).
|
|
•
|
Restructuring charges and certain acquisition-related costs—
$57 million
in 2012 (in
Restructuring charges and certain acquisition-related costs
).
|
|
•
|
Other costs associated with cost reduction/productivity initiatives—additional depreciation associated with asset restructuring—
$2 million
and
$13 million
in 2013 and 2012, respectively (
$2 million
and
$4 million
in 2013 and 2012, respectively, in
Selling, general and administrative expenses
and
$9 million
in 2012 in
Research and development expenses
).
|
|
•
|
Other costs associated with cost reduction/productivity initiatives—implementation costs—
$1 million
and
$9 million
in 2013 and 2012, respectively (
$1 million
and
$8 million
in 2013 and 2012, respectively, in
Selling, general and administrative expenses;
and
$1 million
in 2012 in
Research and development expenses
).
|
|
•
|
Share-based compensation expense—
$3 million
and
$33 million
in 2013 and 2012, respectively (
$1 million
and
$7 million
in 2013 and 2012, respectively, in
Cost of sales
;
$2 million
and
$21 million
in 2013 and 2012, respectively, in
Selling, general and administrative expenses
; and
$5 million
in 2012 in
Research and development expenses
).
|
|
•
|
Compensation-related expenses—
$1 million
and
$12 million
in 2013 and 2012, respectively (
$5 million
in 2012 in
Cost of sales
;
$1 million
and
$5 million
in 2013 and 2012, respectively, in
Selling, general and administrative expenses
; and
$2 million
in 2012 in
Research and development expenses
).
|
|
•
|
Interest expense—
$2 million
and
$31 million
in 2013 and 2012, respectively.
|
|
B.
|
Basis of Presentation After the Separation
|
|
•
|
Goodwill
—goodwill represents the excess of the consideration transferred for an acquired business over the assigned values of its net assets. Goodwill is not amortized.
|
|
•
|
Identifiable intangible assets, less accumulated amortization
—these acquired assets are recorded at our cost. Identifiable intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives. Identifiable intangible assets with indefinite lives that are associated with marketed products are not amortized until a useful life can be determined. Identifiable intangible assets associated with IPR&D projects are not amortized until regulatory approval is obtained. The useful life of an amortizing asset generally is determined by identifying the period in which substantially all of the cash flows are expected to be generated.
|
|
•
|
Property, plant and equipment, less accumulated depreciation
––these assets are recorded at our cost and are increased by the cost of any significant improvements after purchase. Property, plant and equipment assets, other than land and construction-in-progress, are depreciated
|
|
•
|
For finite-lived identifiable intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows associated with the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is found to be greater, we record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we re-evaluate the remaining useful lives of the assets and modify them, as appropriate.
|
|
•
|
For indefinite-lived identifiable intangible assets, such as brands and IPR&D assets, we test for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the indefinite-lived intangible asset is less than its carrying amount. If we conclude it is more likely than not that the fair value is less than the carrying amount, a quantitative test that compares the fair value of the indefinite-lived intangible asset with its carrying value is performed. If the fair value is less than the carrying amount, an impairment loss is recognized. We record an impairment loss, if any, for the excess of book value over fair value. In addition, in all cases of an impairment review other than for IPR&D assets, we re-evaluate whether continuing to characterize the asset as indefinite-lived is appropriate.
|
|
•
|
For goodwill, we test for impairment on at least an annual basis, or more frequently if impairment indicators exist, either by assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, or by performing a quantitative assessment. If we choose to perform a qualitative analysis and conclude it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative fair value test is performed. We determine the implied fair value of goodwill by subtracting the fair value of all the identifiable net assets other than goodwill from the fair value of the reporting unit and record an impairment loss for the excess, if any, of book value of goodwill over the implied fair value. In 2014, we quantitatively assessed, as of September 28, 2014, the fair value of each of our reporting units using the income approach. The fair value of each reporting unit was found to exceed its respective carrying value, therefore no impairments were recorded.
|
|
•
|
Income approach, which is based on the present value of a future stream of net cash flows.
|
|
•
|
Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities.
|
|
•
|
Cost approach, which is based on the cost to acquire or construct comparable assets less an allowance for functional and/or economic obsolescence.
|
|
•
|
Quoted prices for identical assets or liabilities in active markets (Level 1 inputs).
|
|
•
|
Quoted prices for similar assets or liabilities in active markets or quoted prices for identical or similar assets or liabilities in markets that are not active or are directly or indirectly observable (Level 2 inputs).
|
|
•
|
Unobservable inputs that reflect estimates and assumptions (Level 3 inputs).
|
|
6.
|
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Restructuring charges and certain acquisition-related costs:
|
||||||||||||
|
Integration costs
(a)
|
$
|
8
|
|
$
|
21
|
|
$
|
26
|
|
|||
|
Restructuring charges (benefits)
(b)
:
|
||||||||||||
|
Employee termination costs
|
16
|
|
(23
|
)
|
49
|
|
||||||
|
Accelerated depreciation
|
—
|
|
5
|
|
—
|
|
||||||
|
Asset impairment charges
|
—
|
|
19
|
|
4
|
|
||||||
|
Exit costs
|
1
|
|
4
|
|
(1
|
)
|
||||||
|
Total direct
|
25
|
|
26
|
|
78
|
|
||||||
|
Integration costs
(a)
|
—
|
|
—
|
|
21
|
|
||||||
|
Restructuring charges
(b)
:
|
||||||||||||
|
Employee termination costs
|
—
|
|
—
|
|
19
|
|
||||||
|
Asset impairment charges
|
—
|
|
—
|
|
10
|
|
||||||
|
Exit costs
|
—
|
|
—
|
|
7
|
|
||||||
|
Total allocated
|
—
|
|
—
|
|
57
|
|
||||||
|
Total
Restructuring charges and certain acquisition-related costs
|
25
|
|
26
|
|
135
|
|
||||||
|
Other costs associated with cost-reduction/productivity initiatives:
|
||||||||||||
|
Additional depreciation associated with asset restructuring––direct
(c)
|
1
|
|
1
|
|
11
|
|
||||||
|
Additional depreciation associated with asset restructuring––allocated
(c)
|
—
|
|
2
|
|
13
|
|
||||||
|
Implementation costs––allocated
(d)
|
—
|
|
1
|
|
9
|
|
||||||
|
Total costs associated with restructuring, acquisitions and cost-reduction/productivity initiatives
|
$
|
26
|
|
$
|
30
|
|
$
|
168
|
|
|||
|
(a)
|
Integration costs represent external, incremental costs directly related to integrating acquired businesses and primarily include expenditures for consulting and the integration of systems and processes, as well as product transfer costs.
|
|
(b)
|
The restructuring charges for the year ended
December 31, 2014
, are primarily related to:
|
|
•
|
restructuring charges related to the exiting of certain leased manufacturing and research facilities consisting of employee termination expenses ($
2 million
), exit costs ($
4 million
), and accelerated depreciation ($
5 million
).
|
|
•
|
For the year ended
December 31, 2014
––EuAfME (
$12 million
) and manufacturing/research/corporate (
$5 million
).
|
|
•
|
For the year ended
December 31, 2013
—EuAfME (
$4 million
), CLAR (
$4 million
) and manufacturing/research/corporate (
$3 million
income).
|
|
•
|
For the year ended December 31, 2012––EuAfME (
$51 million
), CLAR (
$3 million
), APAC (
$1 million
income) and manufacturing/research/corporate (
$1 million
income).
|
|
(c)
|
Additional depreciation associated with asset restructuring represents the impact of changes in the estimated lives of assets involved in restructuring actions. In 2014, included in
Research and development expenses
. In 2013, included in
Cost of sales
($
1 million
) and
Selling, general and administrative expenses
($
2 million
). In 2012, included in
Cost of sales
(
$10 million
),
Selling, general and administrative expenses
(
$5 million
) and
Research and development expenses
(
$9 million
).
|
|
(d)
|
Implementation costs—allocated represent external, incremental costs directly related to implementing cost reduction/productivity initiatives, and primarily include expenditures related to system and process standardization and the expansion of shared services. Included in
Selling, general and administrative expenses
.
|
|
Employee
|
|
Asset
|
|
|||||||||||||||||
|
Termination
|
|
Impairment
|
|
Accelerated
|
|
Exit
|
|
|||||||||||||
|
(MILLIONS OF DOLLARS)
|
Costs
|
|
Charges
|
|
Depreciation
|
|
Costs
|
|
Accrual
|
|
||||||||||
|
Balance, December 31, 2011
|
$
|
70
|
|
$
|
—
|
|
$
|
—
|
|
$
|
11
|
|
$
|
81
|
|
|||||
|
Provision/(Benefit)
|
49
|
|
4
|
|
—
|
|
(1
|
)
|
52
|
|
||||||||||
|
Utilization and other
(a)
|
(51
|
)
|
(4
|
)
|
—
|
|
(4
|
)
|
(59
|
)
|
||||||||||
|
Balance, December 31, 2012
|
68
|
|
—
|
|
—
|
|
6
|
|
74
|
|
||||||||||
|
Provision/(Benefit)
|
(23
|
)
|
19
|
|
5
|
|
4
|
|
5
|
|
||||||||||
|
Utilization and other
(a)
|
(16
|
)
|
—
|
|
—
|
|
(4
|
)
|
(20
|
)
|
||||||||||
|
Non-cash activity
|
—
|
|
(19
|
)
|
(5
|
)
|
—
|
|
(24
|
)
|
||||||||||
|
Separation adjustment
(b)
|
(14
|
)
|
—
|
|
—
|
|
—
|
|
(14
|
)
|
||||||||||
|
Balance, December 31, 2013
(c)
|
15
|
|
—
|
|
—
|
|
6
|
|
21
|
|
||||||||||
|
Provision/(Benefit)
|
16
|
|
—
|
|
—
|
|
1
|
|
17
|
|
||||||||||
|
Utilization and other
(a)
|
(13
|
)
|
—
|
|
(6
|
)
|
(19
|
)
|
||||||||||||
|
Balance, December 31, 2014
(c)
|
$
|
18
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1
|
|
$
|
19
|
|
|||||
|
(a)
|
Includes adjustments for foreign currency translation.
|
|
(b)
|
See
Note 2B. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
Adjustments Associated with the Separation.
|
|
(c)
|
At
December 31, 2014
and 2013, included in
Other current liabilities
(
$13 million
for both years) and
Other noncurrent liabilities
(
$6 million
and $
8 million
, respectively).
|
|
7.
