|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the fiscal year ended December 31, 2016
|
||
|
or
|
||
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from __________ to __________
|
||
|
Zoetis Inc.
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
46-0696167
|
|
|
(State or other jurisdiction of
|
(I.R.S. Employer Identification No.)
|
|
|
incorporation or organization)
|
||
|
10 Sylvan Way, Parsippany, New Jersey
|
07054
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
(973) 822-7000
|
|
(Registrant’s telephone number, including area code)
|
|
Title of each class
|
Name of each exchange on which registered
|
||
|
Common Stock, $0.01 par value per share
|
New York Stock Exchange
|
||
|
Large accelerated filer
x
|
Accelerated filer
¨
|
Non-accelerated filer
¨
|
Smaller reporting company
¨
|
|||
|
Page
|
||||
|
Item 1.
|
||||
|
Item 1A.
|
||||
|
Item 1B.
|
||||
|
Item 2.
|
||||
|
Item 3.
|
||||
|
Item 4.
|
||||
|
Item 5.
|
||||
|
Item 6.
|
||||
|
Item 7.
|
||||
|
Item 7A.
|
||||
|
Item 8.
|
||||
|
Item 9.
|
||||
|
Item 9A.
|
||||
|
Item 9B.
|
||||
|
Item 10.
|
||||
|
Item 11.
|
||||
|
Item 12.
|
||||
|
Item 13.
|
||||
|
Item 14.
|
||||
|
Item 15.
|
||||
|
Item 16.
|
||||
|
•
|
economic differences, such as standards of living in developed markets as compared to emerging markets;
|
|
•
|
cultural differences, such as dietary preferences for different animal proteins, pet ownership preferences and pet care standards;
|
|
•
|
epidemiological differences, such as the prevalence of certain bacterial and viral strains and disease dynamics;
|
|
•
|
treatment differences, such as utilization of different types of medicines and vaccines, as well as the pace of adoption of new technologies;
|
|
•
|
environmental differences, such as seasonality, climate and the availability of arable land and fresh water; and
|
|
•
|
regulatory differences, such as standards for product approval and manufacturing.
|
|
•
|
United States
with revenue of $
2,447 million
, or
50%
of total revenue for the year ended
December 31, 2016
; and
|
|
•
|
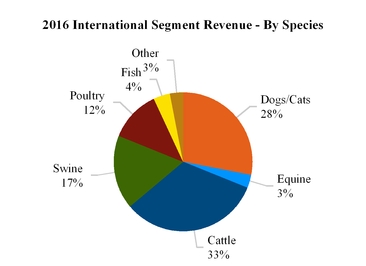
International
with revenue of
$2,390 million
, or
49%
of total revenue for the year ended
December 31, 2016
.
|
|
(MILLIONS OF DOLLARS)
|
Revenue
|
Livestock
|
Companion Animal
|
|
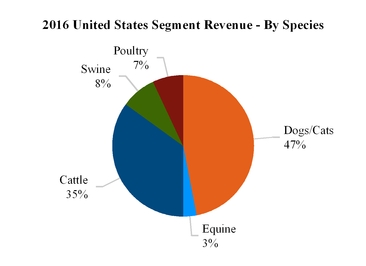
United States
|
$2,447
|
50%
|
50%
|
|
Australia
|
$157
|
62%
|
38%
|
|
Brazil
|
$245
|
82%
|
18%
|
|
Canada
|
$173
|
61%
|
39%
|
|
China
|
$145
|
73%
|
27%
|
|
France
|
$117
|
62%
|
38%
|
|
Germany
|
$125
|
53%
|
47%
|
|
Italy
|
$83
|
50%
|
50%
|
|
Japan
|
$127
|
47%
|
53%
|
|
Mexico
|
$76
|
85%
|
15%
|
|
Spain
|
$82
|
72%
|
28%
|
|
United Kingdom
|
$151
|
48%
|
52%
|
|
Other Developed
|
$302
|
66%
|
34%
|
|
Other Emerging
|
$607
|
84%
|
16%
|
|
•
|
anti-infectives
: products that prevent, kill or slow the growth of bacteria, fungi or protozoa;
|
|
•
|
vaccines
: biological preparations that help prevent diseases of the respiratory, gastrointestinal and reproductive tracts or induce a specific immune response;
|
|
•
|
parasiticides
: products that prevent or eliminate external and internal parasites such as fleas, ticks and worms;
|
|
•
|
medicated feed additives
: products added to animal feed that provide medicines to livestock; and
|
|
•
|
other pharmaceutical products
: pain and sedation, oncology, antiemetic, allergy and dermatology, and reproductive products.
|
|
•
|
Apoquel
®
, the first Janus kinase inhibitor for use in veterinary medicine, was approved for the control of pruritus associated with allergic dermatitis and the control of atopic dermatitis in dogs at least 12 months of age. Since January 2014, we launched Apoquel in all key markets including the United States, Europe, Japan, Brazil, and Australia and expect ongoing market launches throughout 2017;
|
|
•
|
Cerenia
®
, the first and only product on the market to prevent vomiting due to motion sickness in dogs, was first launched in Europe in 2006, followed by the United States in 2007; it was approved to prevent vomiting in cats in 2012 in the United States and European countries. In January 2016, it was approved in the United States for intravenous administration in dogs and cats four months of age and older and for the prevention of vomiting caused by emetogenic or chemotherapeutic agents in dogs four months of age or older;
|
|
•
|
Cytopoint
TM
, the first canine monoclonal antibody to help reduce the clinical signs such as itching of atopic dermatitis in dogs of any age, licensed in the United States in 2016. An injection given once every four to eight weeks, Cytopoint neutralizes interleukin - 31, a protein that has been demonstrated to trigger itching in dogs. This therapy is conditionally licensed in Canada and an application for approval in the European Union is under review;
|
|
•
|
Fostera
®
PCV MH was introduced in November 2013 in the United States and approved in the European Union in 2015. It was developed to help protect pigs from PCVAD and enzootic pneumonia caused by
M. hyopneumoniae
. The one-bottle formulation of Fostera PCV MH allows the convenience of a one-dose program or the flexibility of a two-dose program;
|
|
•
|
Improvac
®
/Improvest
®
/Vivax
®
, a protein product that works like an immunization, is currently the only product that provides a safe and effective alternative to physical castration to manage unpleasant aromas that can occur when cooking pork; launched in Australia and New Zealand in 2004, in Brazil in 2007, in certain European countries beginning in 2008, and in the United States in 2011;
|
|
•
|
Inforce
®
3, the first vaccine for cattle that prevents respiratory disease caused by bovine respiratory syncytial virus (BRSV) while also aiding in the prevention of infectious bovine rhinotracheitis (IBR) and parainfluenza
3
(PI
3
), launched in 2010;
|
|
•
|
Palladia
®
, the first drug to be approved by the U.S. Food and Drug Administration (FDA) for treating cancer in dogs, launched in 2009;
|
|
•
|
Simparica
®
(sarolaner) Chewables, a monthly chewable tablet for dogs to control fleas and ticks, was approved in the European Union and New Zealand in 2015, and in the United States, Canada, Australia, and Brazil (Simparic) in 2016; and
|
|
•
|
Vanguard
®
and Versican Plus
®
are market leading vaccine lines for dogs intended to help prevent a range of diseases including those that are zoonotic. The Versican Plus line was launched in the European Union in 2014. Zoetis added new and innovative vaccines to its Vanguard line of canine vaccines with Vanguard crLyme, Vanguard Rapid Resp Intranasal, Vanguard B Oral, and Vanguard CIV H3N2.
|
|
Product line / product
|
Description
|
Primary species
|
||
|
Anti-infectives
|
||||
|
Ceftiofur injectable line
|
Broad-spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria, including ß-lactamase-producing strains, with some formulations producing a single course of therapy in one injection
|
Cattle, sheep, swine
|
||
|
Draxxin
®
|
Single-dose low-volume antibiotic for the treatment and prevention of bovine and swine respiratory disease, infectious bovine keratoconjunctivitis and bovine foot rot
|
Cattle, swine, sheep
|
||
|
Spectramast
®
|
Treatment of subclinical or clinical mastitis in dry or lactating dairy cattle, delivered via intramammary infusion; same active ingredient as the ceftiofur line
|
Cattle
|
||
|
Terramycin
®
line
|
Antibiotic for the treatment of susceptible infections
|
Cattle, poultry, sheep, swine
|
||
|
Vaccines
|
||||
|
Bovi-Shield
®
line
|
Aids in preventing diseases, including infectious bovine rhinotracheitis (IBR), bovine viral diarrhea (BVD) Types 1 and 2, parainfluenza
3
(PI
3
), bovine respiratory syncytial virus (BRSV), and leptospirosis caused by
Leptospira borgpetersenii
,
L.canicola, L grippotyphosa, L. hardjo, L. icterohaemorrhagiae, and L. pamona
, depending on formulation
|
Cattle
|
||
|
Rispoval
®
line
|
Aids in preventing three key viruses involved in cattle pneumonia-BRSV, PI
3
virus and BVD-viruses as well as other respiratory diseases, depending on formulation
|
Cattle
|
||
|
Suvaxyn
®
/ Fostera
®
|
Aids in preventing or controlling disease associated with major pathogens in swine such as porcine circovirus type 2 (PCV2), porcine reproductive and respiratory syndrome virus (PRRSv) and Mycoplasma hyopneumoniae, depending on formulation
|
Swine
|
||
|
Parasiticides
|
||||
|
Cydectin
®
|
Injectable or pour-on endectocide to treat and control internal and external cattle parasites, including gastrointestinal roundworms, lungworms, cattle grubs, mites and lice
|
Cattle, sheep
|
||
|
Dectomax
®
|
Injectable or pour-on endectocide, characterized by extended duration of activity, for the treatment and control of internal and external parasite infections
|
Cattle, swine
|
||
|
Medicated Feed Additives
|
||||
|
Aureomycin
®
|
Provides livestock producers control, treatment and convenience against a wide range of respiratory, enteric and reproductive diseases
|
Cattle, poultry, sheep, swine
|
||
|
BMD
®
|
Aids in preventing and controlling enteritis; and increases rate of weight gain and improves feed efficiency in poultry and swine
|
Poultry, swine
|
||
|
Lasalocid line
|
Controls coccidiosis in poultry (Avatec
®
) and cattle (Bovatec
®
) and for increased rate of weight gain and improved feed efficiency in cattle
|
Poultry, cattle
|
||
|
Lincomycin line
|
Controls necrotic enteritis; treatment of dysentery (bloody scours), control of ileitis and treatment/reduction in severity of mycoplasmal pneumonia
|
Swine, poultry
|
||
|
Other
|
||||
|
Eazi-Breed
TM
CIDR
®
|
A vaginal insert that contains progesterone, used in reproductive management programs to synchronize estrus within a herd and help cows, heifers and ewes become pregnant
|
Cattle, sheep
|
||
|
Embrex
®
devices
|
Devices for enhancing hatchery operations' efficiency through
in ovo
detection and vaccination
|
Poultry
|
||
|
Lutalyse
®
|
For estrus control or in the induction of parturition or abortion
|
Cattle, swine
|
||
|
Product line / product
|
Description
|
Primary species
|
||
|
Anti-infectives
|
||||
|
Clavamox
®
/ Synulox
®
|
A broad-spectrum antibiotic and the first and only potentiated penicillin approved for use in dogs and cats
|
Cats, dogs
|
||
|
Convenia
®
|
Anti-infective for the treatment of common bacterial skin infections that provides a course of treatment in a single injection
|
Cats, dogs
|
||
|
Vaccines
|
||||
|
Vanguard
®
L4 (4-way Lepto)
|
Compatible with the Vanguard line and helps protect against leptospirosis caused by
Leptospira canicola
,
L. grippotyphosa
,
L. icterohaemorrhagiae
and
L. pomona
|
Dogs
|
||
|
Vanguard
®
line
|
Aids in preventing canine distemper caused by canine distemper virus; infectious canine hepatitis caused by canine adenovirus type 1; respiratory disease caused by canine adenovirus type 2; canine parainfluenza caused by canine parainfluenza virus; canine parvoviral enteritis caused by canine parvovirus; Lyme disease and subclinical arthritis associated with
Borrelia burgdorferi
, the causative agent of Lyme disease; and Rapid Resp - a group of three vaccines combating infections in dogs caused by
Bordetella bronchiseptica
, canine parainfluenza and canine adenovirus; canine influenza vaccines; and an oral vaccine for
Bordatella bronchiseptica
|
Dogs
|
||
|
Parasiticides
|
||||
|
ProHeart
®
|
Prevents heartworm infestation; also for treatment of existing larval and adult hookworm infections
|
Dogs
|
||
|
Revolution
®
/ Stronghold
®
|
An antiparasitic for protection against fleas, heartworm disease and ear mites in cats and dogs; sarcoptic mites and American dog tick in dogs and roundworms and hookworms for cats
|
Cats, dogs
|
||
|
Other
|
||||
|
Apoquel
®
|
A selective inhibitor of the Janus Kinase 1 enzyme that controls pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age
|
Dogs
|
||
|
Cerenia
®
|
A medication that prevents and treats acute vomiting in dogs, treats acute vomiting in cats and prevents vomiting due to motion sickness in dogs
|
Cats, dogs
|
||
|
Rimadyl
®
|
For the relief of pain and inflammation associated with osteoarthritis and for the control of postoperative pain associated with soft tissue and orthopedic surgeries
|
Dogs
|
||
|
Site
|
Location
|
Site
|
Location
|
|||
|
Campinas
|
Brazil
|
Medolla
|
Italy
|
|||
|
Catania
|
Italy
|
Melbourne
|
Australia
|
|||
|
Charles City
|
Iowa, U.S.
|
Olot
|
Spain
|
|||
|
Chicago Heights
|
Illinois, U.S.
|
Oslo
|
Norway
|
|||
|
Durham
|
North Carolina, U.S.
|
Overhalla
|
Norway
|
|||
|
Eagle Grove
|
Iowa, U.S.
|
Salisbury
|
Maryland, U.S.
|
|||
|
Farum
(a)
|
Denmark
|
San Diego
|
California, U.S.
|
|||
|
Guarulhos
|
Brazil
|
Suzhou
|
China
|
|||
|
Jilin
(b)
|
China
|
Wellington
|
New Zealand
|
|||
|
Kalamazoo
|
Michigan, U.S.
|
White Hall
|
Illinois, U.S.
|
|||
|
Lincoln
|
Nebraska, U.S.
|
Willow Island
|
West Virginia, U.S.
|
|||
|
London
|
Ontario, Canada
|
Yantai
|
China
|
|||
|
Louvain-la-Neuve
|
Belgium
|
|||||
|
(a)
|
In August 2016, Zoetis acquired a veterinary diagnostics business in Denmark.
|
|
(b)
|
This site is operated by our China joint venture, Jilin Zoetis Guoyuan Animal Health Company, Ltd.
|
|
•
|
Establish and implement harmonized technical requirements for the registration of veterinary medicinal products in the VICH regions, which meet high quality, safety and efficacy standards and minimize the use of test animals and costs of product development.
|
|
•
|
Provide a basis for wider international harmonization of registration requirements through the VICH Outreach Forum.
|
|
•
|
Monitor and maintain existing VICH guidelines, taking particular note of the ICH work program and, where necessary, update these VICH guidelines.
|
|
•
|
Ensure efficient processes for maintaining and monitoring consistent interpretation of data requirements following the implementation of VICH guidelines.
|
|
•
|
By means of a constructive dialogue between regulatory authorities and industry, provide technical guidance enabling response to significant emerging global issues and science that impact on regulatory requirements within the VICH regions.
|
|
•
|
environmental-related capital expenditures - $4 million; and
|
|
•
|
other environmental-related expenditures - $16 million.
|
|
•
|
our historical combined financial data does not reflect the separation from Pfizer;
|
|
•
|
our historical combined financial data reflects expense allocations for certain support functions that are provided on a centralized basis within Pfizer, such as expenses for business technology, facilities, legal, finance, human resources, business development, public affairs and procurement, as well as certain manufacturing and supply costs incurred by manufacturing sites that are shared with other Pfizer business units that may be higher or lower than the comparable expenses we would have actually incurred, or will incur, as an independent company;
|
|
•
|
our cost of debt and our capital structure is different from that reflected in our historical combined financial data;
|
|
•
|
significant increases may occur in our cost structure as a result of our being an independent public company, including costs related to public company reporting, investor relations and compliance with the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley Act); and
|
|
•
|
loss of economies of scale as a result of our no longer being a part of Pfizer.
|
|
•
|
the failure of us or any of our vendors or suppliers, including logistical service providers, to comply with applicable regulations and quality assurance guidelines;
|
|
•
|
construction delays;
|
|
•
|
equipment malfunctions;
|
|
•
|
shortages of materials;
|
|
•
|
labor problems;
|
|
•
|
natural disasters;
|
|
•
|
power outages;
|
|
•
|
criminal and terrorist activities;
|
|
•
|
changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, shipping distributions or physical limitations; and
|
|
•
|
the outbreak of any highly contagious diseases near our production sites.