|
Other (Income)/Deductions—Net
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Royalty-related income
|
$
|
(32
|
)
|
$
|
(23
|
)
|
$
|
(32
|
)
|
|||
|
Identifiable intangible asset impairment charges
(a)
|
7
|
|
1
|
|
5
|
|
||||||
|
Net gain on sale of assets
(b)
|
(9
|
)
|
(6
|
)
|
—
|
|
||||||
|
Certain legal matters, net
(c)
|
10
|
|
1
|
|
(19
|
)
|
||||||
|
Foreign currency (gain)/loss
(d)
|
28
|
|
20
|
|
—
|
|
||||||
|
Other, net
(e)
|
3
|
|
(2
|
)
|
—
|
|
||||||
|
Other (income)/deductions—net
|
$
|
7
|
|
$
|
(9
|
)
|
$
|
(46
|
)
|
|||
|
(a)
|
In 2014, the intangible asset impairment charges primarily include (i) approximately $
6 million
related to the impairment of IPR&D assets related to a pharmaceutical product for dogs acquired with the FDAH acquisition in 2009, as a result of the termination of the development program due to a re-assessment of economic viability; and (ii) approximately $
1 million
related to finite-lived developed technology rights and IPR&D due to negative market conditions and the re-assessment of economic viability. In 2012, the intangible asset impairment charges include (i) approximately $
2 million
of finite-lived companion animal developed technology rights; (ii) approximately $
1 million
of finite-lived trademarks related to genetic testing services; and (iii) approximately $
2 million
of finite-lived patents related to poultry technology. The asset impairment charges for 2012 reflect, among other things, loss of revenue as a result of negative market conditions and, with respect to the poultry technology, a re-assessment of economic viability.
|
|
(b)
|
In 2014, represents the net gain on sale of land in our Taiwan joint venture of $
6 million
and the net gain on the government-mandated sale of certain product rights in Argentina and China that were associated with the FDAH acquisition in 2009 of $
3 million
. In 2013, represents the net gain on the government-mandated sale of certain product rights in Brazil that were acquired with the FDAH acquisition in
2009
.
|
|
(c)
|
In July 2014, we reached a commercial settlement with several large poultry customers in Mexico associated with specific lots of a Zoetis poultry vaccine. Although there have been no quality or efficacy issues with the manufacturing of this vaccine, certain shipments from several lots in Mexico may have experienced an issue in storage with a third party in Mexico that could have impacted their efficacy. We issued a recall of these lots in July 2014 and the product is currently unavailable in Mexico. For 2014, includes a
$13 million
charge recorded in the second quarter of 2014, which was partially offset by a $
1 million
insurance recovery recorded in the third quarter of 2014, related to the commercial settlement in Mexico. We do not expect any significant additional charges related to this issue. For 2014, also includes an insurance recovery of other litigation-related charges of $
2 million
. For 2012, represents income from a favorable legal settlement related to an intellectual property matter ($
14 million
) and a change in estimate for an environmental-related reserve due to a favorable settlement ($
7 million
income) partially offset by litigation-related charges ($
2 million
).
|
|
(d)
|
For 2014, primarily represents costs related to hedging and exposures to certain emerging market currencies, as well as losses related to the depreciation of the Argentine peso in the first quarter of 2014. For 2013, includes a foreign currency loss of $
9 million
incurred in the first quarter of 2013 related to the Venezuela currency devaluation in February 2013 and other foreign currency losses in the fourth quarter of 2013 primarily related to Argentina.
|
|
(e)
|
Includes interest income and other miscellaneous income and charges. For 2014, also includes a pension plan settlement charge related to the sale of a manufacturing plant of
$4 million
.
|
|
8.
|
Tax Matters
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
United States
|
$
|
455
|
|
$
|
238
|
|
$
|
340
|
|
|||
|
International
|
365
|
|
452
|
|
370
|
|
||||||
|
Income before provision for taxes on income
(a)(b)
|
$
|
820
|
|
$
|
690
|
|
$
|
710
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
United States:
|
||||||||||||
|
Current income taxes:
|
||||||||||||
|
Federal
|
$
|
179
|
|
$
|
63
|
|
$
|
132
|
|
|||
|
State and local
|
13
|
|
12
|
|
5
|
|
||||||
|
Deferred income taxes:
|
||||||||||||
|
Federal
|
(14
|
)
|
10
|
|
(7
|
)
|
||||||
|
State and local
|
(3
|
)
|
2
|
|
11
|
|
||||||
|
Total U.S. tax provision
|
175
|
|
87
|
|
141
|
|
||||||
|
International:
|
||||||||||||
|
Current income taxes
|
90
|
|
89
|
|
211
|
|
||||||
|
Deferred income taxes
|
(32
|
)
|
11
|
|
(78
|
)
|
||||||
|
Total international tax provision
|
58
|
|
100
|
|
133
|
|
||||||
|
Provision for taxes on income
(a)(b)(c)
|
$
|
233
|
|
$
|
187
|
|
$
|
274
|
|
|||
|
(a)
|
In 2014
,
the
Provision for taxes on income
reflects the following:
|
|
•
|
U.S. tax expense of approximately $
2 million
as a result of providing U.S. deferred income taxes on certain current-year income earned outside the United States that will not be indefinitely reinvested overseas (see
C. Deferred Taxes
);
|
|
•
|
U.S. tax benefit related to U.S. Research and Development Tax Credit which was extended on December 19, 2014, and the U.S. Domestic Production Activities deduction;
|
|
•
|
Tax benefit related to the changes in valuation allowances and the resolution of other tax items;
|
|
•
|
Tax expense related to an $
8 million
discrete tax item during the first quarter of 2014 related to an intercompany inventory adjustment; and
|
|
•
|
Tax cost related to changes in uncertain tax positions (see
D. Tax Contingencies).
|
|
(b)
|
In 2013, the
Provision for taxes on income
reflects the following:
|
|
•
|
U.S. tax expense of approximately $
3 million
as a result of providing U.S. deferred income taxes on certain current-year income earned outside the United States that will not be indefinitely reinvested overseas (see
C. Deferred Taxes
);
|
|
•
|
U.S. tax benefit related to U.S. Research and Development Tax Credit which was retroactively extended on January 3, 2013, and the U.S. Domestic Production Activities deduction;
|
|
•
|
Tax expense of approximately $
25 million
related to the establishment of valuation allowance; and
|
|
•
|
Tax cost related to changes in uncertain tax positions (see
D. Tax Contingencies).
|
|
(c)
|
In 2012, the
Provision for taxes on income
reflects the following:
|
|
•
|
U.S. tax benefits of approximately $
29.3 million
, representing tax and interest, resulting from a multi-year settlement with the U.S. Internal Revenue Service with respect to audits for the years 2006 through 2008, and international tax benefits of approximately $
2.7 million
, representing tax and interest, resulting from the resolution of certain tax positions pertaining to prior years with various foreign tax authorities and from the expiration of certain statutes of limitations;
|
|
•
|
U.S. tax expense of approximately $
9 million
as a result of providing U.S. deferred income taxes on certain current-year income earned outside the United States that will not be indefinitely reinvested overseas (see
C. Deferred Taxes)
;
|
|
•
|
The expiration of the U.S. Research and Development Tax Credit on December 31, 2011; and
|
|
•
|
Tax cost related to changes in uncertain tax positions (see
D. Tax Contingencies
)
.
|
|
Year Ended December 31,
|
|||||||||
|
2014
|
|
2013
|
|
2012
|
|
||||
|
U.S. statutory income tax rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
|||
|
State and local taxes, net of federal benefits
|
0.4
|
|
1.0
|
|
1.7
|
|
|||
|
Taxation of non-U.S. operations
(a)(b)
|
(8.9
|
)
|
(6.7
|
)
|
5.6
|
|
|||
|
Unrecognized tax benefits and tax settlements and resolution of certain tax positions
(c)
|
1.0
|
|
1.1
|
|
(4.1
|
)
|
|||
|
U.S. healthcare legislation
(d)
|
—
|
|
—
|
|
(0.4
|
)
|
|||
|
U.S. Research and Development Tax Credit and U.S. Domestic Production Activities deduction
(e)
|
(1.5
|
)
|
(1.2
|
)
|
(0.3
|
)
|
|||
|
Non-deductible / non-taxable items
(f)
|
0.5
|
|
0.5
|
|
0.8
|
|
|||
|
All other—net
|
1.9
|
|
(2.6
|
)
|
0.3
|
|
|||
|
Effective tax rate
|
28.4
|
%
|
27.1
|
%
|
38.6
|
%
|
|||
|
(a)
|
The rate impact of taxation of non-U.S. operations was a decrease to our effective tax rate in 2014 and 2013 due to (i) the jurisdictional mix of earnings as tax rates outside the United States are generally lower than the U.S. statutory income tax rate; and (ii) incentive tax rulings in Belgium effective December 1, 2012, and in Singapore effective October 29, 2012. The rate impact of taxation of non-U.S. operations was an increase to our effective tax rate in 2012 due to (i) the cost of repatriation decisions and other U.S. tax implications that more than offset the impact of the generally lower tax rates outside the United States; (ii) the tax impact of non-deductible items in those jurisdictions; and (iii) the tax impact of changes in uncertain tax positions related to our non-U.S. operations.
|
|
(b)
|
In 2014 and 2013, the impact to the rate due to increases in uncertain tax positions was more than offset by the jurisdictional mix of earnings and other U.S. tax implications of our foreign operations described in the above footnotes. The increase in the rate in 2012 is primarily due to increases in uncertain tax positions (see
D. Tax Contingencies
, for current and prior period increases to uncertain tax positions), of which a significant portion relates to our non-U.S. operations.
|
|
(c)
|
For a discussion about unrecognized tax benefits and tax settlements and resolution of certain tax positions, see
A. Taxes on Income
and
D. Tax Contingencies
.
|
|
(d)
|
The decrease in the rate in 2012 primarily relates to the tax benefit recorded in connection with the establishment of deferred income tax assets related to the Medicare Part D subsidy for retiree prescription drug coverage.
|
|
(e)
|
In 2014 and 2013, the decrease in the rate was due to the benefit associated with the U.S. Research and Development Tax Credit. In 2012, no benefit from the U.S. Research and Development Tax Credit was reflected as the credit expired on December 31, 2011, and was not extended until January 2013. In all years, we received a benefit from the U.S. Domestic Production Activities deduction.
|
|
(f)
|
Non-deductible items include meals and entertainment expenses.
|
|
B.
|
Tax Matters Agreement
|
|
•
|
Pfizer will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments and including those taxes attributable to our business) reportable on a consolidated, combined or unitary return that includes Pfizer or any of its subsidiaries (and us and/or any of our subsidiaries) for any periods or portions thereof ending on or prior to December 31, 2012. We will be responsible for the portion of any such taxes for periods or portions thereof beginning on or after January 1, 2013, as would be applicable to us if we filed the relevant tax returns on a standalone basis.
|
|
•
|
We will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments) that are reportable on returns that include only us and/or any of our subsidiaries, for all tax periods whether before or after the completion of the Separation.