|
|
•
|
volatility in the international financial markets;
|
|
•
|
compliance with governmental controls;
|
|
•
|
difficulties enforcing contractual and intellectual property rights;
|
|
•
|
parallel trade in our products (importation of our products from European Union countries where our products are sold at lower prices into European Union countries where the products are sold at higher prices);
|
|
•
|
compliance with a wide variety of laws and regulations, such as the FCPA and similar non-U.S. laws and regulations;
|
|
•
|
compliance with foreign labor laws;
|
|
•
|
burdens to comply with multiple and potentially conflicting foreign laws and regulations, including those relating to environmental, health and safety requirements;
|
|
•
|
changes in laws, regulations, government controls or enforcement practices with respect to our business and the businesses of our customers, including the imposition of limits on our profitability (e.g., the Venezuelan Law on Fair Pricing);
|
|
•
|
political and social instability, including crime, civil disturbance, terrorist activities and armed conflicts;
|
|
•
|
trade restrictions and restrictions on direct investments by foreign entities, including restrictions administered by the Office of Foreign Assets Control of the U.S. Department of Treasury (OFAC) and the European Union, in relation to our products or the products of farmers and other customers (e.g., restrictions on the importation of agricultural products from the European Union to Russia);
|
|
•
|
government limitations on foreign ownership;
|
|
•
|
government takeover or nationalization of business;
|
|
•
|
changes in tax laws, challenges brought against our incentive tax rulings, and tariffs;
|
|
•
|
imposition of anti-dumping and countervailing duties or other trade-related sanctions;
|
|
•
|
costs and difficulties in staffing, managing and monitoring international operations;
|
|
•
|
longer payment cycles and increased exposure to counterparty risk; and
|
|
•
|
additional limitations on transferring personal information between countries or other restrictions on the processing of personal information.
|
|
•
|
pay monetary damages;
|
|
•
|
obtain a license in order to continue manufacturing or marketing the affected products, which may not be available on commercially reasonable terms, or at all; or
|
|
•
|
stop activities, including any commercial activities, relating to the affected products, which could include a recall of the affected products and/or a cessation of sales in the future.
|
|
•
|
making it more difficult for us to satisfy our obligations with respect to our debt;
|
|
•
|
limiting our ability to obtain additional financing to fund future working capital, capital expenditures, business development or other general corporate requirements, including dividends;
|
|
•
|
increasing our vulnerability to general adverse economic and industry conditions;
|
|
•
|
exposing us to the risk of increased interest rates as certain of our borrowings are and may in the future be at variable rates of interest;
|
|
•
|
limiting our flexibility in planning for and reacting to changes in the animal health industry;
|
|
•
|
placing us at a competitive disadvantage to other, less leveraged competitors;
|
|
•
|
impacting our effective tax rate; and
|
|
•
|
increasing our cost of borrowing.
|
|
•
|
our operating performance and the performance of our competitors;
|
|
•
|
our or our competitors' press releases, other public announcements and filings with the SEC regarding new products or services, enhancements, significant contracts, acquisitions or strategic investments;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts, if any, who cover our common stock;
|
|
•
|
changes in our investor base;
|
|
•
|
failures to meet external expectations or management guidance;
|
|
•
|
fluctuations in our financial results or the financial results of companies perceived to be similar to us;
|
|
•
|
changes in our capital structure or dividend policy, future issuances of securities, sales of large blocks of common stock by our stockholders or the incurrence of additional debt;
|
|
•
|
reputational issues;
|
|
•
|
changes in general economic and market conditions in any of the regions in which we conduct our business;
|
|
•
|
the arrival or departure of key personnel;
|
|
•
|
the actions of speculators and financial arbitrageurs (such as hedge funds);
|
|
•
|
changes in applicable laws, rules or regulations and other dynamics; and
|
|
•
|
other developments or changes affecting us, our industry or our competitors.
|
|
•
|
a Board of Directors that is divided into three classes with staggered terms;
|
|
•
|
rules regarding how our stockholders may present proposals or nominate directors for election at stockholder meetings;
|
|
•
|
the right of our Board of Directors to issue preferred stock without stockholder approval; and
|
|
•
|
limitations on the right of stockholders to remove directors.
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
High
|
Low
|
|
|
2015
|
||
|
First Quarter
|
$47.92
|
$42.29
|
|
Second Quarter
|
$55.38
|
$44.31
|
|
Third Quarter
|
$50.39
|
$37.73
|
|
Fourth Quarter
|
$48.65
|
$38.98
|
|
2016
|
||
|
First Quarter
|
$48.35
|
$38.26
|
|
Second Quarter
|
$49.10
|
$45.01
|
|
Third Quarter
|
$52.64
|
$46.84
|
|
Fourth Quarter
|
$54.15
|
$46.86
|
|
Issuer Purchases of Equity Securities
|
||||
|
Total Number of Shares Purchased
(a)
|
Average Price Paid Per Share
|
Total Number of Shares Purchased as Part of Publicly Announced Programs
|
Approximate Dollar Value of Shares that May Yet Be Purchased Under Plans or Programs
|
|
|
October 3 - October 30, 2016
|
422,445
|
$51.25
|
421,100
|
$53,585,743
|
|
October 31 - November 30, 2016
|
537,290
|
$49.51
|
534,979
|
$27,087,964
|
|
December 1 - December 31, 2016
|
526,849
|
$51.24
|
525,295
|
$1,500,162,603
|
|
Total
|
1,486,584
|
$50.62
|
1,481,374
|
$1,500,162,603
|
|
2016
|
2015
|
|
|
First Quarter
|
$0.095
|
$0.083
|
|
Second Quarter
|
$0.095
|
$0.083
|
|
Third Quarter
|
$0.095
|
$0.083
|
|
Fourth Quarter
|
$0.095
|
$0.083
|
|
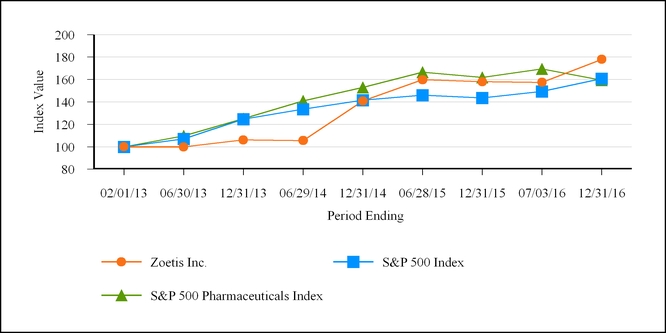
February 1, 2013
|
June 30,
2013
|
December 31, 2013
|
June 29,
2014
|
December 31, 2014
|
June 28,
2015
|
December 31, 2015
|
July 3,
2016
|
December 31, 2016
|
|
|
Zoetis Inc.
|
$100
|
$99.81
|
$106.07
|
$105.56
|
$140.84
|
$159.73
|
$157.98
|
$157.42
|
$177.95
|
|
S&P 500
|
$100
|
$107.14
|
$124.61
|
$133.55
|
$141.67
|
$146.06
|
$143.63
|
$149.46
|
$160.81
|
|
S&P 500 Pharmaceuticals Index
|
$100
|
$109.67
|
$125.16
|
$140.83
|
$152.97
|
$166.53
|
$161.82
|
$169.39
|
$159.29
|
|
Year Ended December 31,
(a)
|
||||||||||||||||||||
|
(MILLIONS, EXCEPT PER SHARE AMOUNTS)
|
2016
|
|
2015
|
|
2014
|
|
2013
|
|
2012
|
|
||||||||||
|
Statement of income data:
|
||||||||||||||||||||
|
Revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
$
|
4,561
|
|
$
|
4,336
|
|
|||||
|
Net income attributable to Zoetis
|
821
|
|
339
|
|
583
|
|
504
|
|
436
|
|
||||||||||
|
Balance sheet data:
|
||||||||||||||||||||
|
Total assets
|
$
|
7,649
|
|
$
|
7,913
|
|
$
|
6,588
|
|
$
|
6,536
|
|
$
|
6,262
|
|
|||||
|
Long-term obligations
(b)
|
4,468
|
|
4,463
|
|
3,624
|
|
3,620
|
|
509
|
|
||||||||||
|
Other data (unaudited):
|
||||||||||||||||||||
|
Adjusted net income
(c)
|
$
|
975
|
|
$
|
889
|
|
$
|
790
|
|
$
|
709
|
|
$
|
539
|
|
|||||
|
Earnings per share attributable to Zoetis Inc. stockholders
(d)
:
|
||||||||||||||||||||
|
Basic
|
$
|
1.66
|
|
$
|
0.68
|
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
|||||
|
Diluted
|
$
|
1.65
|
|
$
|
0.68
|
|
$
|
1.16
|
|
$
|
1.01
|
|
$
|
0.87
|
|
|||||
|
Dividends declared per common share
|
$
|
0.390
|
|
$
|
0.344
|
|
$
|
0.299
|
|
$
|
0.267
|
|
$
|
—
|
|
|||||
|
Weighted average shares outstanding (in thousands)
(d)
:
|
||||||||||||||||||||
|
Basic
|
495,715
|
|
499,707
|
|
501,055
|
|
500,002
|
|
500,000
|
|
||||||||||
|
Diluted
|
498,225
|
|
502,019
|
|
502,025
|
|
500,317
|
|
500,000
|
|
||||||||||
|
(a)
|
Starting in 2015, includes the acquisitions of Pharmaq and certain assets from Abbott Animal Health. See Notes to Consolidated Financial Statements—
|
|
(b)
|
In 2012, primarily includes an allocation of Pfizer debt that was issued to partially finance the acquisition of Wyeth (including Fort Dodge Animal Health (FDAH)) in 2009. The debt has been allocated on a pro-rata basis using the deemed acquisition cost of FDAH as a percentage of the total acquisition cost of Wyeth.
|
|
(c)
|
Adjusted net income (a non-GAAP financial measure) is defined as reported net income attributable to Zoetis excluding purchase accounting adjustments, acquisition-related costs and certain significant items. Management uses adjusted net income, among other factors, to set performance goals and to measure the performance of the overall company, as described in
Item 7.
Management’s Discussion and Analysis of Financial Condition and Results of Operations—Adjusted net income
. We believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. Reconciliations of U.S. GAAP reported net income attributable to Zoetis to non-GAAP adjusted net income for the years ended December 31, 2016, 2015 and 2014 are provided in
Item 7.
Management’s Discussion and Analysis of Financial Condition and Results of Operations—Adjusted net income
. The adjusted net income measure is not, and should not be viewed as, a substitute for U.S. GAAP reported net income attributable to Zoetis.
|
|
(d)
|
The weighted average shares outstanding for both basic and diluted earnings per share for the year ended December 31, 2012 were calculated using 500 million shares of common stock outstanding, which was the number of Zoetis Inc. shares outstanding at the time of the IPO, which was completed on February 6, 2013.
|
|
Years Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
|
15/14
|
|
||||||||
|
Revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
3
|
|
—
|
|
|||||
|
Net income attributable to Zoetis
|
821
|
|
339
|
|
583
|
|
*
|
|
(42
|
)
|
||||||||
|
Adjusted net income
(a)
|
975
|
|
889
|
|
790
|
|
10
|
|
13
|
|
||||||||
|
(a)
|
Adjusted net income is a non-GAAP financial measure. See the
Adjusted net income
section of this MD&A for more information.
|
|
•
|
human population growth and increasing standards of living, particularly in many emerging markets;
|
|
•
|
increasing demand for improved nutrition, particularly animal protein;
|
|
•
|
natural resource constraints, such as scarcity of arable land, fresh water and increased competition for cultivated land, resulting in fewer resources that will be available to meet this increased demand for animal protein;
|
|
•
|
increasing urbanization; and
|
|
•
|
increased focus on food safety and food security.
|
|
•
|
economic development and related increases in disposable income, particularly in many emerging markets;
|
|
•
|
increasing pet ownership; and
|
|
•
|
companion animals living longer, increasing medical treatment of companion animals and advances in companion animal medicines and vaccines.
|
|
•
|
leverage our direct local presence and strong customer relationships
—Through our direct selling commercial model, we can deepen our understanding of our customers’ businesses and can encourage the adoption of more sophisticated animal health products;
|
|
•
|
further penetrate emerging markets
—We seek to maximize our presence where economic development is driving increased demand for animal protein and increased demand for and spending on companion animals;
|
|
•
|
pursue new product research and development and value-added product lifecycle innovation
to extend our product portfolio
—New product R&D and product lifecycle innovation enable us to deliver products to address unmet needs and evolve our product lines so they remain relevant for our customers. We leverage our strong direct presence in many regions and cost-effectively develop new products;
|
|
•
|
remain the partner of choice
for access to new products and technologies
—We support cutting-edge research and secure the right to develop and commercialize new products and technologies;
|
|
•
|
continue to provide high-quality products
and improve manufacturing production margins
—We believe our manufacturing and supply chain provides us with a global platform for continued expansion, including in emerging markets, and that our quality and reliability differentiate us from our competitors; and
|
|
•
|
expand into complementary businesses
to become a more complete, trusted partner in providing solutions
—We believe we have the potential to generate incremental and complementary revenue, in the areas of diagnostics, genetics, devices, dairy data management, e-learning and professional consulting, which could also enhance the loyalty of our customer base and may lead to increased product sales.
|
|
•
|
for sales returns, we perform calculations in each market that incorporate the following, as appropriate: local returns policies and practices; returns as a percentage of revenue; an understanding of the reasons for past returns; estimated shelf life by product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the estimate of future returns, product recalls, discontinuation of products or a changing competitive environment; and
|
|
•
|
for revenue incentives, we use our historical experience with similar incentives programs to estimate the impact of such programs on revenue.
|
|
•
|
a significant adverse change in the extent or manner in which an asset is used. For example, restrictions imposed by the regulatory authorities could affect our ability to manufacture or sell a product, and
|
|
•
|
a projection or forecast that demonstrates losses or reduced profits associated with an asset. This could result, for example, from the introduction of a competitor’s product that results in a significant loss of market share or the inability to achieve the previously projected revenue growth, or from the lack of acceptance of a product by customers.
|
|
•
|
In 2016, the intangible asset impairment charges reflect approximately $1 million of finite-lived trademarks related to a canine pain management product that is no longer marketed.
|
|
•
|
In 2015, the intangible asset impairment charges reflect (i) approximately $27 million of developed technology rights due to product rationalization decisions associated with our operational efficiency initiative; and (ii) approximately $2 million of acquired in-process research and development (IPR&D) assets related to the termination of a canine oncology project.
|
|
•
|
In 2014, the intangible asset impairment charges reflect (i) approximately $6 million of IPR&D assets related to a pharmaceutical product for dogs acquired with the FDAH acquisition in 2009, as a result of the termination of the development program due to a re-assessment of economic viability; and (ii) approximately $1 million related to finite-lived developed technology rights and IPR&D due to negative market conditions and the re-assessment of economic viability.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
|
15/14
|
|
||||||||
|
Revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
3
|
|
—
|
|
|||||
|
Costs and expenses:
|
||||||||||||||||||
|
Cost of sales
(a)
|
1,666
|
|
1,738
|
|
1,717
|
|
(4
|
)
|
1
|
|
||||||||
|
% of revenue
|
34
|
%
|
36
|
%
|
36
|
%
|
||||||||||||
|
Selling, general and administrative expenses
(a)
|
1,364
|
|
1,532
|
|
1,643
|
|
(11
|
)
|
(7
|
)
|
||||||||
|
% of revenue
|
28
|
%
|
32
|
%
|
34
|
%
|
||||||||||||
|
Research and development expenses
(a)
|
376
|
|
364
|
|
396
|
|
3
|
|
(8
|
)
|
||||||||
|
% of revenue
|
8
|
%
|
8
|
%
|
8
|
%
|
||||||||||||
|
Amortization of intangible assets
(a)
|
85
|
|
61
|
|
60
|
|
39
|
|
2
|
|
||||||||
|
Restructuring charges and certain acquisition-related costs
|
5
|
|
320
|
|
25
|
|
(98
|
)
|
*
|
|
||||||||
|
Interest expense, net of capitalized interest
|
166
|
|
124
|
|
117
|
|
34
|
|
6
|
|
||||||||
|
Other (income)/deductions—net
|
(2
|
)
|
81
|
|
7
|
|
*
|
|
*
|
|
||||||||
|
Income before provision for taxes on income
|
1,228
|
|
545
|
|
820
|
|
*
|
|
(34
|
)
|
||||||||
|
% of revenue
|
25
|
%
|
11
|
%
|
17
|
%
|
||||||||||||
|
Provision for taxes on income
|
409
|
|
206
|
|
233
|
|
99
|
|
(12
|
)
|
||||||||
|
Effective tax rate
|
33.3
|
%
|
37.8
|
%
|
28.4
|
%
|
||||||||||||
|
Net income before allocation to noncontrolling interests
|
819
|
|
339
|
|
587
|
|
*
|
|
(42
|
)
|
||||||||
|
Less: Net income attributable to noncontrolling interests
|
(2
|
)
|
—
|
|
4
|
|
*
|
|
*
|
|
||||||||
|
Net income attributable to Zoetis
|
$
|
821
|
|
$
|
339
|
|
$
|
583
|
|
*
|
|
(42
|
)
|
|||||
|
% of revenue
|
17
|
%
|
7
|
%
|
12
|
%
|
||||||||||||
|
(a)
|
Amortization expense related to finite-lived acquired intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property is included in
Amortization of intangible assets
as these intangible assets benefit multiple business functions. Amortization expense related to finite-lived acquired intangible assets that are associated with a single function is included in
Cost of sales
,
Selling, general and administrative expenses
or
Research and development expenses
, as appropriate.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
15/14
|
|
||||||||
|
U.S.