|
|
•
|
Pfizer will be responsible for certain specified foreign taxes directly resulting from certain aspects of the Separation.
|
|
As of December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
(MILLIONS OF DOLLARS)
|
Assets (Liabilities)
|
|||||||
|
Prepaid/deferred items
|
$
|
56
|
|
$
|
59
|
|
||
|
Inventories
|
25
|
|
29
|
|
||||
|
Intangibles
|
(98
|
)
|
(111
|
)
|
||||
|
Property, plant and equipment
|
(89
|
)
|
(92
|
)
|
||||
|
Employee benefits
|
19
|
|
11
|
|
||||
|
Restructuring and other charges
|
5
|
|
4
|
|
||||
|
Legal and product liability reserves
|
19
|
|
13
|
|
||||
|
Net operating loss/credit carryforwards
|
65
|
|
51
|
|
||||
|
Unremitted earnings
|
(5
|
)
|
(3
|
)
|
||||
|
All other
|
(3
|
)
|
(10
|
)
|
||||
|
Subtotal
|
(6
|
)
|
(49
|
)
|
||||
|
Valuation allowance
|
(119
|
)
|
(128
|
)
|
||||
|
Net deferred tax liability
(a)(b)
|
$
|
(125
|
)
|
$
|
(177
|
)
|
||
|
(a)
|
The decrease in the total net deferred tax liability from December 31, 2013, to December 31, 2014, is primarily attributable to an increase in deferred tax assets related to net operating loss/credit carryforwards and other prepaid/deferred items, partially offset by a decrease in valuation allowances representing the amounts determined to be unrecoverable.
|
|
(b)
|
In 2014, included in
Current deferred tax assets
($
109 million
),
Noncurrent deferred tax assets
($
54 million
),
Other current liabilities
($
11 million
) and
Noncurrent deferred tax liabilities
($
277 million
). In 2013, included in
Current deferred tax assets
($
97 million
),
Noncurrent deferred tax assets
($
63 million
),
Other current liabilities
($
15 million
) and
Noncurrent deferred tax liabilities
($
322 million
).
|
|
•
|
Tax assets associated with uncertain tax positions primarily represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction. As of December 31, 2014 and 2013, we had approximately $
1 million
and $
1 million
, respectively, in assets associated with uncertain tax positions recorded in
Other noncurrent assets
.
|
|
•
|
Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the amounts taken or expected to be taken in a tax return because of the uncertainties described above. These unrecognized tax benefits relate primarily to issues common among multinational corporations. Substantially all of these unrecognized tax benefits, if recognized, would impact our effective income tax rate.
|
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Balance, January 1
|
$
|
(45
|
)
|
$
|
(144
|
)
|
$
|
(114
|
)
|
|||
|
Adjustments associated with the Separation
(a)
|
—
|
|
115
|
|
—
|
|
||||||
|
Increases based on tax positions taken during a prior period
(b)
|
(1
|
)
|
(2
|
)
|
(2
|
)
|
||||||
|
Decreases based on tax positions taken during a prior period
(b)(c)
|
6
|
|
—
|
|
40
|
|
||||||
|
Decreases based on cash payments for a prior period
|
—
|
|
1
|
|
3
|
|
||||||
|
Increases based on tax positions taken during the current period
(b)
|
(15
|
)
|
(16
|
)
|
(73
|
)
|
||||||
|
Lapse in statute of limitations
|
1
|
|
1
|
|
2
|
|
||||||
|
Balance, December 31
(d)
|
$
|
(54
|
)
|
$
|
(45
|
)
|
$
|
(144
|
)
|
|||
|
(a)
|
The significant decrease in the total gross unrecognized tax benefits from December 31, 2012, to December 31, 2013, is primarily attributable to the elimination of net tax liabilities associated with uncertain tax positions that were retained by Pfizer. See
Note 2B. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer— Adjustments Associated with the Separation.
|
|
(b)
|
Primarily included in
Provision for taxes on income.
|
|
(c)
|
In 2014, the decreases are primarily related to movements in foreign translation adjustments on prior year positions and effective settlement of certain issues with the U.S. tax authorities. In 2012, the decreases are primarily a result of effectively settling certain issues with the U.S. and non-U.S. tax authorities. See
A. Tax Matters—Taxes on Income.
|
|
(d)
|
In 2014, included in
Noncurrent deferred tax assets
($
6 million
) and
Other taxes payable
($
48 million
). In 2013, included in
Noncurrent deferred tax assets
($
6 million
) and
Other taxes payable
($
39 million
).
|
|
•
|
Interest related to our unrecognized tax benefits is recorded in accordance with the laws of each jurisdiction and is recorded in
Provision for taxes on income
in our consolidated and combined statements of income. In 2014, we recorded a net interest expense of $
1 million
; in 2013, we recorded a net interest expense of $
3 million
; and in 2012, we recorded a net interest expense of
$1 million
. Gross accrued interest totaled $
4 million
and $
11 million
as of December 31, 2014 and 2013, respectively, and were included in
Other taxes payable
. Accrued penalties are not significant.
|
|
9.
|
Financial Instruments
|
|
A.
|
Debt
|
|
December 31,
|
|
December 31,
|
|
|||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
||||
|
Lines of credit, due 2016-2018
|
$
|
3
|
|
$
|
2
|
|
||
|
1.150% Senior Notes due 2016
|
400
|
|
400
|
|
||||
|
1.875% Senior Notes due 2018
|
750
|
|
750
|
|
||||
|
3.250% Senior Notes due 2023
|
1,350
|
|
1,350
|
|
||||
|
4.700% Senior Notes due 2043
|
1,150
|
|
1,150
|
|
||||
|
3,653
|
|
3,652
|
|
|||||
|
Unamortized debt discount
|
(10
|
)
|
(10
|
)
|
||||
|
Long-term debt / Allocated long-term debt
|
$
|
3,643
|
|
$
|
3,642
|
|
||
|
After
|
|
|||||||||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2015
|
|
2016
|
|
2017
|
|
2018
|
|
2018
|
|
Total
|
|
||||||||||||
|
Maturities
|
$
|
—
|
|
$
|
401
|
|
$
|
1
|
|
$
|
751
|
|
$
|
2,500
|
|
$
|
3,653
|
|
||||||
|
B.
|
Derivative Financial Instruments
|
|
December 31,
|
|
December 31,
|
|
||||
|
(MILLIONS OF DOLLARS)
|
Balance Sheet Location
|
2014
|
|
2013
|
|
||
|
Foreign currency forward-exchange contracts
|
Other current assets
|
$
|
9
|
|
$
|
10
|
|
|
Foreign currency forward-exchange contracts
|
Other current liabilities
|
(4
|
)
|
(5
|
)
|
||
|
Total foreign currency forward-exchange contracts
|
$
|
5
|
|
$
|
5
|
|
|
|
10.
|
Inventories
|
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
||||
|
Finished goods
|
$
|
688
|
|
$
|
862
|
|
||
|
Work-in-process
|
340
|
|
218
|
|
||||
|
Raw materials and supplies
|
261
|
|
213
|
|
||||
|
Inventories
|
$
|
1,289
|
|
$
|
1,293
|
|
||
|
Useful Lives
|
As of December 31,
|
|||||||||
|
(MILLIONS OF DOLLARS)
|
(Years)
|
2014
|
|
2013
|
|
|||||
|
Land
|
—
|
$
|
36
|
|
$
|
36
|
|
|||
|
Buildings
|
33
1
/
3
- 50
|
918
|
|
883
|
|
|||||
|
Machinery, equipment and fixtures
|
3 - 20
|
1,342
|
|
1,205
|
|
|||||
|
Construction-in-progress
|
—
|
167
|
|
199
|
|
|||||
|
2,463
|
|
2,323
|
|
|||||||
|
Less: Accumulated depreciation
|
1,145
|
|
1,028
|
|
||||||
|
Property, plant and equipment
|
$
|
1,318
|
|
$
|
1,295
|
|
||||
|
12.
|
Goodwill and Other Intangible Assets
|
|
A.
|
Goodwill
|
|
(MILLIONS OF DOLLARS)
|
U.S.
|
|
EuAfME
|
|
CLAR
|
|
APAC
|
|
Total
|
|
||||||||||
|
Balance, December 31, 2012
|
$
|
502
|
|
$
|
157
|
|
$
|
163
|
|
$
|
163
|
|
$
|
985
|
|
|||||
|
Other
(a)
|
(1
|
)
|
—
|
|
(1
|
)
|
(1
|
)
|
(3
|
)
|
||||||||||
|
Balance, December 31, 2013
|
$
|
501
|
|
$
|
157
|
|
$
|
162
|
|
$
|
162
|
|
$
|
982
|
|
|||||
|
Other
(a)
|
—
|
|
(4
|
)
|
(1
|
)
|
(1
|
)
|
(6
|
)
|
||||||||||
|
Balance, December 31, 2014
|
$
|
501
|
|
$
|
153
|
|
$
|
161
|
|
$
|
161
|
|
$
|
976
|
|
|||||
|
(a)
|
Primarily reflects adjustments for foreign currency translation.
|
|
B.
|
Other Intangible Assets
|
|
As of December 31, 2014
|
As of December 31, 2013
|
|||||||||||||||||||||||
|
Identifiable
|
|
Identifiable
|
|
|||||||||||||||||||||
|
Gross
|
|
Intangible Assets,
|
|
Gross
|
|
Intangible Assets,
|
|
|||||||||||||||||
|
Carrying
|
|
Accumulated
|
|
Less Accumulated
|
|
Carrying
|
|
Accumulated
|
|
Less Accumulated
|
|
|||||||||||||
|
(MILLIONS OF DOLLARS)
|
Amount
|
|
Amortization
|
|
Amortization
|
|
Amount
|
|
Amortization
|
|
Amortization
|
|
||||||||||||
|
Finite-lived intangible assets:
|
||||||||||||||||||||||||
|
Developed technology rights
|
$
|
744
|
|
$
|
(259
|
)
|
$
|
485
|
|
$
|
762
|
|
$
|
(219
|
)
|
$
|
543
|
|
||||||
|
Brands
|
216
|
|
(111
|
)
|
105
|
|
216
|
|
(100
|
)
|
116
|
|
||||||||||||
|
Trademarks and tradenames
|
60
|
|
(41
|
)
|
19
|
|
59
|
|
(38
|
)
|
21
|
|
||||||||||||
|
Other
|
119
|
|
(116
|
)
|
3
|
|
121
|
|
(116
|
)
|
5
|
|
||||||||||||
|
Total finite-lived intangible assets
|
1,139
|
|
(527
|
)
|
612
|
|
1,158
|
|
(473
|
)
|
685
|
|
||||||||||||
|
Indefinite-lived intangible assets:
|
||||||||||||||||||||||||
|
Brands
|
38
|
|
—
|
|
38
|
|
39
|
|
—
|
|
39
|
|
||||||||||||
|
Trademarks and trade names
|
67
|
|
—
|
|
67
|
|
67
|
|
—
|
|
67
|
|
||||||||||||
|
In-process research and development
(a)
|
2
|
|
—
|
|
2
|
|
12
|
|
—
|
|
12
|
|
||||||||||||
|
Product rights
|
8
|
|
—
|
|
8
|
|
—
|
|
—
|
|
—
|
|
||||||||||||
|
Total indefinite-lived intangible assets
|
115
|
|
—
|
|
115
|
|
118
|
|
—
|
|
118
|
|
||||||||||||
|
Identifiable intangible assets
|
$
|
1,254
|
|
$
|
(527
|
)
|
$
|
727
|
|
$
|
1,276
|
|
$
|
(473
|
)
|
$
|
803
|
|
||||||
|
C.
|
Amortization
|
|
(MILLIONS OF DOLLARS)
|
2015
|
|
2016
|
|
2017
|
|
2018
|
|
2019
|
|
||||||||||
|
Amortization expense
|
$
|
60
|
|
$
|
60
|
|
$
|
60
|
|
$
|
58
|
|
$
|
55
|
|
|||||
|
As of and for the
|
||||||||
|
Year Ended December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
||||
|
Change in benefit obligation:
|
||||||||
|
Projected benefit obligation, beginning
|
$
|
73
|
|
$
|
39
|
|
||
|
Separation adjustments
(a)
|
78
|
|
28
|
|
||||
|
Changes in actuarial assumptions and other
|
17
|
|
(2
|
)
|
||||
|
Plan settlement
|
(38
|
)
|
—
|
|
||||
|
Adjustments for foreign currency translation
|
(7
|
)
|
2
|
|
||||
|
Other––net
|
6
|
|
6
|
|
||||
|
Benefit obligation, ending
|
129
|
|
73
|
|
||||
|
Change in plan assets:
|
||||||||
|
Fair value of plan assets, beginning
|
45
|
|
35
|
|
||||
|
Separation adjustments
(a)
|
56
|
|
7
|
|
||||
|
Actual return on plan assets
|
3
|
|
—
|
|
||||
|
Company contributions
|
3
|
|
2
|
|
||||
|
Plan settlement
|
(38
|
)
|
—
|
|
||||
|
Adjustments for foreign currency translation
|
(3
|
)
|
2
|
|
||||
|
Other––net
|
(3
|
)
|
(1
|
)
|
||||
|
Fair value of plan assets, ending
|
63
|
|
45
|
|
||||
|
Funded status—Projected benefit obligation in excess of plan assets at end of year
(b)
|
$
|
(66
|
)
|
$
|
(28
|
)
|
||
|
(a)
|
Represents the benefit obligations and plan assets transferred to us in 2014 and 2013 (net obligation of approximately
$22 million
and
21 million
, respectively) from Pfizer as part of the Separation, as described above.
|
|
(b)
|
Included in
Other noncurrent liabilities.
|
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
||||
|
Pension plans with an accumulated benefit obligation in excess of plan assets:
|
||||||||
|
Fair value of plan assets
|
$
|
35
|
|
$
|
37
|
|
||
|
Accumulated benefit obligation
|
73
|
|
58
|
|
||||
|
Pension plans with a projected benefit obligation in excess of plan assets:
|
||||||||
|
Fair value of plan assets
|
63
|
|
42
|
|
||||
|
Projected benefit obligation
|
129
|
|
70
|
|
||||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Service cost
|
$
|
4
|
|
$
|
2
|
|
$
|
1
|
|
|||
|
Interest cost
|
2
|
|
3
|
|
2
|
|
||||||
|
Expected return on plan assets
|
(1
|
)
|
(2
|
)
|
(1
|
)
|
||||||
|
Special termination benefits
|
1
|
|
—
|
|
—
|
|
||||||
|
Settlement loss
|
5
|
|
1
|
|
—
|
|
||||||
|
Net periodic benefit cost
|
$
|
11
|
|
$
|
4
|
|
$
|
2
|
|
|||
|
As of December 31,
|
|||||||||
|
(PERCENTAGES)
|
2014
|
|
2013
|
|
2012
|
|
|||
|
Weighted average assumptions used to determine benefit obligations:
|
|||||||||
|
Discount rate
|
2.8
|
%
|
5.0
|
%
|
4.6
|
%
|
|||
|
Rate of compensation increase
|
3.6
|
%
|
4.4
|
%
|
5.3
|
%
|
|||
|
Weighted average assumptions used to determine net benefit cost for the year ended December 31:
|
|||||||||
|
Discount rate
|
5.0
|
%
|
4.6
|
%
|
5.8
|
%
|
|||
|
Expected return on plan assets
|
4.0
|
%
|
4.5
|
%
|
3.6
|
%
|
|||
|
Rate of compensation increase
|
4.4
|
%
|
5.3
|
%
|
2.7
|
%
|
|||
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
||||
|
Cash and cash equivalents
|
$
|
1
|
|
$
|
—
|
|
||
|
Equity securities: Equity commingled funds
|
27
|
|
7
|
|
||||
|
Debt securities: Government bonds
|
26
|
|
31
|
|
||||
|
Other investments
|
9
|
|
7
|
|
||||
|
Total
(a)
|
$
|
63
|
|
$
|
45
|
|
||
|
(a)
|
Fair values are determined based on valuation inputs categorized as Level 1, 2 or 3 (see
|
|
•
|
Equity commingled funds––observable market prices.
|
|
•
|
Government bonds and other investments––principally observable market prices.
|
|
As of December 31,
|
|||||||||
|
Target allocation
|
|
||||||||
|
percentage
|
|
Percentage of Plan Assets
|
|||||||
|
(PERCENTAGES)
|
2014
|
|
2014
|
|
2013
|
|
|||
|
Cash and cash equivalents
|
0-10%
|
|
2.1
|
%
|
—
|
%
|
|||
|
Equity securities
|
0-50%
|
|
42.5
|
%
|
14.2
|
%
|
|||
|
Debt securities
|
20-70%
|
|
41.3
|
%
|
70.1
|
%
|
|||
|
Other investments
|
0-40%
|
|
14.1
|
%
|
15.7
|
%
|
|||
|
Total
|
100
|
%
|
100
|
%
|
100
|
%
|
|||
|
14.
|
Share-Based Payments
|
|
A.
|
Share-Based Compensation Expense
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Stock options / stock appreciation rights
|
$
|
18
|
|
$
|
9
|
|
$
|
—
|
|
|||
|
RSUs / DSUs
|
14
|
|
9
|
|
—
|
|
||||||
|
Pfizer stock benefit plans—direct
|
—
|
|
25
|
|
28
|
|
||||||
|
Share-based compensation expense—direct
|
32
|
|
43
|
|
28
|
|
||||||
|
Share-based compensation expense—indirect
|
—
|
|
—
|
|
5
|
|
||||||
|
Share-based compensation expense—total
|
$
|
32
|
|
$
|
43
|
|
$
|
33
|
|
|||
|
Tax benefit for share-based compensation expense
|
(8
|
)
|
(6
|
)
|
(10
|
)
|
||||||
|
Share-based compensation expense, net of tax
|
$
|
24
|
|
$
|
37
|
|
$
|
23
|
|
|||
|
B.
|
Stock Options
|
|
Year Ended December 31,
|
||||||
|
2014
|
|
2013
|
|
|||
|
Expected dividend yield
(a)
|
0.93
|
%
|
1.00
|
%
|
||
|
Risk-free interest rate
(b)
|
2.01
|
%
|
1.30
|
%
|
||
|
Expected stock price volatility
(c)
|
24.72
|
%
|
28.21
|
%
|
||
|
Expected term
(d)
(years)
|
6.5
|
|
6.5
|
|
||
|
(a)
|
Determined using a constant dividend yield during the expected term of the Zoetis stock option.
|
|
(b)
|
Determined using the interpolated yield on U.S. Treasury zero-coupon issues.
|
|
(c)
|
Determined using implied volatility.
|
|
(d)
|
Determined using expected exercise and post-vesting termination patterns.
|
|
Weighted-Average
|
|||||||||||||
|
Weighted-Average
|
|
Remaining
|
Aggregate
|
|
|||||||||
|
Exercise Price
|
|
Contractual Term
|
Intrinsic Value
(a)
|
|
|||||||||
|
Shares
|
|
Per Share
|
|
(Years)
|
(MILLIONS)
|
|
|||||||
|
Outstanding, December 31, 2013
|
2,879,564
|
|
$
|
26.11
|
|
||||||||
|
Granted
|
3,006,351
|
|
30.97
|
|
|||||||||
|
Exercised
|
(58,536
|
)
|
26.00
|
|
|||||||||
|
Forfeited
|
(286,066
|
)
|
29.75
|
|
|||||||||
|
Outstanding, December 31, 2014
|
5,541,313
|
|
$
|
28.56
|
|
8.7
|
$
|
66
|
|
||||
|
Exercisable, December 31, 2014
|
63,751
|
|
$
|
27.39
|
|
8.4
|
$
|
1
|
|
||||
|
(a)
|
Market price of underlying Zoetis common stock less exercise price.
|
|
Year Ended/As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS, EXCEPT PER STOCK OPTION AMOUNTS)
|
2014
|
|
2013
|
|
||||
|
Weighted-average grant date fair value per stock option
|
$
|
8.01
|
|
$
|
7.05
|
|
||
|
Aggregate intrinsic value on exercise
|
1
|
|
—
|
|
||||
|
Cash received upon exercise
|
2
|
|
—
|
|
||||
|
Tax benefits realized related to exercise
|
1
|
|
—
|
|
||||
|
Total compensation cost related to nonvested stock options not yet recognized, pre-tax
|
17
|
|
11
|
|
||||
|
Weighted-average period over which stock option compensation is expected to be recognized (years)
|
1.8
|
|
2.0
|
|
||||
|
C.
|
Restricted Stock and Restricted Stock Units (RSUs)
|
|
Weighted-Average
|
|
||||||
|
Grant Date Fair Value
|
|
||||||
|
Shares
|
|
Per Share
|
|
||||
|
Nonvested, December 31, 2013
|
949,370
|
|
$
|
26.82
|
|
||
|
Granted
|
844,079
|
|
31.03
|
|
|||
|
Vested
|
(58,306
|
)
|
27.81
|
|
|||
|
Reinvested dividend equivalents
|
10,545
|
|
28.92
|
|
|||
|
Forfeited
|
(122,714
|
)
|
28.77
|
|
|||
|
Nonvested, December 31, 2014
|
1,622,974
|
|
$
|
28.85
|
|
||
|
Year Ended/As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
||||
|
Total compensation cost related to nonvested RSU awards not yet recognized, pre-tax
|
$
|
24
|
|
$
|
17
|
|
||
|
Weighted-average period over which RSU cost is expected to be recognized (years)
|
1.8
|
|
2.1
|
|
||||
|
D.