|
$
|
2,447
|
|
$
|
2,328
|
|
$
|
2,059
|
|
5
|
13
|
|
|||||
|
International
|
2,390
|
|
2,386
|
|
2,676
|
|
—
|
(11
|
)
|
||||||||
|
Total operating segments
|
4,837
|
|
4,714
|
|
4,735
|
|
3
|
—
|
|
||||||||
|
Contract manufacturing
|
51
|
|
51
|
|
50
|
|
—
|
2
|
|
||||||||
|
Total Revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
3
|
—
|
|
|||||
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
|
15/14
|
|
||||||||
|
Livestock
|
$
|
2,881
|
|
$
|
2,958
|
|
$
|
3,103
|
|
(3
|
)
|
(5
|
)
|
|||||
|
Companion animal
|
1,956
|
|
1,756
|
|
1,632
|
|
11
|
|
8
|
|
||||||||
|
Contract manufacturing
|
51
|
|
51
|
|
50
|
|
—
|
|
2
|
|
||||||||
|
Total Revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
3
|
|
—
|
|
|||||
|
•
|
increased sales of Apoquel
®
and new product launches, which contributed approximately 5%;
|
|
•
|
growth of our in-line products, which contributed approximately 3%, of which price comprised 2% and volume comprised 1%; and
|
|
•
|
recent acquisitions, primarily Pharmaq and the acquisition of certain assets of Abbott Animal Health, which contributed approximately 2%,
|
|
•
|
our product and market rationalization as part of the operational efficiency initiative, which resulted in a decline of approximately 5%.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
|
15/14
|
||||||||
|
Cost of sales
|
$
|
1,666
|
|
$
|
1,738
|
|
$
|
1,717
|
|
(4
|
)
|
1
|
|||||
|
% of revenue
|
34
|
%
|
36
|
%
|
36
|
%
|
|||||||||||
|
•
|
favorable product mix;
|
|
•
|
favorable foreign exchange;
|
|
•
|
a reduction in the amount of costs related to becoming an independent public company;
|
|
•
|
lower global manufacturing and supply costs; and
|
|
•
|
business model changes in Venezuela,
|
|
•
|
the inclusion of the cost of products for Pharmaq, as well as charges reflecting fair value adjustments to inventory related to the acquisition of Pharmaq;
|
|
•
|
an increase in sales volume; and
|
|
•
|
an increase in inventory obsolescence, scrap and other charges.
|
|
•
|
an increase in sales volume of products with less favorable margins;
|
|
•
|
higher global manufacturing and supply costs;
|
|
•
|
charges related to our operational efficiency initiative and supply network strategy; and
|
|
•
|
charges reflecting the fair value adjustments to inventory acquired from Abbott Animal Health and Pharmaq in 2015,
|
|
•
|
favorable foreign exchange.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
|
15/14
|
|
||||||||
|
Selling, general and administrative expenses
|
$
|
1,364
|
|
$
|
1,532
|
|
$
|
1,643
|
|
(11
|
)
|
(7
|
)
|
|||||
|
% of revenue
|
28
|
%
|
32
|
%
|
34
|
%
|
||||||||||||
|
•
|
a reduction in marketing and general and administrative expense driven by our operational efficiency initiative;
|
|
•
|
a reduction in the amount of additional costs related to becoming an independent public company;
|
|
•
|
favorable foreign exchange; and
|
|
•
|
a reduction in consulting charges relating to our operational efficiency initiative,
|
|
•
|
higher advertising and promotional spending associated with new products;
|
|
•
|
the inclusion of Pharmaq; and
|
|
•
|
an increase in depreciation associated with the implementation of our enterprise resource planning system.
|
|
•
|
favorable foreign exchange; and
|
|
•
|
a reduction in marketing and other spending driven by our operational efficiency initiative,
|
|
•
|
higher costs associated with our enabling functions, including higher business technology costs; and
|
|
•
|
an increase in bad debt expense.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
15/14
|
|
||||||||
|
Research and development expenses
|
$
|
376
|
|
$
|
364
|
|
$
|
396
|
|
3
|
(8
|
)
|
|||||
|
% of revenue
|
8
|
%
|
8
|
%
|
8
|
%
|
|||||||||||
|
•
|
higher development expenses for late-stage projects; and
|
|
•
|
the inclusion of Pharmaq;
|
|
•
|
a reduction in spending driven by our operational efficiency initiative.
|
|
•
|
favorable foreign exchange;
|
|
•
|
a reduction in spending driven by our operational efficiency initiative; and
|
|
•
|
lower expenses associated with our business development activities.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
15/14
|
||||||||
|
Amortization of intangible assets
|
$
|
85
|
|
$
|
61
|
|
$
|
60
|
|
39
|
2
|
|||||
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
|
15/14
|
||||||||
|
Restructuring charges and certain acquisition-related costs
|
$
|
5
|
|
$
|
320
|
|
$
|
25
|
|
(98
|
)
|
*
|
|||||
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
15/14
|
||||||||
|
Interest expense, net of capitalized interest
|
$
|
166
|
|
$
|
124
|
|
$
|
117
|
|
34
|
6
|
|||||
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
15/14
|
||||||||
|
Other (income)/deductions—net
|
$
|
(2
|
)
|
$
|
81
|
|
$
|
7
|
|
*
|
*
|
|||||
|
•
|
charges of $89 million in 2015 related to the revaluation of the net monetary assets in Venezuela; and
|
|
•
|
a net gain of $26 million in 2016 related to sales of certain manufacturing sites and products,
|
|
•
|
a charge of $14 million in 2016 related to a commercial settlement in Mexico; and
|
|
•
|
a charge of $15 million related to the devaluation of the Egyptian pound in November 2016.
|
|
•
|
charges of $89 million in 2015 related to the revaluation of the net monetary assets in Venezuela;
|
|
•
|
impairment charges of $6 million in 2015 related to assets held by our joint venture in Taiwan, classified as held for sale in 2015 and subsequently sold in 2016; and
|
|
•
|
an impairment of IPR&D assets related to the impairment of a canine oncology project in 2015,
|
|
•
|
lower charges for legal and other matters as a result of the commercial settlement of $13 million in Mexico in 2014; and
|
|
•
|
lower foreign currency losses primarily as a result of the depreciation of the Argentine peso in the first quarter of 2014.
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
15/14
|
|
||||||||
|
Provision for taxes on income
|
$
|
409
|
|
$
|
206
|
|
$
|
233
|
|
99
|
(12
|
)
|
|||||
|
Effective tax rate
|
33.3
|
%
|
37.8
|
%
|
28.4
|
%
|
|||||||||||
|
•
|
the impact of the extent and location of restructuring charges related to the operational efficiency initiative, supply network strategy, asset impairments and gains and losses on asset divestitures;
|
|
•
|
a $15 million discrete tax benefit recorded in the fourth quarter of 2016 related to prior period tax adjustments;
|
|
•
|
a $10 million discrete tax benefit recorded in the first quarter of 2016 related to a revaluation of deferred taxes as a result of a change in statutory tax rates;
|
|
•
|
a $7 million discrete tax benefit related to the impact of a new accounting standard adopted in 2016 requiring the excess tax benefits for share-based payments to be recognized as a component of
Provision for taxes on income
. See Notes to Consolidated Financial Statements—
|
|
•
|
a $2 million discrete tax benefit recorded in the second half of 2016 related to a revaluation of the company’s deferred tax assets and liabilities using the tax rates expected to be in place going forward
as a result of the implementation of operational changes,
|
|
•
|
changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings from operations and repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and operating fluctuations in the normal course of business and the impact of non-deductible items;
|
|
•
|
a net tax expense of approximately $35 million mainly recorded in the first half of 2016 related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. This net charge represents the recovery of prior tax benefits for the periods 2013 through 2015 offset by the revaluation of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision;
|
|
•
|
changes in valuation allowances and resolution of other tax items; and
|
|
•
|
tax expense related to changes in uncertain tax positions, see Notes to Consolidated Financial Statements—
|
|
•
|
the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings from (i) operations and (ii) restructuring charges related to the operational efficiency initiative and supply network strategy, as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures;
|
|
•
|
the tax expense related to the non-deductible revaluation of the net monetary assets in Venezuela to the SIMADI exchange rate recorded in the fourth quarter of 2015;
|
|
•
|
changes in valuation allowances and resolution of other tax items;
|
|
•
|
the tax expense related to changes in uncertain tax positions, see Notes to Consolidated Financial Statements—
|
|
•
|
a $9 million discrete tax benefit recorded in the first quarter of 2015 related to a revaluation of deferred taxes as a result of a change in tax rates; and
|
|
•
|
a $6 million discrete tax benefit recorded in the second quarter of 2015 related to prior period tax adjustments.
|
|
% Change
|
||||||||||||||||||||||||||
|
16/15
|
15/14
|
|||||||||||||||||||||||||
|
Related to
|
Related to
|
|||||||||||||||||||||||||
|
Year Ended December 31,
|
Foreign
|
|
Foreign
|
|
||||||||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
Total
|
|
Exchange
|
|
Operational
|
|
Total
|
|
Exchange
|
|
Operational
|
|||||||||
|
U.S.
|
||||||||||||||||||||||||||
|
Livestock
|
$
|
1,227
|
|
$
|
1,251
|
|
$
|
1,163
|
|
(2
|
)
|
—
|
|
(2
|
)
|
8
|
|
—
|
|
8
|
||||||
|
Companion animal
|
1,220
|
|
1,077
|
|
896
|
|
13
|
|
—
|
|
13
|
|
20
|
|
—
|
|
20
|
|||||||||
|
2,447
|
|
2,328
|
|
2,059
|
|
5
|
|
—
|
|
5
|
|
13
|
|
—
|
|
13
|
||||||||||
|
International
|
||||||||||||||||||||||||||
|
Livestock
|
1,654
|
|
1,707
|
|
1,940
|
|
(3
|
)
|
(6
|
)
|
3
|
|
(12
|
)
|
(15
|
)
|
3
|
|||||||||
|
Companion animal
|
736
|
|
679
|
|
736
|
|
8
|
|
(5
|
)
|
13
|
|
(8
|
)
|
(15
|
)
|
7
|
|||||||||
|
2,390
|
|
2,386
|
|
2,676
|
|
—
|
|
(5
|
)
|
5
|
|
(11
|
)
|
(15
|
)
|
4
|
||||||||||
|
Total
|
||||||||||||||||||||||||||
|
Livestock
|
2,881
|
|
2,958
|
|
3,103
|
|
(3
|
)
|
(4
|
)
|
1
|
|
(5
|
)
|
(9
|
)
|
4
|
|||||||||
|
Companion animal
|
1,956
|
|
1,756
|
|
1,632
|
|
11
|
|
(2
|
)
|
13
|
|
8
|
|
(6
|
)
|
14
|
|||||||||
|
Contract manufacturing
|
51
|
|
51
|
|
50
|
|
—
|
|
(3
|
)
|
3
|
|
2
|
|
(9
|
)
|
11
|
|||||||||
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
3
|
|
(2
|
)
|
5
|
|
—
|
|
(8
|
)
|
8
|
|||||||
|
% Change
|
|||||||||||||||||||||
|
16/15
|
15/14
|
||||||||||||||||||||
|
Related to
|
Related to
|
||||||||||||||||||||
|
Year Ended December 31,
|
Foreign
|
|
Foreign
|
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
Total
|
|
Exchange
|
|
Operational
|
Total
|
|
Exchange
|
|
Operational
|
|||||
|
U.S.
|
$
|
1,508
|
|
$
|
1,390
|
|
$
|
1,176
|
|
8
|
|
—
|
|
8
|
18
|
|
—
|
|
18
|
||
|
International
|
1,054
|
|
941
|
|
1,025
|
|
12
|
|
(5
|
)
|
17
|
(8
|
)
|
(18
|
)
|
10
|
|||||
|
Total reportable segments
|
2,562
|
|
2,331
|
|
2,201
|
|
10
|
|
(2
|
)
|
12
|
6
|
|
(8
|
)
|
14
|
|||||
|
Other business activities
|
(309
|
)
|
(293
|
)
|
(318
|
)
|
5
|
|
(8
|
)
|
|||||||||||
|
Reconciling Items:
|
|||||||||||||||||||||
|
Corporate
|
(684
|
)
|
(606
|
)
|
(559
|
)
|
13
|
|
8
|
|
|||||||||||
|
Purchase accounting adjustments
|
(99
|
)
|
(57
|
)
|
(51
|
)
|
74
|
|
12
|
|
|||||||||||
|
Acquisition-related costs
|
(4
|
)
|
(21
|
)
|
(8
|
)
|
(81
|
)
|
*
|
|
|||||||||||
|
Certain significant items
|
(57
|
)
|
(592
|
)
|
(205
|
)
|
(90
|
)
|
*
|
|
|||||||||||
|
Other unallocated
|
(181
|
)
|
(217
|
)
|
(240
|
)
|
(17
|
)
|
(10
|
)
|
|||||||||||
|
Income before income taxes
|
$
|
1,228
|
|
$
|
545
|
|
$
|
820
|
|
*
|
|
(34
|
)
|
||||||||
|
•
|
Livestock revenue declined primarily due to
product rationalizations as part of the company’s operational efficiency initiative, which impacted both poultry and swine. Additionally, sales of cattle products were impacted by unfavorable market conditions, while swine was impacted by increased competition.
|
|
•
|
Companion animal revenue growth was driven by increased sales of Apoquel
®
, new product launches, and initial sales of products into expanded distribution relationships. Partially offsetting growth was a decline in the company’s surgical fluid products.
|
|
•
|
Livestock revenue growth was driven primarily by the acquisition of Pharmaq, with sales primarily in Chile and Norway. Growth also benefited from swine performance in China, as well as cattle performance in certain emerging markets. Growth was partially offset by our operational efficiency initiative, which includes product rationalization and the impact of our business decisions in Venezuela and India.
|
|
•
|
Companion animal revenue growth resulted from increased sales of Apoquel
®
, other new product launches, and demand for our vaccines portfolio in China, due to increased field force expansions and positive medicalization rates.
|
|
•
|
Livestock revenue growth was driven by increased sales across the cattle, poultry, and swine portfolios. Sales of cattle products grew across multiple categories, including premium brands, as a result of favorable market conditions. Cattle sales also benefited from new product launches. Growth in sales of poultry products was driven by the re-introduction of a medicated feed additive. Sales of swine products grew due to the continued recovery in the pig population following the PEDv outbreak in the previous year.
|
|
•
|
Companion animal revenue growth was driven by the addition of products acquired from Abbott Animal Health, as well as the solid performance of Apoquel
®
. This growth was partially offset by competitive pressure in other parts of the companion animal portfolio.
|
|
•
|
Operational revenue
increased
$99 million
, or
4%
, reflecting
growth
of approximately
$49 million
in livestock products and
growth
of approximately
$50 million
in companion animal products.
|
|
•
|
Livestock revenue growth was driven primarily by sales of swine products, particularly in China due to favorable market conditions. Sales of cattle products benefited from growth in Brazil and Mexico, partially offset by the impact of business reduction decisions in Venezuela. Livestock revenue in France also declined due to the anti-infective legislative changes in 2014.
|
|
•
|
Companion animal revenue growth resulted from increased sales of Apoquel
®
, the addition of products acquired from Abbott Animal Health, and the non-recurrence of a prior year inventory buyback related to the termination of a distributor agreement in Japan.
|
|
•
|
Corporate,
which includes certain costs associated with business technology, facilities, legal, finance, human resources, business development, and communications, among others. These costs also include certain compensation costs and other miscellaneous operating expenses that are not charged to our operating segments, as well as interest income and expense;
|
|
•
|
Certain transactions and events such as (i)
Purchase accounting adjustments
, which includes expenses associated with the amortization of fair value adjustments to inventory, intangible assets and property, plant and equipment; (ii)
Acquisition-related activities
, which includes costs for acquisition and integration; and (iii)
Certain significant items
, which includes non-acquisition-related restructuring charges, certain asset impairment charges, stand-up costs, certain legal and commercial settlements, and costs associated with cost reduction/productivity initiatives; and
|
|
•
|
Other unallocated
, which includes (i) certain overhead expenses associated with our global manufacturing operations not charged to our operating segments; (ii) certain costs associated with business technology and finance that specifically support our global manufacturing operations; certain supply chain and global logistics costs; and (iv) procurement costs.
|
|
•
|
senior management receives a monthly analysis of our operating results that is prepared on an adjusted net income basis;
|
|
•
|
our annual budgets are prepared on an adjusted net income basis; and
|
|
•
|
other goal setting and performance measurements.
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
2015
|
2014
|
16/15
|
|
15/14
|
|
|||||||||||
|
GAAP reported net income attributable to Zoetis
|
$
|
821
|
|
$
|
339
|
|
$
|
583
|
|
*
|
|
(42
|
)
|
|||||
|
Purchase accounting adjustments—net of tax
|
60
|
|
39
|
|
34
|
|
54
|
|
15
|
|
||||||||
|
Acquisition-related costs—net of tax
|
4
|
|
22
|
|
5
|
|
(82
|
)
|
*
|
|
||||||||
|
Certain significant items—net of tax
|
90
|
|
489
|
|
168
|
|
(82
|
)
|
*
|
|
||||||||
|
Non-GAAP adjusted net income
(a)(b)
|
$
|
975
|
|
$
|
889
|
|
$
|
790
|
|
10
|
|
13
|
|
|||||
|
(a)
|
The effective tax rate on adjusted pretax income is
29.9%
,
26.8%
and
26.8%
for full year 2016, 2015 and 2014, respectively. The higher effective tax rate in 2016 compared to 2015 is primarily due to changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs, offset by a $15 million discrete tax benefit related to prior period tax adjustments, a $7 million discrete tax benefit related to the impact of a new accounting standard adopted in 2016 requiring the excess tax benefits for share-based payments to be recognized as a component of
Provision for taxes on income
, and a $4 million discrete tax benefit recorded in the first quarter of 2015 related to prior period deferred tax adjustments. The change in the effective tax rate in 2015 compared to 2014 is primarily due to changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs, changes in valuation allowances and resolution of other tax items.
|
|
(b)
|
The impact of the incentive tax rulings in Belgium and Singapore were a component of the 2015 and 2014 effective tax rate, but are no longer a component of the 2016 effective tax rate. For additional information on the impact of the European Commission’s negative decision on the Belgium excess profits ruling on January 11, 2016, see Notes to Consolidated Financial Statements—
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||||||
|
2016
|
2015
|
2014
|
16/15
|
|
15/14
|
|
||||||||||||
|
Earnings per share—diluted
(a)(b)
:
|
||||||||||||||||||
|
GAAP reported EPS attributable to Zoetis—diluted
|
$
|
1.65
|
|
$
|
0.68
|
|
$
|
1.16
|
|
*
|
|
(41
|
)
|
|||||
|
Purchase accounting adjustments—net of tax
|
0.12
|
|
0.08
|
|
0.07
|
|
50
|
|
14
|
|
||||||||
|
Acquisition-related costs—net of tax
|
0.01
|
|
0.04
|
|
0.01
|
|
(75
|
)
|
*
|
|
||||||||
|
Certain significant items—net of tax
|
0.18
|
|
0.97
|
|
0.33
|
|
(81
|
)
|
*
|
|
||||||||
|
Non-GAAP adjusted EPS—diluted
|
$
|
1.96
|
|
$
|
1.77
|
|
$
|
1.57
|
|
11
|
|
13
|
|
|||||
|
(a)
|
Diluted earnings per share was computed using the weighted-average common shares outstanding during the period plus the common stock equivalents related to stock options, RSUs, PSUs and DSUs.
|
|
(b)
|
EPS amounts may not add due to rounding.