|
Deferred Stock Units (DSUs)
|
|
E.
|
Other Equity-Based or Cash-Based Awards.
|
|
F.
|
Treatment of Outstanding Pfizer Equity Awards
|
|
15.
|
Stockholders' Equity
|
|
(MILLIONS OF DOLLARS AND SHARES)
|
Common Shares Issued
|
|
Treasury Stock
(a)
|
|
Cost of Treasury Stock
|
|
||||
|
Balance, December 31, 2012
|
—
|
|
—
|
|
$
|
—
|
|
|||
|
IPO
|
500.000
|
|
—
|
|
—
|
|
||||
|
Stock-based compensation
|
0.008
|
|
—
|
|
—
|
|
||||
|
Defined contribution plan
|
—
|
|
—
|
|
—
|
|
||||
|
Balance, December 31, 2013
|
500.008
|
|
—
|
|
—
|
|
||||
|
Stock-based compensation
|
0.104
|
|
0.015
|
|
0.5
|
|
||||
|
Defined contribution plans
|
1.230
|
|
—
|
|
—
|
|
||||
|
Balance, December 31, 2014
|
501.342
|
|
0.015
|
|
$
|
0.5
|
|
|||
|
(a)
|
Treasury shares are reacquired from employees for withholding tax purposes in connection with the vesting and exercise of awards under our equity compensation plan. For additional information regarding share-based compensation, see
|
|
Currency Translation
|
|
Accumulated
|
|
|||||||||
|
Adjustment
|
|
Benefit Plans
|
|
Other
|
|
|||||||
|
Net Unrealized
|
|
Actuarial
|
|
Comprehensive
|
|
|||||||
|
(MILLIONS OF DOLLARS)
|
Losses
|
|
Gains/(Losses)
|
|
Loss
|
|
||||||
|
Balance, December 31, 2011
|
$
|
(59
|
)
|
$
|
(6
|
)
|
$
|
(65
|
)
|
|||
|
Other comprehensive loss, net of tax
|
(93
|
)
|
1
|
|
(92
|
)
|
||||||
|
Balance, December 31, 2012
|
(152
|
)
|
(5
|
)
|
(157
|
)
|
||||||
|
Other comprehensive loss, net of tax
|
(54
|
)
|
(2
|
)
|
(56
|
)
|
||||||
|
Separation adjustments
(a)
|
(6
|
)
|
—
|
|
(6
|
)
|
||||||
|
Balance, December 31, 2013
|
(212
|
)
|
(7
|
)
|
(219
|
)
|
||||||
|
Other comprehensive loss, net of tax
|
(124
|
)
|
(7
|
)
|
(b)
|
(131
|
)
|
|||||
|
Pension plan transfers from Pfizer Inc.
(c)
|
—
|
|
(11
|
)
|
(11
|
)
|
||||||
|
Balance, December 31, 2014
|
$
|
(336
|
)
|
$
|
(25
|
)
|
$
|
(361
|
)
|
|||
|
(a)
|
See
Note 2B. The Separation, Adjustments Associated with the Separation, Senior Notes Offering, Initial Public Offering and Exchange Offer
—
Adjustments Associated with the Separation
.
|
|
(b)
|
Includes the 2014 settlement charge associated with the 2012 sale of our Netherlands manufacturing facility. See
Note 13. Benefit Plans.
|
|
(c)
|
Relates to 2014 transfers of defined benefit pension plans from Pfizer Inc. and the reclassification from
Additional Paid in Capital
to
Accumulated Other Comprehensive Loss
. See
|
|
16.
|
Earnings per Share
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS AND SHARES, EXCEPT PER SHARE DATA)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Numerator
|
||||||||||||
|
Net income before allocation to noncontrolling interests
|
$
|
587
|
|
$
|
503
|
|
$
|
436
|
|
|||
|
Net income/(loss) attributable to noncontrolling interests
|
4
|
|
(1
|
)
|
—
|
|
||||||
|
Net income attributable to Zoetis Inc.
|
$
|
583
|
|
$
|
504
|
|
$
|
436
|
|
|||
|
Denominator
|
||||||||||||
|
Weighted-average common shares outstanding
|
501.055
|
|
500.002
|
|
500.000
|
|
||||||
|
Common stock equivalents: stock options, RSUs and DSUs
|
0.970
|
|
0.315
|
|
|
|
||||||
|
Weighted-average common and potential dilutive shares outstanding
|
502.025
|
|
500.317
|
|
500.000
|
|
||||||
|
Earnings per share attributable to Zoetis Inc. stockholders—basic
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
|||
|
Earnings per share attributable to Zoetis Inc. stockholders—diluted
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
|||
|
17.
|
Commitments and Contingencies
|
|
A.
|
Legal Proceedings
|
|
•
|
Product liability and other product-related litigation, which can include injury, consumer, off-label promotion, antitrust and breach of contract claims.
|
|
•
|
Commercial and other matters, which can include product-pricing claims and environmental claims and proceedings.
|
|
•
|
Patent litigation, which typically involves challenges to the coverage and/or validity of our patents or those of third parties on various products or processes.
|
|
•
|
Government investigations, which can involve regulation by national, state and local government agencies in the United States and in other countries.
|
|
After
|
|
|||||||||||||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2015
|
|
2016
|
|
2017
|
|
2018
|
|
2019
|
|
2019
|
|
Total
|
|
||||||||||||||
|
Maturities
|
$
|
27
|
|
$
|
23
|
|
$
|
17
|
|
$
|
12
|
|
$
|
8
|
|
$
|
17
|
|
$
|
104
|
|
|||||||
|
18.
|
Segment, Geographic and Other Revenue Information
|
|
A.
|
Segment Information
|
|
•
|
The U.S.
|
|
•
|
EuAfME—Includes, among others, the United Kingdom, Germany, France, Italy, Spain, Northern Europe and Central Europe as well as Russia, Turkey and South Africa.
|
|
•
|
CLAR––Includes Canada, Brazil, Mexico, Central America and other South American countries.
|
|
•
|
APAC––Includes Australia, Japan, New Zealand, South Korea, India, China/Hong Kong, Northeast Asia, Southeast Asia and South Asia.
|
|
•
|
Other business activities
, includes our CSS contract manufacturing results, as well as expenses associated with our dedicated veterinary medicine research and development organization, research alliances, U.S. regulatory affairs and other operations focused on the development of our products. Other R&D-related costs associated with non-U.S. market and regulatory activities are generally included in the respective regional segment.
|
|
•
|
Corporate
, which is responsible for platform functions such as business technology, facilities, legal, finance, human resources, business development, public affairs and procurement, among others. These costs also include compensation costs and other miscellaneous operating expenses not charged to our operating segments, as well as interest income and expense.
|
|
•
|
Certain transactions and events such as (i)
Purchase accounting adjustments
, where we incur expenses associated with the amortization of fair value adjustments to inventory, intangible assets and property, plant and equipment; (ii)
Acquisition-related activities
, where we incur costs for restructuring and integration; and (iii)
Certain significant items
, which includes non-acquisition-related restructuring charges, certain asset impairment charges, stand-up costs and costs associated with cost reduction/productivity initiatives.
|
|
•
|
Other unallocated
includes certain overhead expenses associated with our global manufacturing operations not charged to our operating segments. Effective January 1, 2014,
Other unallocated
also includes certain costs associated with business technology and finance that specifically support our global manufacturing operations. These costs were previously reported in
Corporate
. Also, beginning in the first quarter of 2014, certain supply chain and global logistics costs that were previously reported in the four reportable segments are reported in
Other unallocated
. This presentation better reflects how we measure the performance of the global manufacturing organization.
|
|
Depreciation
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
Revenue
(a)
|
Earnings
(b)
|
and Amortization
(c)
|
|||||||||
|
Year Ended December 31, 2014
|
||||||||||||
|
U.S.
|
$
|
2,059
|
|
$
|
1,176
|
|
$
|
33
|
|
|||
|
EuAfME
|
1,141
|
|
437
|
|
20
|
|
||||||
|
CLAR
|
815
|
|
310
|
|
13
|
|
||||||
|
APAC
|
720
|
|
278
|
|
17
|
|
||||||
|
Total reportable segments
|
4,735
|
|
2,201
|
|
83
|
|
||||||
|
Other business activities
(d)
|
50
|
|
(314
|
)
|
28
|
|
||||||
|
Reconciling Items:
|
||||||||||||
|
Corporate
(e)
|
—
|
|
(571
|
)
|
31
|
|
||||||
|
Purchase accounting adjustments
(f)
|
—
|
|
(51
|
)
|
51
|
|
||||||
|
Acquisition-related costs
(g)
|
—
|
|
(8
|
)
|
—
|
|
||||||
|
Certain significant items
(h)
|
—
|
|
(205
|
)
|
5
|
|
||||||
|
Other unallocated
(i)
|
—
|
|
(232
|
)
|
6
|
|
||||||
|
$
|
4,785
|
|
$
|
820
|
|
$
|
204
|
|
||||
|
Year Ended December 31, 2013
|
||||||||||||
|
U.S.
|
$
|
1,902
|
|
$
|
1,045
|
|
$
|
43
|
|
|||
|
EuAfME
|
1,115
|
|
412
|
|
22
|
|
||||||
|
CLAR
|
778
|
|
266
|
|
18
|
|
||||||
|
APAC
|
713
|
|
271
|
|
13
|
|
||||||
|
Total reportable segments
|
4,508
|
|
1,994
|
|
96
|
|
||||||
|
Other business activities
(d)
|
53
|
|
(312
|
)
|
28
|
|
||||||
|
Reconciling Items:
|
||||||||||||
|
Corporate
(e)
|
—
|
|
(567
|
)
|
23
|
|
||||||
|
Purchase accounting adjustments
(f)
|
—
|
|
(48
|
)
|
48
|
|
||||||
|
Acquisition-related costs
(g)
|
—
|
|
(22
|
)
|
—
|
|
||||||
|
Certain significant items
(h)
|
—
|
|
(240
|
)
|
5
|
|
||||||
|
Other unallocated
(i)
|
—
|
|
(115
|
)
|
9
|
|
||||||
|
$
|
4,561
|
|
$
|
690
|
|
$
|
209
|
|
||||
|
Year Ended December 31, 2012
|
||||||||||||
|
U.S.