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Interest expense, net of capitalized interest
|
$
|
166
|
|
$
|
124
|
|
$
|
117
|
|
|||
|
Interest income
|
8
|
|
6
|
|
6
|
|
||||||
|
Income taxes
|
415
|
|
326
|
|
290
|
|
||||||
|
Depreciation
|
133
|
|
124
|
|
131
|
|
||||||
|
Amortization
|
16
|
|
16
|
|
17
|
|
||||||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Purchase accounting adjustments:
|
||||||||||||
|
Amortization and depreciation
(a)
|
$
|
76
|
|
$
|
48
|
|
$
|
47
|
|
|||
|
Cost of sales
(b)
|
23
|
|
9
|
|
4
|
|
||||||
|
Total purchase accounting adjustments—pre-tax
|
99
|
|
57
|
|
51
|
|
||||||
|
Income taxes
(c)
|
39
|
|
18
|
|
17
|
|
||||||
|
Total purchase accounting adjustments—net of tax
|
60
|
|
39
|
|
34
|
|
||||||
|
Acquisition-related costs:
|
||||||||||||
|
Integration costs
|
3
|
|
10
|
|
8
|
|
||||||
|
Transaction costs
|
—
|
|
9
|
|
—
|
|
||||||
|
Other
|
1
|
|
2
|
|
—
|
|
||||||
|
Total acquisition-related costs—pre-tax
|
4
|
|
21
|
|
8
|
|
||||||
|
Income taxes
(c)
|
—
|
|
(1
|
)
|
3
|
|
||||||
|
Total acquisition-related costs—net of tax
|
4
|
|
22
|
|
5
|
|
||||||
|
Certain significant items:
|
||||||||||||
|
Operational efficiency initiative
(d)
|
(9
|
)
|
346
|
|
—
|
|
||||||
|
Supply network strategy
(e)
|
19
|
|
27
|
|
—
|
|
||||||
|
Other restructuring charges and cost-reduction/productivity initiatives
(f)
|
(1
|
)
|
—
|
|
18
|
|
||||||
|
Certain asset impairment charges
(g)
|
1
|
|
5
|
|
6
|
|
||||||
|
Net gains on sale of assets
(h)
|
—
|
|
—
|
|
(5
|
)
|
||||||
|
Stand-up costs
(i)
|
23
|
|
118
|
|
168
|
|
||||||
|
Foreign currency loss related to Venezuela revaluation
(j)
|
—
|
|
93
|
|
—
|
|
||||||
|
Other
(k)
|
24
|
|
3
|
|
18
|
|
||||||
|
Total certain significant items—pre-tax
|
57
|
|
592
|
|
205
|
|
||||||
|
Income taxes
(c)
|
(33
|
)
|
103
|
|
37
|
|
||||||
|
Total certain significant items—net of tax
|
90
|
|
489
|
|
168
|
|
||||||
|
Total purchase accounting adjustments, acquisition-related costs, and certain significant items—net of tax
|
$
|
154
|
|
$
|
550
|
|
$
|
207
|
|
|||
|
(a)
|
Amortization and depreciation expenses related to
Purchase accounting adjustments
with respect to identifiable intangible assets and property, plant and equipment
.
|
|
(b)
|
Amortization and depreciation expense, as well as fair value adjustments to acquired inventory
.
|
|
(c)
|
Income taxes include the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying that jurisdiction's applicable tax rate. Income taxes in
Purchase accounting adjustments
for 2016, includes a tax benefit related to the revaluation of deferred taxes as a result of a change in tax rates. Income taxes in
Acquisition-related costs
for 2016, includes a tax charge related to the acquisition of certain assets of Abbott Animal Health. Income taxes in
Certain significant items
for 2016, also includes (i) a net tax charge of approximately $20 million recorded in the second half of 2016, as a result of the implementation of certain operational changes, which represents an increase to current income tax expense of approximately $22 million offset by a $2 million tax benefit related to a revaluation of the company’s deferred tax assets and liabilities using the tax rates expected to be in place going forward, and (ii) a net tax charge of approximately $35 million mainly recorded in the first half of 2016, related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium which represents the recovery of prior tax benefits for the periods 2013 through 2015 offset by the revaluation of the company’s deferred tax assets and liabilities, using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision.
|
|
(d)
|
For 2016, includes a reduction in employee termination accruals of $8 million, an increase in exit costs of $5 million, inventory write-offs of $5 million, accelerated depreciation of $1 million, consulting fees of $14 million, and a $26 million net gain related to divestitures.
|
|
(e)
|
For 2016, represents restructuring charges of $6 million related to employee termination costs, accelerated depreciation of $6 million, inventory write-offs of $1 million and consulting fees of $6 million.
|
|
(f)
|
Represents charges incurred for restructuring and cost-reduction/productivity initiatives. For 2014, primarily represents employee termination costs in Europe and our global manufacturing operations.
|
|
(g)
|
For 2016, represents an impairment of finite-lived trademarks related to a canine pain management product. For 2015, primarily represents impairment charges related to assets held by our joint venture in Taiwan, which was subsequently sold in 2016. For 2015, also includes an impairment of IPR&D assets related to the termination of a canine oncology project. For 2014, amounts primarily represent an impairment charge related to an IPR&D project acquired with the FDAH acquisition in 2009.
|
|
(h)
|
For 2014, primarily represents the Zoetis portion of a net gain on the sale of land by our Taiwan joint venture and the net gain on the government-mandated sale of certain product rights in Argentina that were acquired with the FDAH acquisition in 2009.
|
|
(i)
|
Represents certain non-recurring costs related to becoming an independent public company, such as the creation of standalone systems and infrastructure, site separation, new branding (including changes to the manufacturing process for required new packaging),and certain legal registration and patent assignment costs.
|
|
(j)
|
For 2015, represents charges primarily related to the foreign currency losses associated with our Venezuela business. For additional information, see Notes to Consolidated Financial Statements—
|
|
(k)
|
For 2016, represents costs associated with changes to our operating model ($10 million) and a charge associated with a commercial settlement in Mexico ($14 million).
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
2015
|
2014
|
|||||||||
|
Cost of sales:
|
||||||||||||
|
Purchase accounting adjustments
|
$
|
23
|
|
$
|
9
|
|
$
|
4
|
|
|||
|
Accelerated depreciation
|
6
|
|
1
|
|
—
|
|
||||||
|
Inventory write-offs
|
5
|
|
13
|
|
—
|
|
||||||
|
Consulting fees
|
6
|
|
16
|
|
—
|
|
||||||
|
Stand-up costs
|
1
|
|
27
|
|
32
|
|
||||||
|
Other
|
1
|
|
—
|
|
—
|
|
||||||
|
Total Cost of sales
|
42
|
|
66
|
|
36
|
|
||||||
|
Selling, general & administrative expenses:
|
||||||||||||
|
Purchase accounting adjustments
|
5
|
|
—
|
|
—
|
|
||||||
|
Accelerated depreciation
|
1
|
|
—
|
|
—
|
|
||||||
|
Consulting fees
|
14
|
|
40
|
|
—
|
|
||||||
|
Stand-up costs
|
22
|
|
90
|
|
131
|
|
||||||
|
Other
|
10
|
|
—
|
|
—
|
|
||||||
|
Total Selling, general & administrative expenses
|
52
|
|
130
|
|
131
|
|
||||||
|
Research & development expenses:
|
||||||||||||
|
Purchase accounting adjustments
|
2
|
|
2
|
|
2
|
|
||||||
|
Accelerated depreciation
|
—
|
|
2
|
|
—
|
|
||||||
|
Total Research & development expenses
|
2
|
|
4
|
|
2
|
|
||||||
|
Amortization of intangible assets:
|
||||||||||||
|
Purchase accounting adjustments
|
69
|
|
46
|
|
45
|
|
||||||
|
Total Amortization of intangible assets
|
69
|
|
46
|
|
45
|
|
||||||
|
Restructuring (benefits)/charges and certain acquisition-related costs:
|
||||||||||||
|
Integration costs
|
3
|
|
10
|
|
8
|
|
||||||
|
Transaction costs
|
—
|
|
9
|
|
—
|
|
||||||
|
Employee termination costs
|
(2
|
)
|
262
|
|
18
|
|
||||||
|
Asset impairments
|
—
|
|
39
|
|
—
|
|
||||||
|
Exit costs
|
4
|
|
—
|
|
—
|
|
||||||
|
Total Restructuring (benefits)/charges and certain acquisition-related costs
|
5
|
|
320
|
|
26
|
|
||||||
|
Other (income)/deductions—net:
|
||||||||||||
|
Net gain on sale of assets
|
(26
|
)
|
—
|
|
(5
|
)
|
||||||
|
Acquisition-related costs
|
1
|
|
—
|
|
—
|
|
||||||
|
Asset impairments
|
1
|
|
5
|
|
6
|
|
||||||
|
Stand-up costs
|
—
|
|
1
|
|
5
|
|
||||||
|
Foreign currency loss related to Venezuela revaluation
|
—
|
|
93
|
|
—
|
|
||||||
|
Other
|
14
|
|
5
|
|
18
|
|
||||||
|
Total Other (income)/deductions—net
|
(10
|
)
|
104
|
|
24
|
|
||||||
|
Provision for taxes on income
|
6
|
|
120
|
|
57
|
|
||||||
|
Total purchase accounting adjustments, acquisition-related costs, and certain significant items—net of tax
|
$
|
154
|
|
$
|
550
|
|
$
|
207
|
|
|||
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
16/15
|
|
15/14
|
||||||||
|
Net cash provided by (used in):
|
|||||||||||||||||
|
Operating activities
|
$
|
713
|
|
$
|
664
|
|
$
|
626
|
|
7
|
|
6
|
|||||
|
Investing activities
|
(214
|
)
|
(1,115
|
)
|
(187
|
)
|
(81
|
)
|
*
|
||||||||
|
Financing activities
|
(903
|
)
|
755
|
|
(154
|
)
|
*
|
|
*
|
||||||||
|
Effect of exchange-rate changes on cash and cash equivalents
|
(23
|
)
|
(32
|
)
|
(13
|
)
|
(28
|
)
|
*
|
||||||||
|
Net (decrease)/increase in cash and cash equivalents
|
$
|
(427
|
)
|
$
|
272
|
|
$
|
272
|
|
*
|
|
—
|
|||||
|
December 31,
|
|
December 31,
|
|
||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
|||
|
Cash and cash equivalents
|
$
|
727
|
|
$
|
1,154
|
|
|
|
Accounts receivable, net
(a)
|
913
|
|
937
|
|
|||
|
Short-term borrowings
|
—
|
|
5
|
|
|||
|
Current portion of long-term debt
|
—
|
|
400
|
|
|||
|
Long-term debt
|
4,468
|
|
4,463
|
|
|||
|
Working capital
|
2,273
|
|
2,049
|
|
|||
|
Ratio of current assets to current liabilities
|
3.03:1
|
|
2.15:1
|
|
|||
|
(a)
|
Accounts receivable are usually collected over a period of 60 to 90 days
.
For the year ended
December 31, 2016
, compared to the year ended December 31, 2015, the number of days that accounts receivables are outstanding remained approximately the same. We regularly monitor our accounts receivable for collectability, particularly in markets where economic conditions remain uncertain. We believe that our allowance for doubtful accounts is appropriate. Our assessment is based on such factors as past due aging, historical and expected collection patterns, the financial condition of our customers, the robust nature of our credit and collection practices and the economic environment.
|
|
2018-
|
|
2020-
|
|
There-
|
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
Total
|
|
2017
|
|
2019
|
|
2021
|
|
after
|
|
||||||||||
|
Long-term debt, including current portion and interest obligations
(a)
|
$
|
6,558
|
|
$
|
163
|
|
$
|
1,049
|
|
$
|
778
|
|
$
|
4,568
|
|
|||||
|
Other long-term liabilities reflected on our consolidated balance sheets
|
||||||||||||||||||||
|
under U.S. GAAP
(b)
|
105
|
|
54
|
|
19
|
|
9
|
|
23
|
|
||||||||||
|
Operating lease commitments
|
154
|
|
29
|
|
45
|
|
26
|
|
54
|
|
||||||||||
|
Purchase obligations and other
(c)
|
312
|
|
198
|
|
65
|
|
38
|
|
11
|
|
||||||||||
|
Benefit plans - continuing service credit obligations
(d)
|
23
|
|
4
|
|
8
|
|
8
|
|
3
|
|
||||||||||
|
Uncertain tax positions
(e)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
(a)
|
Long-term debt consists of senior notes and other notes. Our calculations of expected interest payments incorporate only current period assumptions for interest rates, foreign currency translation rates and Zoetis hedging strategies. See Notes to Consolidated Financial Statements—
|
|
(b)
|
Includes expected employee termination payments that represent contractual obligations, expected payments related to our unfunded U.S. supplemental (non-qualified) savings plans, deferred compensation and expected payments relating to our future benefit payments net of plan assets (included in the determination of the projected benefit obligation) for pension plans that are dedicated to Zoetis employees and those transferred to us from Pfizer. See Notes to Consolidated
|
|
(c)
|
Includes agreements to purchase goods and services that are enforceable and legally binding and includes amounts relating to advertising, contract manufacturing, information technology services and potential milestone payments deemed reasonably likely to occur.
|
|
(d)
|
Includes the cost of service credit continuation for certain Zoetis employees in the Pfizer U.S. qualified defined benefit pension and U.S. retiree medical plans, in accordance with the employee matters agreement. See Notes to Consolidated Financial Statements—
Note 13. Benefit Plans.
|
|
(e)
|
Except for amounts reflected in
Income taxes payable
, we are unable to predict the timing of tax settlements, as tax audits can involve complex issues and the resolution of those issues may span multiple years, particularly if subject to negotiation or litigation.
|
|
Description
|
Principal Amount
|
Interest Rate
|
Terms
|
|
2013 Senior Note due 2018
|
$750 million
|
1.875%
|
Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2018
|
|
2015 Senior Note due 2020
|
$500 million
|
3.450%
|
Interest due semi annually, not subject to amortization, aggregate principal due on November 13, 2020
|
|
2013 Senior Note due 2023
|
$1,350 million
|
3.250%
|
Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2023
|
|
2015 Senior Note due 2025
|
$750 million
|
4.500%
|
Interest due semi annually, not subject to amortization, aggregate principal due on November 13, 2025
|
|
2013 Senior Note due 2043
|
$1,150 million
|
4.700%
|
Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2043
|
|
Commercial
|
||||||||
|
Paper
|
Long-term Debt
|
Date of
|
||||||
|
Name of Rating Agency
|
Rating
|
Rating
|
Outlook
|
Last Action
|
||||
|
Moody’s
|
P-2
|
Baa2
|
Stable
|
November 2015
|
||||
|
S&P
|
A-2
|
BBB
|
Stable
|
December 2016
|
||||
|
•
|
emerging restrictions and bans on the use of antibacterials in food-producing animals;
|
|
•
|
perceived adverse effects on human health linked to the consumption of food derived from animals that utilize our products;
|
|
•
|
increased regulation or decreased governmental support relating to the raising, processing or consumption of food-producing animals;
|
|
•
|
fluctuations in foreign exchange rates and potential currency controls;
|
|
•
|
changes in tax laws and regulations;
|
|
•
|
legal factors, including product liability claims, antitrust litigation and governmental investigations, including tax disputes, environmental concerns, commercial disputes and patent disputes with branded and generic competitors, any of which could preclude commercialization of products or negatively affect the profitability of existing products;
|
|
•
|
failure to protect our intellectual property rights or to operate our business without infringing the intellectual property rights of others;
|
|
•
|
an outbreak of infectious disease carried by animals;
|
|
•
|
adverse weather conditions and the availability of natural resources;
|
|
•
|
adverse global economic conditions;
|
|
•
|
failure of our R&D, acquisition and licensing efforts to generate new products;
|
|
•
|
the possible impact of competing products, including generic alternatives, on our products and our ability to compete against such products;
|
|
•
|
quarterly fluctuations in demand and costs;
|
|
•
|
governmental laws and regulations affecting domestic and foreign operations, including without limitation, tax obligations and changes affecting the tax treatment by the United States of income earned outside the United States that may result from pending and possible future proposals; and
|
|
•
|
governmental laws and regulations affecting our interactions with veterinary healthcare providers.