|
$
|
1,776
|
|
$
|
921
|
|
$
|
28
|
|
|||
|
EuAfME
|
1,068
|
|
376
|
|
27
|
|
||||||
|
CLAR
|
769
|
|
253
|
|
23
|
|
||||||
|
APAC
|
695
|
|
236
|
|
17
|
|
||||||
|
Total reportable segments
|
4,308
|
|
1,786
|
|
95
|
|
||||||
|
Other business activities
(d)
|
28
|
|
(276
|
)
|
17
|
|
||||||
|
Reconciling Items:
|
||||||||||||
|
Corporate
(e)
|
—
|
|
(506
|
)
|
25
|
|
||||||
|
Purchase accounting adjustments
(f)
|
—
|
|
(52
|
)
|
52
|
|
||||||
|
Acquisition-related costs
(g)
|
—
|
|
(53
|
)
|
10
|
|
||||||
|
Certain significant items
(h)
|
—
|
|
(96
|
)
|
1
|
|
||||||
|
Other unallocated
(i)
|
—
|
|
(93
|
)
|
—
|
|
||||||
|
$
|
4,336
|
|
$
|
710
|
|
$
|
200
|
|
||||
|
(a)
|
Revenue denominated in euros were
$710 million
in 2014,
$693 million
in 2013, and
$639 million
in 2012.
|
|
(b)
|
Defined as income before provision for taxes on income.
|
|
(c)
|
Certain production facilities are shared. Depreciation and amortization is allocated to the reportable operating segments based on estimates of where the benefits of the related assets are realized.
|
|
(d)
|
Other business activities
reflects R&D costs managed by our Research and Development organization and not allocated to the operating segments, as well as our contract manufacturing business.
|
|
(e)
|
Corporate
includes, among other things, administration expenses, interest expense, certain compensation and other costs not charged to our operating segments.
|
|
(f)
|
Purchase accounting adjustments
include certain charges related to intangible assets, property, plant and equipment not charged to our operating segments.
|
|
(g)
|
Acquisition-related costs
can include costs associated with acquiring, integrating and restructuring acquired businesses, such as allocated transaction costs, integration costs, restructuring charges and additional depreciation associated with asset restructuring. For additional information, see
|
|
(h)
|
Certain significant items
are substantive, unusual items that, either as a result of their nature or size, would not be expected to occur as part of our normal business on a regular basis. Such items primarily include certain costs related to becoming an independent public company, restructuring charges and implementation costs associated with our cost-reduction/productivity initiatives that are not associated with an acquisition, certain legal and commercial settlements and the impact of divestiture-related gains and losses. For additional information, see
|
|
•
|
For 2014, certain significant items primarily includes: (i) Zoetis stand-up costs of $
168 million
; (ii) charges related to a commercial settlement in Mexico of
$13 million
, partially offset by the insurance recovery of
$1 million
; (iii) restructuring charges of $
12 million
related to employee termination costs in EuAfME and
$6 million
related to employee termination costs in our global manufacturing operations, partially offset by a
$2 million
benefit related to the reversal of a previously established reserve as a result of a change in estimate of employee termination costs; (iv) intangible asset impairment charges related to an IPR&D project acquired with the FDAH acquisition in 2009 of $
6 million
; (v) costs of
$5 million
due to unusual investor-related activities; (vi) the Zoetis portion of a net gain on the sale of land by our Taiwan joint venture of $
3 million
income, and the net gain on the government-mandated sale of certain product rights in Argentina that were acquired with the FDAH acquisition in 2009 of
$2 million
income; (vii) additional depreciation associated with asset restructuring of $
1 million
; (viii) a pension plan settlement charge related to a divestiture of a manufacturing plant of
$4 million
; and (ix) an insurance recovery of other litigation related charges of $
2 million
income. Stand-up costs include certain nonrecurring costs related to becoming an independent public company, such as new branding (including changes to the manufacturing process for required new packaging), the creation of standalone systems and infrastructure, site separation, accelerated vesting and associated cash payment related to certain Pfizer equity awards, and certain legal registration and patent assignment costs.
|
|
•
|
For 2013, certain significant items includes: (i) Zoetis stand-up costs of $
206 million
; (ii) $
20 million
income primarily related to a reversal of certain employee termination expenses, partially offset by restructuring charges related to exiting certain manufacturing and research facilities; (iii) $
6 million
income on the government-mandated sale of certain product rights in Brazil that were acquired with the FDAH acquisition in
2009
; (iv) asset impairment charges associated with asset restructuring of $
19 million
; (v) additional depreciation associated with asset restructuring of $
8 million
; (vi) write-offs of inventory and intercompany accounts that were transferred to us as part of the Separation from Pfizer of $
24 million
; and (vii) litigation-related charges of $
5 million
.
|
|
•
|
In 2012, certain significant items includes: (i)
$115 million
for restructuring charges and implementation costs associated with our cost-reduction/productivity initiatives that are not associated with an acquisition; (ii)
$14 million
income related to a favorable legal settlement for an intellectual property matter; and (iii)
$4 million
income due to a change in estimate related to transitional manufacturing purchase agreements associated with divestitures.
|
|
(i)
|
Includes overhead expenses associated with our manufacturing operations.
|
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
||||
|
U.S.
|
$
|
867
|
|
$
|
827
|
|
||
|
EuAfME
|
217
|
|
233
|
|
||||
|
CLAR
|
104
|
|
114
|
|
||||
|
APAC
|
130
|
|
121
|
|
||||
|
Property, plant and equipment, less accumulated depreciation
|
$
|
1,318
|
|
$
|
1,295
|
|
||
|
C.
|
Other Revenue Information
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Livestock:
|
||||||||||||
|
Cattle
|
$
|
1,747
|
|
$
|
1,628
|
|
$
|
1,603
|
|
|||
|
Swine
|
695
|
|
652
|
|
592
|
|
||||||
|
Poultry
|
568
|
|
551
|
|
506
|
|
||||||
|
Other
|
93
|
|
85
|
|
94
|
|
||||||
|
3,103
|
|
2,916
|
|
2,795
|
|
|||||||
|
Companion Animal:
|
||||||||||||
|
Equine
|
182
|
|
179
|
|
185
|
|
||||||
|
Dogs and Cats
|
1,450
|
|
1,413
|
|
1,328
|
|
||||||
|
1,632
|
|
1,592
|
|
1,513
|
|
|||||||
|
Contract Manufacturing
|
50
|
|
53
|
|
28
|
|
||||||
|
Total revenue
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2014
|
|
2013
|
|
2012
|
|
||||||
|
Anti-infectives
|
$
|
1,398
|
|
$
|
1,295
|
|
$
|
1,268
|
|
|||
|
Vaccines
|
1,212
|
|
1,189
|
|
1,108
|
|
||||||
|
Parasiticides
|
708
|
|
691
|
|
675
|
|
||||||
|
Medicated feed additives
|
479
|
|
446
|
|
403
|
|
||||||
|
Other pharmaceuticals
|
783
|
|
739
|
|
710
|
|
||||||
|
Other non-pharmaceuticals
|
155
|
|
148
|
|
144
|
|
||||||
|
Contract manufacturing
|
50
|
|
53
|
|
28
|
|
||||||
|
Total revenue
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
|||
|
19.
|
Transactions and Agreements with Pfizer
|
|
A.
|
Pre-Separation Period
|
|
B.
|
Agreements with Pfizer
|
|
•
|
Global separation agreement
. This agreement governs the relationship between Pfizer and us following the IPO and includes provisions related to the allocation of assets and liabilities, indemnification, delayed transfers and further assurances, mutual releases, insurance and certain covenants.
|
|
•
|
Transitional services agreement
. This agreement grants us the right to continue to use certain of Pfizer's services and resources related to our corporate functions, such as business technology, facilities, finance, human resources, public affairs and procurement, in exchange for mutually agreed-upon fees based on Pfizer's costs of providing these services.
|
|
•
|
Tax matters agreement
. This agreement governs ours and Pfizer's respective rights, responsibilities and obligations with respect to tax liabilities and benefits, tax attributes, the preparation and filing of tax returns, the control of audits and other tax proceedings and other matters regarding taxes. Pursuant to this agreement, we have also agreed to certain covenants that contain restrictions intended to preserve the tax-free status of certain transactions, and we have agreed to indemnify Pfizer and its affiliates against any and all tax-related liabilities incurred by them relating to these transactions to the extent caused by an acquisition of our stock or assets or by any other action undertaken by us.
|
|
•
|
Research and development collaboration and license agreement
. This agreement permits certain of our employees to be able to review a Pfizer database to identify compounds that may be of interest to the animal health field. Pfizer has granted to us an option to enter into a license agreement subject to certain restrictions and requirements and we will make payments to Pfizer.
|
|
•
|
Employee matters agreement
. This agreement governs ours and Pfizer's respective rights, responsibilities and obligations with respect to the following matters: employees and former employees (and their respective dependents and beneficiaries) who are or were associated with Pfizer, us or the parties' respective subsidiaries or affiliates; the allocation of assets and liabilities generally relating to employees, employment or service-related matters and employee benefit plans; and other human resources, employment and employee benefits matters.
|
|
•
|
Master manufacturing and supply agreements
. These two agreements govern our manufacturing and supply arrangements with Pfizer. Under one of these agreements, Pfizer will manufacture and supply us with animal health products. Under this agreement, our manufacturing and supply chain leadership will have oversight responsibility over product quality and other key aspects of the manufacturing process with respect to the Pfizer-supplied products. Under the other agreement, we will manufacture and supply certain human health products to Pfizer.
|
|
•
|
Environmental matters agreement
. This agreement governs the performance of remedial actions for liabilities allocated to each party under the global separation agreement; addresses our substitution for Pfizer with respect to animal health assets and remedial actions allocated to us (including substitution related to, for example, permits, financial assurances and consent orders); allows our conditional use of Pfizer's consultants and contractors to assist in the conduct of remedial actions; and addresses the exchange of related information between the parties. The agreement also sets forth standards of conduct for remedial activities at the co-located facilities: Guarulhos, Brazil; Catania, Italy; Hsinchu, Taiwan; and Kalamazoo, Michigan, in the United States. In addition, the agreement sets forth site-specific terms to govern conduct at several of these co-located facilities.
|
|
•
|
Screening services agreement
. This agreement requires us to provide certain high throughput screening services to Pfizer's R&D organization for which Pfizer pays to us agreed-upon fees.
|
|
•
|
Intellectual property license agreements
. Under these agreements (i) Pfizer and certain of its affiliates licensed to us and certain of our affiliates the right to use certain intellectual property rights in the animal health field; (ii) we licensed to Pfizer and certain of its affiliates certain rights to intellectual property in all fields outside the animal health field; and (iii) Pfizer granted us rights with respect to certain trademarks and copyrighted works.
|
|
•
|
Intellectual Property
. As part of the Separation, Pfizer assigned to us ownership of certain animal health related patents, pending patent applications, and trademark applications and registrations. In addition, Pfizer licensed to us the right to use certain intellectual property rights in the animal health field. We licensed to Pfizer the right to use certain of our trademarks and substantially all of our other intellectual property rights in the human health field and all other fields outside of animal health. In addition, Pfizer granted us a transitional license to use certain of Pfizer's trademarks and we granted Pfizer a transitional license to use certain of our trademarks for a period of time following the completion of the IPO.
|
|
•
|
Manufacturing Facilities
. Our global manufacturing network consists of
13
“anchor” manufacturing sites and
14
“satellite” manufacturing sites. Ownership of, or the existing leasehold interest in, these facilities were conveyed to us by Pfizer as part of the Separation. Among these
27
manufacturing sites is our facility in Guarulhos, Brazil, which we leased back to Pfizer. Certain of our products are currently manufactured at
11
manufacturing sites that were retained by Pfizer. The products manufactured by Pfizer at these sites and at our Guarulhos, Brazil facility continue to be supplied to us under the terms of a manufacturing and supply agreement we entered into with Pfizer.
|
|
•
|
R&D Facilities
. We have R&D operations co-located with certain of our manufacturing sites in Australia, Belgium, Brazil, Spain and the United States to facilitate the efficient transfer of production processes from our laboratories to manufacturing sites. In addition, we maintain R&D operations at non-manufacturing locations in Belgium, Brazil, India and the United States. As part of the Separation, Pfizer conveyed to us its interest in each of these R&D facilities, with the exception of our Mumbai, India facility, which we expect Pfizer to transfer to us after the completion of the Separation for cash consideration to be agreed upon, and, in the interim, we are leasing this facility from Pfizer.
|
|
•
|
Employees
. In general, as part of the Separation, employees of Pfizer who were substantially dedicated to the animal health business became our employees. However, labor and employment laws or other business considerations in some jurisdictions delayed Pfizer from transferring to us employees who are substantially dedicated to the animal health business. In those instances, to the extent permissible under applicable law, we and Pfizer entered into mutually-acceptable arrangements to provide for continued operation of the business until such time as the employees in those jurisdictions can be transferred to us.
|
|
(MILLIONS OF DOLLARS)
|
||||
|
Transitional services agreement
|
$
|
63
|
|
|
|
Master manufacturing and supply agreements
|
130
|
|
||
|
Employee matters agreement
|
99
|
|
||
|
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA)
|
FIRST
|
|
SECOND
|
|
THIRD
|
|
FOURTH
|
|
||||||||
|
2014:
|
||||||||||||||||
|
Revenue
|
$
|
1,097
|
|
$
|
1,158
|
|
$
|
1,210
|
|
$
|
1,320
|
|
||||
|
Costs and expenses
(a)
|
867
|
|
953
|
|
970
|
|
1,150
|
|
||||||||
|
Restructuring charges and certain acquisition-related costs
|
3
|
|
5
|
|
2
|
|
15
|
|
||||||||
|
Income before provision for taxes on income
|
227
|
|
200
|
|
238
|
|
155
|
|
||||||||
|
Provision for taxes on income
|
72
|
|
61
|
|
71
|
|
29
|
|
||||||||
|
Net income before allocation to noncontrolling interests
|
155
|
|
139
|
|
167
|
|
126
|
|
||||||||
|
Net income/(loss) attributable to noncontrolling interests
|
—
|
|
3
|
|
1
|
|
—
|
|
||||||||
|
Net income/(loss) attributable to Zoetis
|
$
|
155
|
|
$
|
136
|
|
$
|
166
|
|
$
|
126
|
|
||||
|
Earnings per common share--basic
(b)
|
$
|
0.31
|
|
$
|
0.27
|
|
$
|
0.33
|
|
$
|
0.25
|
|
||||
|
Earnings per common share--diluted
(b)
|
$
|
0.31
|
|
$
|
0.27
|
|
$
|
0.33
|
|
$
|
0.25
|
|
||||
|
2013:
|
||||||||||||||||
|
Revenue
|
$
|
1,090
|
|
$
|
1,114
|
|
$
|
1,103
|
|
$
|
1,254
|
|
||||
|
Costs and expenses
(a)
|
891
|
|
947
|
|
915
|
|
1,092
|
|
||||||||
|
Restructuring charges and certain acquisition-related costs
|
7
|
|
(20
|
)
|
3
|
|
36
|
|
||||||||
|
Income before provision for taxes on income
|
192
|
|
187
|
|
185
|
|
126
|
|
||||||||
|
Provision for taxes on income
|
52
|
|
59
|
|
54
|
|
22
|
|
||||||||
|
Net income before allocation to noncontrolling interests
|
140
|
|
128
|
|
131
|
|
104
|
|
||||||||
|
Net income/(loss) attributable to noncontrolling interests
|
—
|
|
—
|
|
—
|
|
(1
|
)
|
||||||||
|
Net income attributable to Zoetis
|
$
|
140
|
|
$
|
128
|
|
$
|
131
|
|
$
|
105
|
|
||||
|
Earnings per common share--basic
(b)
|
$
|
0.28
|
|
$
|
0.26
|
|
$
|
0.26
|
|
$
|
0.21
|
|
||||
|
Earnings per common share--diluted
(b)
|
$
|
0.28
|
|
$
|
0.26
|
|
$
|
0.26
|
|
$
|
0.21
|
|
||||
|
(a)
|
Costs and expenses in the fourth quarter reflect seasonal trends as well as specific costs associated with the build-up of our capabilities as an independent company.
|
|
(b)
|
The weighted average common shares outstanding for both basic and diluted earnings per share for 2012 was calculated using an aggregate of
500 million
shares of common stock outstanding, which was the number of Zoetis Inc. shares outstanding at the time of the IPO. There were no Zoetis RSUs, stock options or performance shares outstanding prior to the IPO.
|
|
21.
|
Subsequent Events
|
|
Balance,
|
|
Balance,
|
|
|||||||||||||
|
Beginning of
|
|
End of
|
|
|||||||||||||
|
(MILLIONS OF DOLLARS)
|
Period
|
|
Additions
|
|
Deductions
|
|
Period
|
|
||||||||
|
Year Ended December 31, 2014
|
||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
31
|
|
$
|
5
|
|
$
|
(4
|
)
|
$
|
32
|
|
||||
|
Year Ended December 31, 2013
|
||||||||||||||||
|
Allowance for doubtful accounts
|
49
|
|
6
|
|
(24
|
)
|
(a)
|
31
|
|
|||||||
|
Year Ended December 31, 2012
|
||||||||||||||||
|
Allowance for doubtful accounts
|
29
|
|
23
|
|
(3
|
)
|
49
|
|
||||||||
|
Item 9A.
|
Controls and Procedures
|
|
Item 12.
|
Security Ownership Of Certain Beneficial Owners And Management And Related Stockholder Matters.
|
|
•
|
warranty obligations created as part of the animal health business;
|
|
•
|
product liability claims with respect to any animal health product;
|
|
•
|
environmental liabilities relating to the animal health business and environmental liabilities at the real property that we acquired from Pfizer;
|
|
•
|
liabilities related to animal health businesses or operations that were discontinued or divested by Pfizer;
|
|
•
|
litigation liabilities; and
|
|
•
|
our debt obligations, including under the senior notes offering.
|
|
•
|
Pfizer is responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments and including those taxes attributable to our business) reportable on a consolidated, combined or unitary return that includes Pfizer or any of its subsidiaries (and us and/or any of our subsidiaries) for any periods or portions thereof ending on or prior to December 31, 2012. We are responsible for the portion of any such taxes for periods or portions thereof beginning on or after January 1, 2013, as would be applicable to us if we filed the relevant tax returns on a standalone basis.
|
|
•
|
We are responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments) that are reportable on returns that include only us and/or any of our subsidiaries, for all tax periods whether before or after the Separation date.
|
|
•
|
Pfizer is responsible for certain specified foreign taxes directly resulting from certain aspects of the Separation.
|
|
•
|
issuance or sale of stock or other securities (including securities convertible into our stock but excluding certain compensatory arrangements);
|
|
•
|
sales of assets outside the ordinary course of business; and
|
|
•
|
entering into any other corporate transaction which would cause us to undergo a 40% or greater change in our stock ownership.
|
|
•
|
employees and former employees (and their respective dependents and beneficiaries) who are or were associated with Pfizer, us or the parties' respective subsidiaries or affiliates;
|
|
•
|
the allocation of assets and liabilities generally relating to employees, employment or service-related matters and employee benefit plans; and
|
|
•
|
other human resources, employment and employee benefits matters.
|
|
A.
|
(1) The financial statements and notes to financial statements are filed as part of this report in Item 8. Financial Statements and Supplementary Data.
|
|
Zoetis Inc.
|
|
|
By:
|
/S/ JUAN RAMÓN ALAIX
|
|
Juan Ramón Alaix
|
|
|
Chief Executive Officer and Director
|
|
|
Name
|
Title
|
Date
|
||
|
/S/ JUAN RAMÓN ALAIX
|
Chief Executive Officer and Director
|
February 27, 2015
|
||
|
Juan Ramón Alaix
|
(Principal Executive Officer)
|
|||
|
/S/ PAUL S. HERENDEEN
|
Executive Vice President and
|
February 27, 2015
|
||
|
Paul S. Herendeen
|
Chief Financial Officer
(Principal Financial Officer and
Principal Accounting Officer)
|
|||
|
/S/ MICHAEL B. MCCALLISTER
|
Chairman and Director
|
February 27, 2015
|
||
|
Michael B. McCallister
|
||||
|
/S/ FRANK A. D'AMELIO
|
Director
|
February 27, 2015
|
||
|
Frank A. D’Amelio
|
||||
|
/S/ WILLIAM F. DOYLE
|
Director
|
February 27, 2015
|
||
|
William F. Doyle
|
||||
|
/S/ SANJAY KHOSLA
|
Director
|
February 27, 2015
|
||
|
Sanjay Khosla
|
||||
|
/s/ GREGORY NORDEN
|
Director
|
February 27, 2015
|
||
|
Gregory Norden
|
||||
|
/S/ LOUISE M. PARENT
|
Director
|
February 27, 2015
|
||
|
Louise M. Parent
|
||||
|
/S/ WILLIE M. REED
|
Director
|
February 27, 2015
|
||
|
Willie M. Reed
|
||||
|
/s/ ROBERT W. SCULLY
|
Director
|
February 27, 2015
|
||
|
Robert W. Scully
|
||||
|
/S/ WILLIAM C. STEERE, JR.
|
Director
|
February 27, 2015
|
||
|
William C. Steere, Jr.