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
|
Page
|
|
|
Audited Consolidated Financial Statements of Zoetis Inc. and Subsidiaries:
|
|
|
Consolidated Statements of Income for the Years Ended December 31, 2016, 2015 and 2014
|
|
|
Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2016, 2015 and 2014
|
|
|
Consolidated Balance Sheets as of December 31, 2016 and 2015
|
|
|
Consolidated Statements of Equity for the Years Ended December 31, 2016, 2015 and 2014
|
|
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2016, 2015 and 2014
|
|
|
Schedule II—Valuation and Qualifying Accounts
|
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS AND SHARES, EXCEPT PER SHARE DATA)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
|||
|
Costs and expenses:
|
||||||||||||
|
Cost of sales
(a)
|
1,666
|
|
1,738
|
|
1,717
|
|
||||||
|
Selling, general and administrative expenses
(a)
|
1,364
|
|
1,532
|
|
1,643
|
|
||||||
|
Research and development expenses
(a)
|
376
|
|
364
|
|
396
|
|
||||||
|
Amortization of intangible assets
(a)
|
85
|
|
61
|
|
60
|
|
||||||
|
Restructuring charges and certain acquisition-related costs
|
5
|
|
320
|
|
25
|
|
||||||
|
Interest expense, net of capitalized interest
|
166
|
|
124
|
|
117
|
|
||||||
|
Other (income)/deductions––net
|
(2
|
)
|
81
|
|
7
|
|
||||||
|
Income before provision for taxes on income
|
1,228
|
|
545
|
|
820
|
|
||||||
|
Provision for taxes on income
|
409
|
|
206
|
|
233
|
|
||||||
|
Net income before allocation to noncontrolling interests
|
819
|
|
339
|
|
587
|
|
||||||
|
Less: Net (loss)/income attributable to noncontrolling interests
|
(2
|
)
|
—
|
|
4
|
|
||||||
|
Net income attributable to Zoetis
|
$
|
821
|
|
$
|
339
|
|
$
|
583
|
|
|||
|
Earnings per share attributable to Zoetis Inc. stockholders:
|
||||||||||||
|
Basic
|
$
|
1.66
|
|
$
|
0.68
|
|
$
|
1.16
|
|
|||
|
Diluted
|
$
|
1.65
|
|
$
|
0.68
|
|
$
|
1.16
|
|
|||
|
Weighted-average common shares outstanding:
|
||||||||||||
|
Basic
|
495.715
|
|
499.707
|
|
501.055
|
|
||||||
|
Diluted
|
498.225
|
|
502.019
|
|
502.025
|
|
||||||
|
Dividends declared per common share
|
$
|
0.390
|
|
$
|
0.344
|
|
$
|
0.299
|
|
|||
|
(a)
|
Exclusive of amortization of intangible assets, except as disclosed in
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Net income before allocation to noncontrolling interests
|
$
|
819
|
|
$
|
339
|
|
$
|
587
|
|
|||
|
Other comprehensive income/(loss), net of tax and reclassification adjustments:
|
||||||||||||
|
Unrealized gain/(loss) on derivatives, net
(a)
|
10
|
|
(2
|
)
|
—
|
|
||||||
|
Foreign currency translation adjustments, net
|
17
|
|
(269
|
)
|
(123
|
)
|
||||||
|
Benefit plans: Actuarial (losses)/gains, net
(a)
|
(6
|
)
|
9
|
|
(10
|
)
|
||||||
|
Plan settlement, net
(b)
|
—
|
|
—
|
|
3
|
|
||||||
|
Total other comprehensive income/(loss), net of tax
|
21
|
|
(262
|
)
|
(130
|
)
|
||||||
|
Comprehensive income before allocation to noncontrolling interests
|
840
|
|
77
|
|
457
|
|
||||||
|
Comprehensive (loss)/income attributable to noncontrolling interests
|
(3
|
)
|
(1
|
)
|
5
|
|
||||||
|
Comprehensive income attributable to Zoetis
|
$
|
843
|
|
$
|
78
|
|
$
|
452
|
|
|||
|
(a)
|
Presented net of reclassification adjustments and tax impacts, which are not significant in any period presented. Reclassification adjustments related to benefit plans are generally reclassified, as part of net periodic pension cost, into
Cost of sales, Selling, general and administrative expenses,
and/or
Research and development expenses,
as appropriate, in the consolidated statements of income.
|
|
(b)
|
Reflects the 2014 settlement charge associated with the 2012 sale of our Netherlands manufacturing facility which was recorded to
Other (income)/deductions—net
. See
|
|
December 31,
|
|
December 31,
|
|
|||||
|
(MILLIONS OF DOLLARS, EXCEPT PER SHARE DATA)
|
2016
|
|
2015
|
|
||||
|
Assets
|
||||||||
|
Cash and cash equivalents
|
$
|
727
|
|
$
|
1,154
|
|
||
|
Accounts receivable, less allowance for doubtful accounts of $30 in 2016 and $34 in 2015
|
913
|
|
937
|
|
||||
|
Inventories
|
1,502
|
|
1,467
|
|
||||
|
Assets held for sale
|
—
|
|
71
|
|
||||
|
Other current assets
|
248
|
|
201
|
|
||||
|
Total current assets
|
3,390
|
|
3,830
|
|
||||
|
Property, plant and equipment, less accumulated depreciation of $1,358 in 2016 and $1,208 in 2015
|
1,381
|
|
1,307
|
|
||||
|
Goodwill
|
1,481
|
|
1,455
|
|
||||
|
Identifiable intangible assets, less accumulated amortization
|
1,228
|
|
1,190
|
|
||||
|
Noncurrent deferred tax assets
|
96
|
|
82
|
|
||||
|
Other noncurrent assets
|
73
|
|
49
|
|
||||
|
Total assets
|
$
|
7,649
|
|
$
|
7,913
|
|
||
|
Liabilities and Equity
|
||||||||
|
Short-term borrowings
|
$
|
—
|
|
$
|
5
|
|
||
|
Current portion of long-term debt
|
—
|
|
400
|
|
||||
|
Accounts payable
|
265
|
|
293
|
|
||||
|
Dividends payable
|
52
|
|
47
|
|
||||
|
Accrued expenses
|
464
|
|
676
|
|
||||
|
Accrued compensation and related items
|
224
|
|
234
|
|
||||
|
Income taxes payable
|
71
|
|
63
|
|
||||
|
Liabilities associated with assets held for sale
|
—
|
|
4
|
|
||||
|
Other current liabilities
|
41
|
|
59
|
|
||||
|
Total current liabilities
|
1,117
|
|
1,781
|
|
||||
|
Long-term debt, net of discount and issuance costs
|
4,468
|
|
4,463
|
|
||||
|
Noncurrent deferred tax liabilities
|
244
|
|
264
|
|
||||
|
Other taxes payable
|
73
|
|
63
|
|
||||
|
Other noncurrent liabilities
|
248
|
|
251
|
|
||||
|
Total liabilities
|
6,150
|
|
6,822
|
|
||||
|
Commitments and contingencies
(Note 17)
|
—
|
|
—
|
|
||||
|
Stockholders' equity:
|
||||||||
|
Preferred stock, $0.01 par value; 1,000,000,000 authorized, none issued
|
—
|
|
—
|
|
||||
|
Common stock, $0.01 par value: 6,000,000,000 authorized, 501,891,243 and 501,808,229 shares issued;
492,855,297 and 497,400,113 shares outstanding at December 31, 2016 and 2015, respectively
|
5
|
|
5
|
|
||||
|
Treasury stock, at cost, 9,035,946 and 4,408,116 shares of common stock at December 31, 2016 and 2015, respectively
|
(421
|
)
|
(203
|
)
|
||||
|
Additional paid-in capital
|
1,024
|
|
1,012
|
|
||||
|
Retained earnings
|
1,477
|
|
876
|
|
||||
|
Accumulated other comprehensive loss
|
(598
|
)
|
(622
|
)
|
||||
|
Total Zoetis Inc. equity
|
1,487
|
|
1,068
|
|
||||
|
Equity attributable to noncontrolling interests
|
12
|
|
23
|
|
||||
|
Total equity
|
1,499
|
|
1,091
|
|
||||
|
Total liabilities and equity
|
$
|
7,649
|
|
$
|
7,913
|
|
||
|
Zoetis
|
||||||||||||||||||||||||||||
|
Accumulated
|
|
Equity
|
|
|||||||||||||||||||||||||
|
Additional
|
|
Other
|
|
Attributable to
|
|
|||||||||||||||||||||||
|
Common
|
|
Treasury
|
|
Paid-in
|
|
Retained
|
|
Comprehensive
|
|
Noncontrolling
|
|
Total
|
|
|||||||||||||||
|
(MILLIONS OF DOLLARS)
|
Stock
(a)
|
|
Stock
(a)
|
|
Capital
|
|
Earnings
|
|
Loss
|
|
Interests
|
|
Equity
|
|
||||||||||||||
|
Balance, December 31, 2013
|
$
|
5
|
|
$
|
—
|
|
$
|
878
|
|
$
|
276
|
|
$
|
(219
|
)
|
$
|
22
|
|
$
|
962
|
|
|||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
583
|
|
—
|
|
4
|
|
587
|
|
||||||||||||||
|
Other comprehensive income/(loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
(131
|
)
|
1
|
|
(130
|
)
|
||||||||||||||
|
Share-based compensation awards
(b)
|
—
|
|
—
|
|
31
|
|
—
|
|
—
|
|
—
|
|
31
|
|
||||||||||||||
|
Defined contribution plan transactions
(c)
|
—
|
|
—
|
|
36
|
|
—
|
|
—
|
|
—
|
|
36
|
|
||||||||||||||
|
Pension plan transfer from Pfizer Inc.
(d)
|
—
|
|
—
|
|
11
|
|
—
|
|
(11
|
)
|
—
|
|
—
|
|
||||||||||||||
|
Employee benefit plan contribution from Pfizer Inc.
(e)
|
—
|
|
—
|
|
2
|
|
—
|
|
—
|
|
—
|
|
2
|
|
||||||||||||||
|
Dividends declared
|
—
|
|
—
|
|
—
|
|
(150
|
)
|
—
|
|
(1
|
)
|
(151
|
)
|
||||||||||||||
|
Balance, December 31, 2014
|
$
|
5
|
|
$
|
—
|
|
$
|
958
|
|
$
|
709
|
|
$
|
(361
|
)
|
$
|
26
|
|
$
|
1,337
|
|
|||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
339
|
|
—
|
|
—
|
|
339
|
|
||||||||||||||
|
Other comprehensive income/(loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
(261
|
)
|
(1
|
)
|
(262
|
)
|
||||||||||||||
|
Share-based compensation awards
(b)
|
—
|
|
(4
|
)
|
51
|
|
—
|
|
—
|
|
—
|
|
47
|
|
||||||||||||||
|
Treasury stock acquired
(f)
|
—
|
|
(199
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(199
|
)
|
||||||||||||||
|
Employee benefit plan contribution from Pfizer Inc.
(e)
|
—
|
|
—
|
|
3
|
|
—
|
|
—
|
|
—
|
|
3
|
|
||||||||||||||
|
Dividends declared
|
—
|
|
—
|
|
—
|
|
(172
|
)
|
—
|
|
(2
|
)
|
(174
|
)
|
||||||||||||||
|
Balance, December 31, 2015
|
$
|
5
|
|
$
|
(203
|
)
|
$
|
1,012
|
|
$
|
876
|
|
$
|
(622
|
)
|
$
|
23
|
|
$
|
1,091
|
|
|||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
821
|
|
—
|
|
(2
|
)
|
819
|
|
||||||||||||||
|
Other comprehensive income/(loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
22
|
|
(1
|
)
|
21
|
|
||||||||||||||
|
Share-based compensation awards
(b)
|
—
|
|
82
|
|
9
|
|
(27
|
)
|
—
|
|
—
|
|
64
|
|
||||||||||||||
|
Treasury stock acquired
(f)
|
—
|
|
(300
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(300
|
)
|
||||||||||||||
|
Employee benefit plan contribution from Pfizer Inc.
(e)
|
—
|
|
—
|
|
3
|
|
—
|
|
—
|
|
—
|
|
3
|
|
||||||||||||||
|
Divestitures
(g)
|
—
|
|
—
|
|
—
|
|
—
|
|
2
|
|
(8
|
)
|
(6
|
)
|
||||||||||||||
|
Dividends declared
|
—
|
|
—
|
|
—
|
|
(193
|
)
|
—
|
|
—
|
|
(193
|
)
|
||||||||||||||
|
Balance, December 31, 2016
|
$
|
5
|
|
$
|
(421
|
)
|
$
|
1,024
|
|
$
|
1,477
|
|
$
|
(598
|
)
|
$
|
12
|
|
$
|
1,499
|
|
|||||||
|
(a)
|
As of
December 31, 2016
and 2015, respectively, there were
492,855,297
and
497,400,113
outstanding shares of common stock and
9,035,946
and
4,408,116
shares or treasury stock. Treasury stock is recognized at the cost to reacquire the shares. For additional information, see
Note 15. Stockholders' Equity
.
|
|
(b)
|
Includes the issuance of shares of Zoetis Inc. common stock and the reacquisition of shares of treasury stock associated with exercises of employee share-based awards. Treasury shares are reacquired from employees for withholding tax purposes in connection with the vesting and exercise of awards under our equity compensation plan. For additional information, see
Note 14. Share-Based Payments
and
Note 15. Stockholders' Equity.
|
|
(c)
|
Reflects company matching and profit-sharing contributions funded through the issuance of shares of Zoetis Inc. common stock for the year ended December 31, 2014. For additional information, see
|
|
(d)
|
Reflects the 2014 transfers of defined benefit pension plans from Pfizer Inc. and the associated reclassification from
Additional Paid in Capital
to
Accumulated Other Comprehensive Loss.
See
Note 13. Benefit Plans.
|
|
(e)
|
Represents contributed capital from Pfizer Inc. associated with service credit continuation for certain Zoetis Inc. employees in Pfizer Inc.'s U.S. qualified defined benefit and U.S. retiree medical plans. See
Note 13. Benefit Plans.
|
|
(f)
|
Reflects the acquisition of treasury shares in connection with the Share Repurchase Program. For additional information, see
Note 15. Stockholders' Equity
.
|
|
(g)
|
Reflects the divestiture of our share of our Taiwan joint venture. See
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Operating Activities
|
||||||||||||
|
Net income before allocation to noncontrolling interests
|
$
|
819
|
|
$
|
339
|
|
$
|
587
|
|
|||
|
Adjustments to reconcile net income before noncontrolling interests to net cash
|
||||||||||||
|
provided by operating activities:
|
||||||||||||
|
Depreciation and amortization expense
|
240
|
|
199
|
|
204
|
|
||||||
|
Share-based compensation expense
|
37
|
|
43
|
|
32
|
|
||||||
|
Restructuring
|
5
|
|
203
|
|
(2
|
)
|
||||||
|
Asset write-offs and asset impairments
|
5
|
|
60
|
|
10
|
|
||||||
|
Gains on sales of assets
|
(26
|
)
|
—
|
|
—
|
|
||||||
|
Provision for losses on inventory
|
105
|
|
94
|
|
94
|
|
||||||
|
Deferred taxes
|
(55
|
)
|
(85
|
)
|
(49
|
)
|
||||||
|
Foreign currency loss related to Venezuela Revaluation, excluding impact on cash
|
—
|
|
6
|
|
—
|
|
||||||
|
Employee benefit plan contribution from Pfizer Inc.
|
3
|
|
3
|
|
2
|
|
||||||
|
Other non-cash adjustments
|
19
|
|
10
|
|
(3
|
)
|
||||||
|
Other changes in assets and liabilities, net of acquisitions and divestitures and transfers with Pfizer Inc.
|
||||||||||||
|
Accounts receivable
|
15
|
|
(58
|
)
|
69
|
|
||||||
|
Inventories
|
(101
|
)
|
(262
|
)
|
(110
|
)
|
||||||
|
Other assets
|
(50
|
)
|
(9
|
)
|
(2
|
)
|
||||||
|
Accounts payable
|
(28
|
)
|
17
|
|
(210
|
)
|
||||||
|
Other liabilities
|
(295
|
)
|
70
|
|
13
|
|
||||||
|
Other tax accounts, net
|
20
|
|
34
|
|
(9
|
)
|
||||||
|
Net cash provided by operating activities
|
713
|
|
664
|
|
626
|
|
||||||
|
Investing Activities
|
||||||||||||
|
Purchases of property, plant and equipment
|
(216
|
)
|
(224
|
)
|
(180
|
)
|
||||||
|
Milestone payment related to previously acquired intangibles
|
—
|
|
—
|
|
(15
|
)
|
||||||
|
Acquisitions
|
(88
|
)
|
(883
|
)
|
—
|
|
||||||
|
Net proceeds from sales of assets
|
90
|
|
2
|
|
9
|
|
||||||
|
Other investing activities
|
—
|
|
(10
|
)
|
(1
|
)
|
||||||
|
Net cash used in investing activities
|
(214
|
)
|
(1,115
|
)
|
(187
|
)
|
||||||
|
Financing Activities
|
||||||||||||
|
(Decrease)/increase in short-term borrowings, net
|
(5
|
)
|
(2
|
)
|
(8
|
)
|
||||||
|
Principal payments on long-term debt
|
(400
|
)
|
—
|
|
—
|
|
||||||
|
Proceeds from issuance of long-term debt—senior notes, net of discount and fees
|
—
|
|
1,236
|
|
—
|
|
||||||
|
Payment of contingent consideration related to previously acquired assets
|
(32
|
)
|
—
|
|
—
|
|
||||||
|
Share-based compensation-related proceeds, net of taxes paid on withholding shares and excess tax benefits
(a)
|
25
|
|
11
|
|
2
|
|
||||||
|
Purchases of treasury stock
(b)
|
(300
|
)
|
(203
|
)
|
—
|
|
||||||
|
Cash dividends paid
|
(188
|
)
|
(168
|
)
|
(146
|
)
|
||||||
|
Cash paid to settle Pharmaq debt
|
—
|
|
(119
|
)
|
—
|
|
||||||
|
Payment of debt issuance costs
|
(3
|
)
|
—
|
|
—
|
|
||||||
|
Other net financing activities with Pfizer Inc.