|
||||
|
Exhibit 3.1
|
Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to Zoetis Inc.'s Quarterly
|
|
|
Report on Form 10-Q filed on November 10, 2014)
|
||
|
Exhibit 3.2
|
Amended and Restated By-laws of the Registrant (incorporated by reference to Exhibit 3.2 to Zoetis Inc.'s 2012 Annual
|
|
|
Report on Form 10-K filed on March 28, 2013)
|
||
|
Exhibit 3.3
|
Certificate of Designation of Series A Junior Participating Preferred Stock of Zoetis Inc. filed with the Secretary of State
|
|
|
of the State of Delaware on November 17, 2014 (incorporate by reference to Exhibit 3.1 to Zoetis Inc.’s Current Report
|
||
|
on Form 8-K filed on November 17, 2014)
|
||
|
Exhibit 4.1
|
Specimen Class A Common Stock Certificate (incorporated by reference to Exhibit 4.1 of Zoetis Inc.’s registration
|
|
|
statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.2
|
Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company Americas, as trustee
|
|
|
(incorporated by reference to Zoetis Inc.'s registration statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.3
|
First Supplemental Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company
|
|
|
Americas, as trustee (incorporated by reference to Exhibit 4.3 of Zoetis Inc.'s registration statement on Form S-1
|
||
|
(File No. 333-183254))
|
||
|
Exhibit 4.4
|
Form of 1.150% Senior Notes due 2016 (incorporated by reference to Exhibit 4.4 of Zoetis Inc.'s registration statement on
|
|
|
Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.5
|
Form of 1.875% Senior Notes due 2018 (incorporated by reference to Exhibit 4.5 of Zoetis Inc.'s registration statement on
|
|
|
Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.6
|
Form of 3.250% Senior Notes due 2023 (incorporated by reference to Exhibit 4.6 of Zoetis Inc.'s registration statement on
|
|
|
Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.7
|
Form of 4.700% Senior Notes due 2043 (incorporated by reference to Exhibit 4.7 of Zoetis Inc.'s registration statement on
|
|
|
Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.8
|
Rights Agreement, dated November 14, 2014, between Zoetis Inc. and Computershare Trust Company, N.A., as Rights Agent,
|
|
|
including the form of Certificate of Designation as Exhibit A, the form of Rights Certificate as Exhibit B and the form
|
||
|
of Summary of Rights to Purchase Preferred Stock as Exhibit C (incorporated by reference to Exhibit 4.1 to Zoetis Inc.’s
|
||
|
Current Report on Form 8-K filed on November 17, 2014)
|
||
|
Exhibit 10.1
|
Global Separation Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013)
|
||
|
Exhibit 10.2
|
Transitional Services Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.2 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013)
|
||
|
Exhibit 10.3
|
Tax Matters Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.3 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013)
|
||
|
Exhibit 10.4
|
Research and Development Collaboration and License Agreement, dated February 6, 2013, by and between Zoetis Inc.
|
|
|
and Pfizer Inc. (incorporated by reference to Exhibit 10.4 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on
|
||
|
March 28, 2013)
|
||
|
Exhibit 10.5
|
Employee Matters Agreement (incorporated by reference to Exhibit 10.5 of Zoetis Inc.'s registration statement on Form S-1
|
|
|
(File No. 333-183254))
|
||
|
Exhibit 10.6
|
Pfizer Inc. 2004 Stock Plan, as Amended and Restated (incorporated by reference to Exhibit 10.6 of Zoetis Inc.'s registration
|
|
|
statement on Form S-1 (File No. 333-183254))*
|
||
|
Exhibit 10.7
|
Pfizer Inc. Amended and Restated Nonfunded Supplemental Retirement Plan, together with all material Amendments
|
|
|
(incorporated by reference to Exhibit 10.7 of Zoetis Inc.'s registration statement on Form S-1 (File No. 333-183254))*
|
||
|
Exhibit 10.8
|
Patent and Know-How License Agreement (Zoetis as licensor), dated February 6, 2013, by and between Zoetis Inc. and
|
|
|
Pfizer Inc. (incorporated by reference to Exhibit 10.8 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on
|
||
|
March 28, 2013)
|
||
|
Exhibit 10.9
|
Patent and Know-How License Agreement (Pfizer as licensor), dated February 6, 2013, by and between Zoetis Inc. and
|
|
|
Pfizer Inc. (incorporated by reference to Exhibit 10.9 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on
|
||
|
March 28, 2013)
|
||
|
Exhibit 10.10
|
Trademark and Copyright License Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.10 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013)
|
||
|
Exhibit 10.11
|
Private Instrument of Non Residential Lease Agreement and Others, dated September 28, 2012, by and between PAH Brasil
|
|
|
Participações Ltda. and Laboratórios Pfizer Ltda. (incorporated by reference to Exhibit 10.11 of Zoetis Inc.'s registration
|
||
|
statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 10.12
|
Private Instrument of Lease Agreement Movable Assets and Others, dated September 28, 2012, by and between PAH Brasil
|
|
|
Participações Ltda. and Laboratórios Pfizer Ltda. (incorporated by reference to Exhibit 10.12 of Zoetis Inc.'s registration
|
||
|
statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 10.13
|
Environmental Matters Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.13 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013)
|
||
|
Exhibit 10.14
|
Master Manufacturing and Supply Agreement, dated October 1, 2012, by and between Pfizer Inc. and Zoetis Inc. (Pfizer as
|
|
|
as manufacturer) (incorporated by reference to Exhibit 10.14 of Zoetis Inc.'s registration statement on Form S-1
|
||
|
(File No. 333-183254))
|
||
|
Exhibit 10.15
|
Registration Rights Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.15 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013)
|
||
|
Exhibit 10.16
|
Zoetis Inc. 2013 Equity and Incentive Plan (incorporated by reference to Exhibit 10.16 to Zoetis Inc.’s 2012 Annual Report
|
|
|
on Form 10-K filed on March 28, 2013)*
|
||
|
Exhibit 10.17
|
Sale of Business Severance Plan (incorporated by reference to Exhibit 10.17 to Zoetis Inc.’s 2012 Annual Report on
|
|
|
Form 10-K filed on March 28, 2013)*
|
||
|
Exhibit 10.18
|
Revolving Credit Agreement, dated as of December 21, 2012, among Zoetis Inc., the lenders named therein and JPMorgan
|
|
|
Chase Bank, N.A., as administrative agent (incorporated by reference to Exhibit 10.18 of Zoetis Inc.'s registration statement
|
||
|
on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 10.19
|
Form of Indemnification Agreement for directors and officers (incorporated by reference to Exhibit 10.19 of Zoetis Inc.'s
|
|
|
registration statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 10.20
|
Registration Rights Agreement, dated as of January 28, 2013, by and among Zoetis Inc. and Merrill Lynch, Pierce, Fenner &
|
|
|
Smith Incorporated, Barclays Capital Inc., J.P. Morgan Securities LLC and Deutsche Bank Securities Inc., as representatives
|
||
|
of the several initial purchasers (incorporated by reference to Exhibit 10.20 of Zoetis Inc.'s registration statement on Form
|
||
|
S-1 (File No. 333-183254))
|
||
|
Exhibit 10.21
|
Form of Restricted Stock Unit Award agreement (incorporated by reference to Exhibit 10.21 to Zoetis Inc.’s 2012 Annual
|
|
|
Report on Form 10-K filed on March 28, 2013)*
|
||
|
Exhibit 10.22
|
Form of Stock Option Award agreement (incorporated by reference to Exhibit 10.22 to Zoetis Inc.’s 2012 Annual Report
|
|
|
on Form 10-K filed on March 28, 2013)*
|
||
|
Exhibit 10.23
|
Form of Non-Employee Director Deferred Stock Unit Award agreement (incorporated by reference to Exhibit 10.22
|
|
|
on Form 10-K filed on March 28, 2013)*
|
||
|
Exhibit 10.24
|
Form of Cash Award agreement (incorporated by reference to Exhibit 10.24 to Zoetis Inc.’s 2012 Annual Report on
|
|
|
Form 10-K filed on March 28, 2013)*
|
||
|
Exhibit 10.25
|
Non-Employee Director Deferred Compensation Plan (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s
|
|
|
Current Report on Form 8-K filed on May 7, 2013)*
|
||
|
Exhibit 10.26
|
Zoetis Executive Severance Plan (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s Quarterly Report on Form 10-Q
|
|
|
filed on August 14, 2013)*
|
||
|
Exhibit 10.27
|
Zoetis Supplemental Savings Plan, as amended and restated, effective September 15, 2014 (incorporated by reference to
|
|
|
Exhibit 10.4 to Zoetis Inc.'s Quarterly Report on Form 10-Q filed on November 10, 2014*
|
||
|
Exhibit 10.28
|
Severance and Release Agreement between the Registrant and Richard A. Passov, effective April 21, 2014
|
|
|
(incorporated by reference to Exhibit 10.2 to Zoetis Inc.’s Quarterly Report on Form 10-Q filed on August 12, 2014)*
|
||
|
Exhibit 10.29
|
Zoetis Equity Deferral Plan, effective November 1, 2014 (incorporated by reference to Exhibit 10.5 to Zoetis Inc.’s
|
|
|
Quarterly Report on Form 10-Q filed on November 10, 2014)*
|
||
|
Exhibit 10.30
|
Offer Letter between Zoetis Inc. and Paul Herendeen, dated July 31, 2014 (incorporated by reference to Exhibit 10.3
|
|
|
to Zoetis Inc.’s Quarterly Report on Form 10-Q filed on November 10, 2014)*
|
||
|
Exhibit 10.31
|
Letter Agreement, dated as of February 3, 2015, by and among Zoetis and Pershing Square Capital Management, L.P.
|
|
|
and certain affiliates thereof and Sachem Head Capital Management LP and certain affiliates thereof (incorporated by
|
||
|
reference to Exhibit 99.1 to Zoetis Inc.’s Current Report on Form 8-K filed on February 4, 2015
|
||
|
Exhibit 12
|
Computation of Ratio of Earnings to Fixed Charges †
|
|
|
Exhibit 21.1
|
Subsidiaries of the Registrant †
|
|
|
Exhibit 23.1
|
Consent of KPMG LLP †
|
|
|
Exhibit 24.1
|
Power of Attorney (included as part of signature page) †
|
|
|
Exhibit 31.1
|
Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 †
|
|
|
Exhibit 31.2
|
Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 †
|
|
|
Exhibit 32.1
|
Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the
|
|
|
Sarbanes-Oxley Act of 2002 †
|
||
|
Exhibit 32.2
|
Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the
|
|
|
Sarbanes-Oxley Act of 2002 †
|
||
|
EX-101.INS
|
INSTANCE DOCUMENT
|
|
|
EX-101.SCH
|
SCHEMA DOCUMENT
|
|
|
EX-101.CAL
|
CALCULATION LINKBASE DOCUMENT
|
|
|
EX-101.LAB
|
LABELS LINKBASE DOCUMENT
|
|
|
EX-101.PRE
|
PRESENTATION LINKBASE DOCUMENT
|
|
|
EX-101.DEF
|
DEFINITION LINKBASE DOCUMENT
|
|
|
†
|
Filed herewith
|
|
*
|
Management contracts or compensatory plans or arrangements
|