|
—
|
|
—
|
|
(2
|
)
|
||||||
|
Net cash (used in)/provided by financing activities
|
(903
|
)
|
755
|
|
(154
|
)
|
||||||
|
Effect of exchange-rate changes on cash and cash equivalents
|
(23
|
)
|
(32
|
)
|
(13
|
)
|
||||||
|
Net (decrease)/increase in cash and cash equivalents
|
(427
|
)
|
272
|
|
272
|
|
||||||
|
Cash and cash equivalents at beginning of period
|
1,154
|
|
882
|
|
610
|
|
||||||
|
Cash and cash equivalents at end of period
|
$
|
727
|
|
$
|
1,154
|
|
$
|
882
|
|
|||
|
Supplemental cash flow information
|
||||||||||||
|
Cash paid during the period for:
|
||||||||||||
|
Income taxes
|
$
|
408
|
|
$
|
224
|
|
$
|
278
|
|
|||
|
Interest, net of capitalized interest
|
165
|
|
117
|
|
118
|
|
||||||
|
Non-cash transactions:
|
||||||||||||
|
Intangible asset acquisition
(c)
|
$
|
—
|
|
$
|
—
|
|
$
|
8
|
|
|||
|
Purchases of property, plant and equipment
|
8
|
|
11
|
|
9
|
|
||||||
|
Contingent purchase price consideration
(d)
|
27
|
|
23
|
|
—
|
|
||||||
|
Dividends declared, not paid
|
52
|
|
47
|
|
42
|
|
||||||
|
(a)
|
Effective 2016, excess tax benefits are reflected within operating activities. See
|
|
(b)
|
Reflects the acquisition of treasury shares in connection with the share repurchase program. For additional information, see
|
|
(c)
|
Reflects the non-cash portion of the acquisition of product registration and application rights from Pfizer.
|
|
(d)
|
For 2016, relates primarily to the non-cash portion of the acquisition of a livestock business in South America and a veterinary diagnostics business in Denmark. For 2015, relates primarily to the non-cash portion of the acquisition of certain assets of Abbott Animal Health. See
Note 4A. Acquisitions, Divestitures and Certain Investments: Acquisitions.
|
|
2.
|
Basis of Presentation
|
|
•
|
Goodwill
—goodwill represents the excess of the consideration transferred for an acquired business over the assigned values of its net assets. Goodwill is not amortized.
|
|
•
|
Identifiable intangible assets, less accumulated amortization
—these acquired assets are recorded at our cost. Identifiable intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives. Identifiable intangible assets with indefinite lives that are associated with marketed products are not amortized until a useful life can be determined. Identifiable intangible assets associated with IPR&D projects are not amortized until regulatory approval is obtained. The useful life of an amortizing asset generally is determined by identifying the period in which substantially all of the cash flows are expected to be generated.
|
|
•
|
Property, plant and equipment, less accumulated depreciation
––these assets are recorded at our cost and are increased by the cost of any significant improvements after purchase. Property, plant and equipment assets, other than land and construction-in-progress, are depreciated on a straight-line basis over the estimated useful life of the individual assets. Depreciation begins when the asset is ready for its intended use. For tax purposes, accelerated depreciation methods are used as allowed by tax laws.
|
|
•
|
For finite-lived identifiable intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows associated with the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is found to be greater, we record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we re-evaluate the remaining useful lives of the assets and modify them, as appropriate.
|
|
•
|
For indefinite-lived identifiable intangible assets, such as brands and IPR&D assets, we test for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the indefinite-lived intangible asset is less than its carrying amount. If we conclude it is more likely than not that the fair value is less than the carrying amount, a quantitative test that compares the fair value of the indefinite-lived intangible asset with its carrying value is performed. If the fair value is less than the carrying amount, an impairment loss is recognized. We record an impairment loss, if any, for the excess of book value over fair value. In addition, in all cases of an impairment review other than for IPR&D assets, we re-evaluate whether continuing to characterize the asset as indefinite-lived is appropriate.
|
|
•
|
For goodwill, we test for impairment on at least an annual basis, or more frequently if impairment indicators exist, either by assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, or by performing a quantitative assessment. If we choose to perform a qualitative analysis and conclude it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative fair value test is performed. We determine the implied fair value of goodwill by subtracting the fair value of all the identifiable net assets other than goodwill from the fair value of the reporting unit and record an impairment loss for the excess, if any, of book value of goodwill over the implied fair value. In 2016, we qualitatively assessed, as of October 2, 2016, whether it is more likely than not that the respective fair values of our reporting units are less than their carrying amounts, including goodwill. Based on that assessment, we determined that this condition does not exist for any of our reporting units and therefore concluded that a quantitative fair value test was not required and no impairments were recorded. In 2015, we quantitatively assessed, as of September 27, 2015, the fair value of each of our reporting units using the income approach. The fair value of each reporting unit was found to exceed its respective carrying value, therefore no impairments were recorded.
|
|
•
|
Income approach, which is based on the present value of a future stream of net cash flows.
|
|
•
|
Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities.
|
|
•
|
Cost approach, which is based on the cost to acquire or construct comparable assets less an allowance for functional and/or economic obsolescence.
|
|
•
|
Quoted prices for identical assets or liabilities in active markets (Level 1 inputs).
|
|
•
|
Quoted prices for similar assets or liabilities in active markets or quoted prices for identical or similar assets or liabilities in markets that are not active or are directly or indirectly observable (Level 2 inputs).
|
|
•
|
Unobservable inputs that reflect estimates and assumptions (Level 3 inputs).
|
|
A.
|
Acquisitions
|
|
(MILLIONS OF DOLLARS)
|
|||
|
Cash and cash equivalents
|
$
|
16
|
|
|
Accounts receivable
(a)
|
21
|
|
|
|
Inventories
(b)
|
42
|
|
|
|
Other current assets
|
2
|
|
|
|
Property, plant and equipment
|
11
|
|
|
|
Intangible assets
(c)
|
550
|
|
|
|
Accounts payable
|
(4
|
)
|
|
|
Accrued expenses
(d)
|
(39
|
)
|
|
|
Accrued compensation and related items
|
(4
|
)
|
|
|
Long-term debt
(d)
|
(89
|
)
|
|
|
Noncurrent deferred tax liabilities
(e)
|
(139
|
)
|
|
|
Other non-current liabilities
|
(2
|
)
|
|
|
Total net assets acquired
|
365
|
|
|
|
Goodwill
(f)
|
303
|
|
|
|
Total consideration
|
$
|
668
|
|
|
(a)
|
Accounts receivable were measured at fair value as of the acquisition date and are substantially comprised of gross trade receivables of
$21 million
,
$1 million
of which is expected to be uncollectible.
|
|
(b)
|
Inventories recorded as of the acquisition date reflect fair value adjustments of
$17 million
which relates primarily to finished goods. The fair value was calculated based on estimated selling profit margin.
|
|
(c)
|
The acquisition date fair value of intangible assets acquired was determined using the income approach and consists of the following:
$160 million
related to currently marketed vaccine products,
$30 million
related to currently marketed therapeutics,
$80 million
related to customer relationships and
$280 million
related to IPR&D. The most significant IPR&D project acquired, with an acquisition date fair value of
$150 million
, relates to the salmon rickettsial syndrome
|
|
(d)
|
Pharmaq callable bonds and derivative contracts were recorded at acquisition date fair value and settled immediately following the closing.
|
|
(e)
|
The Pharmaq acquisition was structured as a stock purchase therefore we assumed the historical tax bases of its assets and liabilities. We also established net deferred tax assets and liabilities associated with the fair value adjustments recorded as part of the opening balance sheet.
|
|
(f)
|
Goodwill of
$303 million
is the excess of consideration transferred over the value of net assets acquired and was allocated to our existing reportable segments and is primarily attributable to corporate synergies related to platform functions. The primary strategic purpose of the acquisition was to enhance the company’s existing product portfolio by enabling Zoetis to further expand into aquaculture. The goodwill recorded is not deductible for tax purposes.
|
|
B.
|
Divestitures
|
|
C.
|
Certain Investments
|
|
5.
|
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Restructuring charges and certain acquisition-related costs:
|
||||||||||||
|
Integration costs
(a)
|
$
|
3
|
|
$
|
10
|
|
$
|
8
|
|
|||
|
Transaction costs
(b)
|
—
|
|
9
|
|
—
|
|
||||||
|
Restructuring charges/(benefits)
(c)
:
|
||||||||||||
|
Employee termination costs/(benefits)
|
(2
|
)
|
262
|
|
16
|
|
||||||
|
Asset impairment charges
|
—
|
|
39
|
|
—
|
|
||||||
|
Exit costs
|
4
|
|
—
|
|
1
|
|
||||||
|
Total
Restructuring charges and certain acquisition-related costs
|
$
|
5
|
|
$
|
320
|
|
$
|
25
|
|
|||
|
(a)
|
Integration costs represent external, incremental costs directly related to integrating acquired businesses and primarily include expenditures for consulting and the integration of systems and processes, as well as product transfer costs.
|
|
(b)
|
Transaction costs represent external costs directly related to acquiring businesses and primarily include expenditures for banking, legal, accounting and other similar services.
|
|
(c)
|
The restructuring charges/(benefits) for the years ended December 31,
2016
and
2015
, primarily relate to our operational efficiency initiative and supply network strategy.
|
|
Year Ended December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
Operational efficiency initiative:
|
||||||||
|
Employee termination costs
|
$
|
(8
|
)
|
$
|
253
|
|
||
|
Asset impairment
|
—
|
|
38
|
|
||||
|
Exit costs
|
5
|
|
—
|
|
||||
|
Total restructuring charges/(benefits) for the operational efficiency initiative
|
(3
|
)
|
291
|
|
||||
|
Supply network strategy:
|
||||||||
|
Employee termination costs
|
6
|
|
9
|
|
||||
|
Asset impairment charges
|
—
|
|
1
|
|
||||
|
Total restructuring charges for the supply network strategy
|
6
|
|
10
|
|
||||
|
Total restructuring charges/(benefits) related to the operational efficiency initiative and supply network strategy
|
3
|
|
301
|
|
||||
|
Other operational efficiency initiative charges:
|
||||||||
|
Cost of sales:
|
||||||||
|
Inventory write-offs
|
5
|
|
13
|
|
||||
|
Selling, general and administrative expenses:
|
||||||||
|
Accelerated depreciation
|
1
|
|
—
|
|
||||
|
Consulting fees
|
14
|
|
40
|
|
||||
|
Research and development expenses:
|
||||||||
|
Accelerated depreciation
|
—
|
|
2
|
|
||||
|
Other (income)/deductions:
|
||||||||
|
Net gain on sale of assets
(a)
|
(26
|
)
|
—
|
|
||||
|
Total other operational efficiency initiative charges
|
(6
|
)
|
55
|
|
||||
|
Other supply network strategy charges:
|
||||||||
|
Cost of sales:
|
||||||||
|
Accelerated depreciation
|
6
|
|
1
|
|
||||
|
Consulting fees
|
6
|
|
16
|
|
||||
|
Inventory write-offs
|
1
|
|
—
|
|
||||
|
Total other supply network strategy charges
|
13
|
|
17
|
|
||||
|
Total costs associated with the operational efficiency initiative and supply network strategy
|
$
|
10
|
|
$
|
373
|
|
||
|
(a)
|
For the year ended December 31,
2016
, represents the net gain on the sale of certain manufacturing sites and products, partially offset by the loss on the sale of our share of our Taiwan joint venture, as part of our operational efficiency initiative.
|
|
Employee
|
|
Asset
|
|
|||||||||||||
|
Termination
|
|
Impairment
|
|
Exit
|
|
|||||||||||
|
(MILLIONS OF DOLLARS)
|
Costs
|
|
Charges
|
|
Costs
|
|
Accrual
|
|
||||||||
|
Balance, December 31, 2013
|
$
|
15
|
|
$
|
—
|
|
$
|
6
|
|
$
|
21
|
|
||||
|
Provision/(benefit)
|
16
|
|
—
|
|
1
|
|
17
|
|
||||||||
|
Utilization and other
(a)
|
(13
|
)
|
—
|
|
(6
|
)
|
(19
|
)
|
||||||||
|
Balance, December 31, 2014
|
18
|
|
—
|
|
1
|
|
19
|
|
||||||||
|
Provision/(benefit)
|
262
|
|
39
|
|
—
|
|
301
|
|
||||||||
|
Utilization and other
(a)
|
(59
|
)
|
—
|
|
—
|
|
(59
|
)
|
||||||||
|
Non-cash activity
|
—
|
|
(39
|
)
|
—
|
|
(39
|
)
|
||||||||
|
Balance, December 31, 2015
(b)
|
221
|
|
—
|
|
1
|
|
222
|
|
||||||||
|
Provision/(benefit)
|
(2
|
)
|
—
|
|
4
|
|
2
|
|
||||||||
|
Utilization and other
(a)
|
(129
|
)
|
—
|
|
(5
|
)
|
(134
|
)
|
||||||||
|
Balance, December 31, 2016
(b)
|
$
|
90
|
|
$
|
—
|
|
$
|
—
|
|
$
|
90
|
|
||||
|
(a)
|
Includes adjustments for foreign currency translation.
|
|
(b)
|
At
December 31, 2016
and 2015, included in
Accrued Expenses
(
$61 million
and
$162 million
, respectively) and
Other noncurrent liabilities
(
$29 million
and $
60 million
, respectively).
|
|
6.
|
Other (Income)/Deductions—Net
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Royalty-related income
|
$
|
(30
|
)
|
$
|
(24
|
)
|
$
|
(32
|
)
|
|||
|
Identifiable intangible asset impairment charges
(a)
|
1
|
|
2
|
|
7
|
|
||||||
|
Other asset impairment charges
(b)
|
—
|
|
6
|
|
—
|
|
||||||
|
Net gain on sale of assets
(c)
|
(26
|
)
|
—
|
|
(9
|
)
|
||||||
|
Certain legal matters, net
(d)
|
14
|
|
—
|
|
10
|
|
||||||
|
Foreign currency loss
(e)
|
49
|
|
13
|
|
28
|
|
||||||
|
Foreign currency loss related to Venezuela revaluation
(f)
|
—
|
|
89
|
|
—
|
|
||||||
|
Other, net
(g)
|
(10
|
)
|
(5
|
)
|
3
|
|
||||||
|
Other (income)/deductions—net
|
$
|
(2
|
)
|
$
|
81
|
|
$
|
7
|
|
|||
|
(a)
|
In 2016, the intangible asset impairment charge represents an impairment of finite-lived trademarks related to a canine pain management product. In 2015, charges include the impairment of acquired IPR&D assets related to the termination of a canine oncology project. In 2014, charges primarily include (i) approximately
$6 million
of IPR&D assets related to a pharmaceutical product for dogs acquired with the Fort Dodge Animal Health (FDAH) acquisition in 2009, as a result of the termination of the development program due to a re-assessment of economic viability; and (ii) approximately
$1 million
related to finite-lived developed technology rights and IPR&D due to negative market conditions and the re-assessment of economic viability.
|
|
(b)
|
In 2015, represents impairment charges related to assets held by our joint venture in Taiwan, which was subsequently sold in 2016. See
|
|
(c)
|
In 2016, represents the net gain on sales of certain manufacturing sites and products related to our operational efficiency initiative. In 2014, represents the net gain on sale of land in our joint venture in Taiwan of
$6 million
and the net gain on the government-mandated sale of certain product rights in Argentina and China that were associated with the FDAH acquisition in 2009 of
$3 million
.
|
|
(d)
|
In July 2014 and December 2016, we reached commercial settlements with several large poultry customers in Mexico associated with specific lots of a Zoetis poultry vaccine. Although there have been no quality or efficacy issues with the manufacturing of this vaccine, certain shipments from several lots in Mexico may have experienced an issue in storage with a third party in Mexico that could have impacted their efficacy. We issued a recall of these lots in July 2014 and the product is currently unavailable in Mexico. For 2016, includes a
$14 million
charge related to the commercial settlement in Mexico for these products. For 2014, includes a
$13 million
charge recorded in the second quarter of 2014, which was partially offset by a
$1 million
insurance recovery recorded in the third quarter of 2014, related to the commercial settlement in Mexico. We do not expect any significant additional charges related to this issue. For 2014, also includes an insurance recovery of other litigation-related charges of
$2 million
.
|
|
(e)
|
For 2016, primarily represents costs related to hedging and exposures to certain emerging market currencies, as well as losses related to the depreciation of the Egyptian pound in the fourth quarter of 2016. For 2015, primarily driven by costs related to hedging and exposures to certain emerging market currencies. For 2014, primarily represents costs related to hedging and exposures to certain emerging market currencies, as well as losses related to the depreciation of the Argentine peso in the first quarter of 2014.
|
|
(f)
|
For additional information, see
Note 7. Foreign Currency Loss Related to Venezuela Revaluation
.
|
|
(g)
|
For 2016, primarily represents income associated with certain business employment tax incentive credits, as well as interest income. For 2015, primarily represents inventory losses sustained as result of weather damage at storage facilities in Brazil and Australia, partially offset by interest income and other miscellaneous income. For 2014, includes a pension plan settlement charge related to the sale of a manufacturing plant of
$4 million
.
|
|
7.
|
Foreign Currency Loss Related to Venezuela Revaluation
|
|
8.
|
Tax Matters
|
|
A.
|
Taxes on Income
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
United States
|
$
|
723
|
|
$
|
469
|
|
$
|
455
|
|
|||
|
International
|
505
|
|
76
|
|
365
|
|
||||||
|
Income before provision for taxes on income
|
$
|
1,228
|
|
$
|
545
|
|
$
|
820
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
United States:
|
||||||||||||
|
Current income taxes:
|
||||||||||||
|
Federal
|
$
|
281
|
|
$
|
221
|
|
$
|
179
|
|
|||
|
State and local
|
3
|
|
19
|
|
13
|
|
||||||
|
Deferred income taxes:
|
||||||||||||
|
Federal
|
(38
|
)
|
(63
|
)
|
(14
|
)
|
||||||
|
State and local
|
11
|
|
(15
|
)
|
(3
|
)
|
||||||
|
Total U.S. tax provision
|
257
|
|
162
|
|
175
|
|
||||||
|
International:
|
||||||||||||
|
Current income taxes
|
179
|
|
50
|
|
90
|
|
||||||
|
Deferred income taxes
|
(27
|
)
|
(6
|
)
|
(32
|
)
|
||||||
|
Total international tax provision
|
152
|
|
44
|
|
58
|
|
||||||
|
Provision for taxes on income
(a)(b)(c)
|
$
|
409
|
|
$
|
206
|
|
$
|
233
|
|
|||
|
(a)
|
In
2016
,
the
Provision for taxes on income
reflects the following:
|
|
•
|
the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings from (i) operations and (ii) restructuring charges related to the operational efficiency initiative and supply network strategy, as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures;
|
|
•
|
U.S. tax benefit related to U.S. Research and Development Tax Credit and the U.S. Domestic Production Activities deduction;
|
|
•
|
a
$15 million
discrete tax benefit recorded in the fourth quarter of 2016 related to prior period tax adjustments;
|
|
•
|
a
$10 million
discrete tax benefit recorded in the first quarter of 2016 related to a revaluation of deferred taxes as a result of a change in statutory tax rates;
|
|
•
|
a
$7 million
discrete tax benefit related to the impact of a new accounting standard adopted in 2016 requiring the excess tax benefits for share-based payments to be recognized as a component of
Provision for taxes on income
;
|
|
•
|
a
$2 million
discrete tax benefit related to a revaluation of the company’s deferred tax assets and liabilities using the tax rates expected to be in place going forward;
|
|
•
|
a net tax expense of approximately
$35 million
mainly recorded in the first half of 2016 related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. This net charge represents the recovery of prior tax benefits for the periods 2013 through 2015 offset by the revaluation of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision;
|
|
•
|
tax expense related to the changes in valuation allowances and the resolution of other tax items;
|
|
•
|
tax expense related to changes in uncertain tax positions (see
D. Tax Contingencies
).
|
|
(b)
|
In
2015
, the
Provision for taxes on income
reflects the following:
|
|
•
|
the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings from (i) operations and (ii) restructuring charges related to the operational efficiency initiative and supply network strategy, as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures;
|
|
•
|
the tax expense related to the non-deductible revaluation of the net monetary assets in Venezuela to the SIMADI exchange rate recorded in the fourth quarter of 2015;
|
|
•
|
tax expense related to the changes in valuation allowances and the resolution of other tax items;
|
|
•
|
tax expense related to changes in uncertain tax positions (see
D. Tax Contingencies
);
|
|
•
|
a
$9 million
discrete tax benefit recorded in the first quarter of 2015 related to a revaluation of deferred taxes as a result of a change in tax rates;
|
|
•
|
a
$6 million
discrete tax benefit recorded in the second quarter of 2015 related to prior period tax adjustments; and
|
|
•
|
U.S. tax benefit related to U.S. Research and Development Tax Credit which was permanently extended on December 18, 2015, and the U.S. Domestic Production Activities deduction.
|
|
(c)
|
In
2014
, the
Provision for taxes on income
reflects the following:
|
|
•
|
tax expense related to an
$8 million
discrete tax item during the first quarter of 2014 related to an intercompany inventory adjustment;
|
|
•
|
U.S. tax expense of approximately
$2 million
as a result of providing U.S. deferred income taxes on certain current-year income earned outside the United States that will not be indefinitely reinvested overseas;
|
|
•
|
tax expense related to changes in uncertain tax positions (see
D. Tax Contingencies
)
;
|
|
•
|
U.S. tax benefit related to U.S. Research and Development Tax Credit which was extended on December 19, 2014, and the U.S. Domestic Production Activities deduction; and
|
|
•
|
tax benefit related to the changes in valuation allowances and the resolution of other tax items.
|
|
Year Ended December 31,
|
|||||||||
|
2016
|
|
2015
|
|
2014
|
|
||||
|
U.S. statutory income tax rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
|||
|
State and local taxes, net of federal benefits
|
0.8
|
|
(1.1
|
)
|
0.4
|
|
|||
|
Taxation of non-U.S. operations
(a)(b)
|
(3.0
|
)
|
(3.2
|
)
|
(8.9
|
)
|
|||
|
Unrecognized tax benefits and tax settlements and resolution of certain tax positions
(c)
|
0.4
|
|
1.8
|
|
1.0
|
|
|||
|
Venezuela revaluation
(d)
|
—
|
|
5.6
|
|
—
|
|
|||
|
Annulment of Belgium Excess Profit Ruling
(e)
|
2.9
|
|
—
|
|
—
|
|
|||
|
U.S. Research and Development Tax Credit and U.S. Domestic Production Activities deduction
(f)
|
(1.4
|
)
|
(2.8
|
)
|
(1.5
|
)
|
|||
|
Stock-based compensation
(g)
|
(0.5
|
)
|
—
|
|
—
|
|
|||
|
Non-deductible / non-taxable items
(h)
|
0.2
|
|
1.3
|
|
0.5
|
|
|||
|
All other—net
|
(1.1
|
)
|
1.2
|
|
1.9
|
|
|||
|
Effective tax rate
|
33.3
|
%
|
37.8
|
%
|
28.4
|
%
|
|||
|
(a)
|
The rate impact of taxation of non-U.S. operations was a decrease to our effective tax rate in 2014 through 2016 due to (i) the jurisdictional mix of earnings as tax rates outside the United States are generally lower than the U.S. statutory income tax rate; and (ii) incentive tax rulings in Belgium and in Singapore in 2015 and 2014.
|
|
(b)
|
In all years, the impact to the rate due to increases in uncertain tax positions was more than offset by the jurisdictional mix of earnings and other U.S. tax implications of our foreign operations described in the above footnotes.
|
|
(c)
|
For a discussion about unrecognized tax benefits and tax settlements and resolution of certain tax positions, see
A. Taxes on Income
and
D. Tax Contingencies
.
|
|
(d)
|
The rate impact related to the non-deductible revaluation of the net monetary assets in Venezuela to the SIMADI exchange rate was an increase to our effective tax rate in 2015.
|
|
(e)
|
The rate impact related to the European Commission’s negative decision on the excess profits rulings in Belgium was an increase to our effective tax rate in 2016. This net charge represents the recovery of prior tax benefits for the periods 2013 through 2015 offset by the revaluation of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision.
|
|
(f)
|
In all years, the decrease in the rate was due to the benefit associated with the U.S. Research and Development Tax Credit and the U.S. Domestic Production Activities deduction.
|
|
(g)
|
The rate impact of the adoption of a new accounting standard requiring the excess tax benefits for share-based payments to be recognized as a component of
Provision for taxes on income
was a decrease to our effective tax rate in 2016.
|
|
(h)
|
In all years, non-deductible items include meals and entertainment expenses.
|
|
B.
|
Tax Matters Agreement
|
|
•
|
Pfizer will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments and including those taxes attributable to our business) reportable on a consolidated, combined or unitary return that includes Pfizer or any of its subsidiaries (and us and/or any of our subsidiaries) for any periods or portions thereof ending on or prior to December 31, 2012. We will be responsible for the portion of any such taxes for periods or portions thereof beginning on or after January 1, 2013, as would be applicable to us if we filed the relevant tax returns on a standalone basis.
|
|
•
|
We will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments) that are reportable on returns that include only us and/or any of our subsidiaries, for all tax periods whether before or after the completion of the separation from Pfizer.
|
|
•
|
Pfizer will be responsible for certain specified foreign taxes directly resulting from certain aspects of the separation from Pfizer.
|
|
C.
|
Deferred Taxes
|
|
As of December 31,
|
||||||||
|
2016
|
|
2015
|
|
|||||
|
(MILLIONS OF DOLLARS)
|
Assets (Liabilities)
|
|||||||
|
Prepaid/deferred items
|
$
|
52
|
|
$
|
61
|
|
||
|
Inventories
|
47
|
|
19
|
|
||||
|
Intangibles
|
(203
|
)
|
(223
|
)
|
||||
|
Property, plant and equipment
|
(101
|
)
|
(97
|
)
|
||||
|
Employee benefits
|
63
|
|
48
|
|
||||
|
Restructuring and other charges
|
9
|
|
37
|
|
||||
|
Legal and product liability reserves
|
18
|
|
15
|
|
||||
|
Net operating loss/credit carryforwards
|
94
|
|
86
|
|
||||
|
Unremitted earnings
|
—
|
|
(3
|
)
|
||||
|
All other
|
(2
|
)
|
(1
|
)
|
||||
|
Subtotal
|
(23
|
)
|
(58
|
)
|
||||
|
Valuation allowance
|
(125
|
)
|
(124
|
)
|
||||
|
Net deferred tax liability
(a)(b)
|
$
|
(148
|
)
|
$
|
(182
|
)
|
||
|
(a)
|
The decrease in the total net deferred tax liability from December 31, 2015, to December 31, 2016, is primarily attributable to a decrease in deferred tax liabilities related to intangibles, an increase in deferred tax assets related to inventory, employee benefits, and net operating loss/credit carryforwards, partially offset by a decrease in deferred tax assets related to restructuring and other charges, prepaid/deferred items and an increase in valuation allowances representing the amounts determined to be unrecoverable.
|
|
(b)
|
In
2016
, included in
Noncurrent deferred tax assets
($
96 million
) and
Noncurrent deferred tax liabilities
($
244 million
). In
2015
, included in
Noncurrent deferred tax assets
($
82 million
) and
Noncurrent deferred tax liabilities
($
264 million
).
|
|
D.
|
Tax Contingencies
|
|
•
|
Tax assets associated with uncertain tax positions primarily represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction. As of
December 31, 2016
,
2015
and 2014, we had approximately $
3 million
, $
1 million
and
$1 million
, respectively, in assets associated with uncertain tax positions recorded in
Other noncurrent assets
.
|
|
•
|
Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the amounts taken or expected to be taken in a tax return because of the uncertainties described above. These unrecognized tax benefits relate primarily to issues common among multinational corporations. Substantially all of these unrecognized tax benefits, if recognized, would impact our effective income tax rate.
|
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Balance, January 1
|
$
|
(61
|
)
|
$
|
(54
|
)
|
$
|
(45
|
)
|
|||
|
Increases based on tax positions taken during a prior period
(a)(b)
|
(48
|
)
|
—
|
|
(1
|
)
|
||||||
|
Decreases based on tax positions taken during a prior period
(a)(c)
|
2
|
|
6
|
|
6
|
|
||||||
|
Increases based on tax positions taken during the current period
(a)
|
(9
|
)
|
(14
|
)
|
(15
|
)
|
||||||
|
Settlements
(d)
|
46
|
|
—
|
|
—
|
|
||||||
|
Lapse in statute of limitations
|
2
|
|
1
|
|
1
|
|
||||||
|
Balance, December 31
(e)
|
$
|
(68
|
)
|
$
|
(61
|
)
|
$
|
(54
|
)
|
|||
|
(a)
|
Primarily included in
Provision for taxes on income.
|
|
(b)
|
In 2016, the increases are primarily related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. See
A. Taxes on Income.
|
|
(c)
|
In 2016, the decreases are primarily related to movements on prior year positions. In 2015 and 2014, the decreases are primarily related to movements in foreign translation adjustments on prior year positions and effective settlement of certain issues with the U.S. tax authorities and foreign tax authorities. See
A. Taxes on Income.
|
|
(d)
|
In 2016, the decreases are due to cash payments related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. See
A. Taxes on Income.
|
|
(e)
|
In
2016
, included in
Noncurrent deferred tax assets
(
$3 million
) and
Other taxes payable
(
$65 million
). In
2015
, included in
Noncurrent deferred tax assets
(
$6 million
) and
Other taxes payable
(
$55 million
). In 2014, included in
Noncurrent deferred tax assets
(
$6 million
) and
Other taxes payable
(
$48 million
).
|
|
•
|
Interest related to our unrecognized tax benefits is recorded in accordance with the laws of each jurisdiction and is recorded in
Provision for taxes on income
in our consolidated statements of income. In
2016
, we recorded a net interest expense of $
2 million
; in
2015
, we recorded a net interest expense of $
1 million
; and in
2014
, we recorded a net interest expense of
$1 million
. Gross accrued interest totaled $
6 million
, $
4 million
and
$3 million
as of
December 31, 2016
,
2015
and 2014, respectively, and were included in
Other taxes payable
. Gross accrued penalties totaled
$4 million
,
$3 million
and
$4 million
as of December 31, 2016, 2015 and 2014, respectively, and were included in
Other taxes payable
.
|
|
9.
|
Financial Instruments
|
|
A.
|
Debt
|
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
1.150% 2013 senior notes due 2016
|
—
|
|
400
|
|
||||
|
1.875% 2013 senior notes due 2018
|
750
|
|
750
|
|
||||
|
3.450% 2015 senior notes due 2020
|
500
|
|
500
|
|
||||
|
3.250% 2013 senior notes due 2023
|
1,350
|
|
1,350
|
|
||||
|
4.500% 2015 senior notes due 2025
|
750
|
|
750
|
|
||||
|
4.700% 2013 senior notes due 2043
|
1,150
|
|
1,150
|
|
||||
|
4,500
|
|
4,900
|
|
|||||
|
Unamortized debt discount / debt issuance costs
|
(32
|
)
|
(37
|
)
|
||||
|
Less current portion of long-term debt
|
—
|
|
(400
|
)
|
||||
|
Long-term debt
|
$
|
4,468
|
|
$
|
4,463
|
|
||
|
After
|
|
|||||||||||||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2017
|
|
2018
|
|
2019
|
|
2020
|
|
2021
|
|
2021
|
|
Total
|
|
||||||||||||||
|
Maturities
|
$
|
—
|
|
$
|
750
|
|
$
|
—
|
|
$
|
500
|
|
$
|
—
|
|
$
|
3,250
|
|
$
|
4,500
|
|
|||||||
|
B.
|
Derivative Financial Instruments
|
|
Fair Value of Derivatives
|
||||||||||
|
As of December 31,
|
||||||||||
|
(MILLIONS OF DOLLARS)
|
Balance Sheet Location
|
2016
|
|
2015
|
|
|||||
|
Derivatives Not Designated as Hedging Instruments:
|
||||||||||
|
Foreign currency forward-exchange contracts
|
Other current assets
|
$
|
12
|
|
$
|
8
|
|
|||
|
Foreign currency forward-exchange contracts
|
Other current liabilities
|
(8
|
)
|
(10
|
)
|
|||||
|
Total derivatives not designated as hedging instruments
|
4
|
|
(2
|
)
|
||||||
|
Derivatives Designated as Hedging Instruments:
|
||||||||||
|
Interest rate swap contracts
|
Other current assets
|
17
|
|
—
|
|
|||||
|
Total derivatives designated as hedging instruments
|
17
|
|
—
|
|
||||||
|
Total derivatives
|
$
|
21
|
|
$
|
(2
|
)
|
||||
|
Year Ended December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
Foreign currency forward-exchange contracts
|
$
|
(24
|
)
|
$
|
25
|
|
||
|
Year Ended December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
Interest rate swap contracts
|
$
|
10
|
|
$
|
—
|
|
||
|
10.
|
Inventories
|
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
Finished goods
|
$
|
799
|
|
$
|
758
|
|
||
|
Work-in-process
|
499
|
|
384
|
|
||||
|
Raw materials and supplies
|
204
|
|
325
|
|
||||
|
Inventories
|
$
|
1,502
|
|
$
|
1,467
|
|
||
|
Useful Lives
|
As of December 31,
|
|||||||||
|
(MILLIONS OF DOLLARS)
|
(Years)
|
2016
|
|
2015
|
|
|||||
|
Land
|
—
|
$
|
22
|
|
$
|
22
|
|
|||
|
Buildings
|
33
1
/
3
- 50
|
923
|
|
874
|
|
|||||
|
Machinery, equipment and fixtures
|
3 - 20
|
1,582
|
|
1,434
|
|
|||||
|
Construction-in-progress
|
—
|
212
|
|
185
|
|
|||||
|
2,739
|
|
2,515
|
|
|||||||
|
Less: Accumulated depreciation
|
1,358
|
|
1,208
|
|
||||||
|
Property, plant and equipment
|
$
|
1,381
|
|
$
|
1,307
|
|
||||
|
12.
|
Goodwill and Other Intangible Assets
|
|
A.
|
Goodwill
|
|
(MILLIONS OF DOLLARS)
|
U.S.
|
|
International
|
|
Total
|
|
||||||
|
Balance, December 31, 2014
|
$
|
501
|
|
$
|
475
|
|
$
|
976
|
|
|||
|
Additions / Adjustments
(a)
|
164
|
|
341
|
|
505
|
|
||||||
|
Other
(b)
|
—
|
|
(26
|
)
|
(26
|
)
|
||||||
|
Balance, December 31, 2015
|
$
|
665
|
|
$
|
790
|
|
$
|
1,455
|
|
|||
|
Additions / Adjustments
(a)
|
(4
|
)
|
20
|
|
16
|
|
||||||
|
Other
(b)
|
—
|
|
10
|
|
10
|
|
||||||
|
Balance, December 31, 2016
|
$
|
661
|
|
$
|
820
|
|
$
|
1,481
|
|
|||
|
(a)
|
For 2016, primarily includes a
$16 million
purchase price allocation associated with the acquisition of a veterinary diagnostics business in Denmark and a
$12 million
purchase price allocation associated with the acquisition of a livestock business in South America, offset by a
$13 million
reduction in the acquisition date fair value of goodwill associated with the acquisition of certain assets of Abbott Animal Health. For 2015, primarily reflects the allocation to reportable segments
|
|
(b)
|
Includes adjustments for foreign currency translation. For 2015, also includes a reclassification adjustment of
$5 million
to
Assets held for sale
. For additional information, see
|
|
B.
|
Other Intangible Assets
|
|
As of December 31, 2016
|
As of December 31, 2015
|
|||||||||||||||||||||||
|
Identifiable
|
|
Identifiable
|
|
|||||||||||||||||||||
|
Gross
|
|
Intangible Assets,
|
|
Gross
|
|
Intangible Assets,
|
|
|||||||||||||||||
|
Carrying
|
|
Accumulated
|
|
Less Accumulated
|
|
Carrying
|
|
Accumulated
|
|
Less Accumulated
|
|
|||||||||||||
|
(MILLIONS OF DOLLARS)
|
Amount
|
|
Amortization
|
|
Amortization
|
|
Amount
|
|
Amortization
|
|
Amortization
|
|
||||||||||||
|
Finite-lived intangible assets:
|
||||||||||||||||||||||||
|
Developed technology rights
(a)
|
$
|
1,064
|
|
$
|
(342
|
)
|
$
|
722
|
|
$
|
1,010
|
|
$
|
(274
|
)
|
$
|
736
|
|
||||||
|
Brands
|
213
|
|
(132
|
)
|
81
|
|
212
|
|
(121
|
)
|
91
|
|
||||||||||||
|
Trademarks and tradenames
|
62
|
|
(44
|
)
|
18
|
|
63
|
|
(44
|
)
|
19
|
|
||||||||||||
|
Other
(b)
|
222
|
|
(130
|
)
|
92
|
|
214
|
|
(118
|
)
|
96
|
|
||||||||||||
|
Total finite-lived intangible assets
|
1,561
|
|
(648
|
)
|
913
|
|
1,499
|
|
(557
|
)
|
942
|
|
||||||||||||
|
Indefinite-lived intangible assets:
|
||||||||||||||||||||||||
|
Brands
|
37
|
|
—
|
|
37
|
|
36
|
|
—
|
|
36
|
|
||||||||||||
|
Trademarks and trade names
|
66
|
|
—
|
|
66
|
|
66
|
|
—
|
|
66
|
|
||||||||||||
|
In-process research and development
(a)
|
204
|
|
—
|
|
204
|
|
138
|
|
—
|
|
138
|
|
||||||||||||
|
Product rights
|
8
|
|
—
|
|
8
|
|
8
|
|
—
|
|
8
|
|
||||||||||||
|
Total indefinite-lived intangible assets
|
315
|
|
—
|
|
315
|
|
248
|
|
—
|
|
248
|
|
||||||||||||
|
Identifiable intangible assets
|
$
|
1,876
|
|
$
|
(648
|
)
|
$
|
1,228
|
|
$
|
1,747
|
|
$
|
(557
|
)
|
$
|
1,190
|
|
||||||
|
(a)
|
In 2016, includes the acquisition of intangible assets associated with the purchase of a veterinary diagnostics business in Denmark in the third quarter of 2016, the acquisition of intangible assets associated with the purchase of a livestock business in South America in the first quarter of 2016 and an increase in the acquisition date fair value of intangible assets associated with the acquisition of certain assets of Abbott Animal Health, as well as the impact of foreign exchange. For 2015, includes the acquisition of intangible assets associated with the acquisitions of Pharmaq and certain assets of Abbott Animal Health. For additional information, see
Note 4A. Acquisitions, Divestitures and Certain Investments: Acquisitions.
|
|
(b)
|
Includes the acquisition of intangible assets associated with the purchase of a veterinary diagnostics business in Denmark in the third quarter of 2016. In 2015, primarily includes customer relationships associated with the acquisition of Pharmaq. For additional information, see
|
|
C.
|
Amortization
|
|
(MILLIONS OF DOLLARS)
|
2017
|
|
2018
|
|
2019
|
|
2020
|
|
2021
|
|
||||||||||
|
Amortization expense
|
$
|
96
|
|
$
|
94
|
|
$
|
90
|
|
$
|
86
|
|
$
|
86
|
|
|||||
|
D.
|
Impairments
|
|
A.
|
International Pension Plans
|
|
As of and for the
|
||||||||
|
Year Ended December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
Change in benefit obligation:
|
||||||||
|
Projected benefit obligation, beginning
|
$
|
127
|
|
$
|
129
|
|
||
|
Service cost
|
9
|
|
9
|
|
||||
|
Interest cost
|
3
|
|
3
|
|
||||
|
Plan combinations
(a)
|
—
|
|
12
|
|
||||
|
Changes in actuarial assumptions and other
|
9
|
|
(4
|
)
|
||||
|
Settlements and curtailments
(b)
|
(12
|
)
|
(4
|
)
|
||||
|
Benefits paid
|
(3
|
)
|
—
|
|
||||
|
Net transfer out related to divestitures
(c)
|
(4
|
)
|
—
|
|
||||
|
Adjustments for foreign currency translation
|
1
|
|
(18
|
)
|
||||
|
Benefit obligation, ending
|
130
|
|
127
|
|
||||
|
Change in plan assets:
|
||||||||
|
Fair value of plan assets, beginning
|
72
|
|
63
|
|
||||
|
Plan combinations
(a)
|
—
|
|
9
|
|
||||
|
Actual return on plan assets
|
(1
|
)
|
4
|
|
||||
|
Company contributions
|
9
|
|
8
|
|
||||
|
Settlements and curtailments
(b)
|
(6
|
)
|
(3
|
)
|
||||
|
Acquisitions/divestitures
(c)
|
(4
|
)
|
—
|
|
||||
|
Adjustments for foreign currency translation
|
1
|
|
(8
|
)
|
||||
|
Other––net
|
(3
|
)
|
(1
|
)
|
||||
|
Fair value of plan assets, ending
|
68
|
|
72
|
|
||||
|
Funded status—Projected benefit obligation in excess of plan assets at end of year
(d)
|
$
|
(62
|
)
|
$
|
(55
|
)
|
||
|
(a)
|
Represents the benefit obligations and plan assets acquired in 2015 from Pharmaq (net obligation of approximately
$2 million
).
|
|
(b)
|
For 2016, reflects the impact of employee terminations due to our operational efficiency and supply network strategy initiatives
.
|
|
(c)
|
For 2016, reflects the impact of the sale of our share of our Taiwan joint venture.
|
|
(d)
|
Included in
Other noncurrent liabilities.
|
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
Pension plans with an accumulated benefit obligation in excess of plan assets:
|
||||||||
|
Fair value of plan assets
|
$
|
49
|
|
$
|
37
|
|
||
|
Accumulated benefit obligation
|
88
|
|
69
|
|
||||
|
Pension plans with a projected benefit obligation in excess of plan assets:
|
||||||||
|
Fair value of plan assets
|
58
|
|
72
|
|
||||
|
Projected benefit obligation
|
120
|
|
127
|
|
||||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Service cost
|
$
|
9
|
|
$
|
9
|
|
$
|
4
|
|
|||
|
Interest cost
|
3
|
|
3
|
|
2
|
|
||||||
|
Expected return on plan assets
|
(3
|
)
|
(3
|
)
|
(1
|
)
|
||||||
|
Amortization of net (gains) / losses
|
1
|
|
1
|
|
—
|
|
||||||
|
Special termination benefits
|
—
|
|
1
|
|
1
|
|
||||||
|
Settlement and curtailments (gains) / losses
|
(2
|
)
|
2
|
|
5
|
|
||||||
|
Net periodic benefit cost
|
$
|
8
|
|
$
|
13
|
|
$
|
11
|
|
|||
|
As of December 31,
|
|||||||||
|
(PERCENTAGES)
|
2016
|
|
2015
|
|
2014
|
|
|||
|
Weighted average assumptions used to determine benefit obligations:
|
|||||||||
|
Discount rate
|
2.1
|
%
|
2.6
|
%
|
2.8
|
%
|
|||
|
Rate of compensation increase
|
3.1
|
%
|
3.0
|
%
|
3.6
|
%
|
|||
|
Weighted average assumptions used to determine net benefit cost for the year ended December 31:
|
|||||||||
|
Discount rate
|
2.5
|
%
|
2.8
|
%
|
5.0
|
%
|
|||
|
Expected return on plan assets
|
4.1
|
%
|
4.3
|
%
|
4.0
|
%
|
|||
|
Rate of compensation increase
|
2.9
|
%
|
3.6
|
%
|
4.4
|
%
|
|||
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
Cash and cash equivalents
|
$
|
1
|
|
$
|
2
|
|
||
|
Equity securities: Equity commingled funds
|
22
|
|
23
|
|
||||
|
Debt securities: Government bonds
|
27
|
|
26
|
|
||||
|
Other investments
|
18
|
|
21
|
|
||||
|
Total
(a)
|
$
|
68
|
|
$
|
72
|
|
||
|
(a)
|
Fair values are determined based on valuation inputs categorized as Level 1, 2 or 3 (see
|
|
•
|
Equity commingled funds––observable market prices.
|
|
•
|
Government bonds and other investments––principally observable market prices.
|
|
As of December 31,
|
|||||||||
|
Target allocation
|
|
||||||||
|
percentage
|
|
Percentage of Plan Assets
|
|||||||
|
(PERCENTAGES)
|
2016
|
|
2016
|
|
2015
|
|
|||
|
Cash and cash equivalents
|
0-10%
|
|
0.8
|
%
|
2.4
|
%
|
|||
|
Equity securities
|
0-60%
|
|
35.5
|
%
|
31.8
|
%
|
|||
|
Debt securities
|
20-100%
|
|
39.5
|
%
|
36.2
|
%
|
|||
|
Other investments
|
0-100%
|
|
24.2
|
%
|
29.6
|
%
|
|||
|
Total
|
100
|
%
|
100
|
%
|
100
|
%
|
|||
|
B.
|
Postretirement Plans
|
|
C.
|
Defined Contribution Plans
|
|
14.
|
Share-Based Payments
|
|
A.
|
Share-Based Compensation Expense
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Stock options / stock appreciation rights
|
$
|
10
|
|
$
|
20
|
|
$
|
18
|
|
|||
|
RSUs / DSUs
|
22
|
|
21
|
|
14
|
|
||||||
|
PSUs
|
5
|
|
2
|
|
—
|
|
||||||
|
Share-based compensation expense—total
(a)(b)
|
$
|
37
|
|
$
|
43
|
|
$
|
32
|
|
|||
|
Tax benefit for share-based compensation expense
|
(10
|
)
|
(8
|
)
|
(8
|
)
|
||||||
|
Share-based compensation expense, net of tax
|
$
|
27
|
|
$
|
35
|
|
$
|
24
|
|
|||
|
(a)
|
For each of the years ended
December 31, 2016
, and
December 31, 2015
, we capitalized approximately
$1 million
of share-based compensation expense to inventory. We did not capitalize compensation expense to inventory for the year ended December 31, 2014.
|
|
(b)
|
Includes additional share-based compensation expense as a result of accelerated vesting of the outstanding stock options and the settlement, on a pro-rata basis, of other equity awards of terminated employees in connection with our operational efficiency initiative and supply network strategy for the years ended
December 31, 2016
and 2015, of approximately
$1 million
and
$2 million
, respectively, which is included in
Restructuring charges/(benefits) and certain acquisition-related costs
. (See
G. Accelerated Vesting of Outstanding Equity Awards
).
|
|
B.
|
Stock Options
|
|
Year Ended December 31,
|
|||||||||
|
2016
|
|
2015
|
|
2014
|
|
||||
|
Expected dividend yield
(a)
|
0.89
|
%
|
0.72
|
%
|
0.93
|
%
|
|||
|
Risk-free interest rate
(b)
|
1.57
|
%
|
1.79
|
%
|
2.01
|
%
|
|||
|
Expected stock price volatility
(c)
|
26.70
|
%
|
23.92
|
%
|
24.72
|
%
|
|||
|
Expected term
(d)
(years)
|
6.5
|
|
6.5
|
|
6.5
|
|
|||
|
(a)
|
Determined using a constant dividend yield during the expected term of the Zoetis stock option.
|
|
(b)
|
Determined using the interpolated yield on U.S. Treasury zero-coupon issues.
|
|
(c)
|
For 2016 and 2015, determined using an equal weighting between historical volatility of the Zoetis stock price and implied volatility. The selection of the blended historical and implied volatility approach was based on our assessment that this calculation of expected volatility is more representative of future stock price trends. For 2014, determined using implied volatility.
|
|
(d)
|
Determined using expected exercise and post-vesting termination patterns.
|
|
Weighted-Average
|
|||||||||||||
|
Weighted-Average
|
|
Remaining
|
Aggregate
|
|
|||||||||
|
Exercise Price
|
|
Contractual Term
|
Intrinsic Value
(a)
|
|
|||||||||
|
Shares
|
|
Per Share
|
|
(Years)
|
(MILLIONS)
|
|
|||||||
|
Outstanding, December 31, 2015
|
5,947,719
|
|
$
|
31.03
|
|
||||||||
|
Granted
|
952,729
|
|
42.51
|
|
|||||||||
|
Exercised
|
(1,262,751
|
)
|
28.54
|
|
|||||||||
|
Forfeited
|
(266,913
|
)
|
39.41
|
|
|||||||||
|
Outstanding, December 31, 2016
|
5,370,784
|
|
$
|
33.23
|
|
7.2
|
$
|
109
|
|
||||
|
Exercisable, December 31, 2016
|
1,672,115
|
|
$
|
26.51
|
|
5.9
|
$
|
45
|
|
||||
|
(a)
|
Market price of underlying Zoetis common stock less exercise price.
|
|
Year Ended/As of December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS, EXCEPT PER STOCK OPTION AMOUNTS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Weighted-average grant date fair value per stock option
|
$
|
11.34
|
|
$
|
11.70
|
|
$
|
8.01
|
|
|||
|
Aggregate intrinsic value on exercise
|
23
|
|
5
|
|
1
|
|
||||||
|
Cash received upon exercise
|
36
|
|
9
|
|
2
|
|
||||||
|
Tax benefits realized related to exercise
|
15
|
|
2
|
|
1
|
|
||||||
|
C.
|
Restricted Stock Units (RSUs)
|
|
Weighted-Average
|
|
||||||
|
Grant Date Fair Value
|
|
||||||
|
Shares
|
|
Per Share
|
|
||||
|
Nonvested, December 31, 2015
|
1,955,978
|
|
$
|
34.44
|
|
||
|
Granted
|
738,643
|
|
42.17
|
|
|||
|
Vested
|
(747,412
|
)
|
27.74
|
|
|||
|
Reinvested dividend equivalents
|
15,871
|
|
38.08
|
|
|||
|
Forfeited
|
(170,092
|
)
|
40.67
|
|
|||
|
Nonvested, December 31, 2016
|
1,792,988
|
|
$
|
39.86
|
|
||
|
D.
|
Deferred Stock Units (DSUs)
|
|
E.
|
Performance-Vesting Restricted Stock Units (PSUs)
|
|
Weighted-Average
|
|
||||||
|
Grant Date Fair Value
|
|
||||||
|
Shares
|
|
Per Share
|
|
||||
|
Nonvested, December 31, 2015
|
142,433
|
|
$
|
63.14
|
|
||
|
Granted
|
198,445
|
|
50.20
|
|
|||
|
Vested
|
(11,861
|
)
|
61.18
|
|
|||
|
Reinvested dividend equivalents
|
2,240
|
|
56.57
|
|
|||
|
Forfeited
|
(41,368
|
)
|
56.30
|
|
|||
|
Nonvested, December 31, 2016
|
289,889
|
|
$
|
55.29
|
|
||
|
F.
|
Other Equity-Based or Cash-Based Awards.
|
|
G.
|
Accelerated Vesting of Outstanding Equity Awards
|
|
15.
|
Stockholders' Equity
|
|
(MILLIONS OF SHARES)
|
Common Shares Issued
(a)
|
|
Treasury Stock
(a)
|
|
||
|
Balance, December 31, 2013
|
500.01
|
|
—
|
|
||
|
Stock-based compensation
(b)
|
0.10
|
|
0.01
|
|
||
|
Defined contribution plan
|
1.23
|
|
—
|
|
||
|
Balance, December 31, 2014
|
501.34
|
|
0.01
|
|
||
|
Stock-based compensation
(b)
|
0.47
|
|
0.06
|
|
||
|
Share repurchase program
(c)
|
—
|
|
4.34
|
|
||
|
Balance, December 31, 2015
|
501.81
|
|
4.41
|
|
||
|
Stock-based compensation
(b)
|
0.08
|
|
(1.72
|
)
|
||
|
Share repurchase program
(c)
|
—
|
|
6.34
|
|
||
|
Balance, December 31, 2016
|
501.89
|
|
9.04
|
|
||
|
(a)
|
Shares may not add due to rounding.
|
|
(b)
|
Includes the issuance of shares of common stock and, beginning in the first quarter of 2016, the reissuance of shares from treasury stock in connection with the vesting of employee share-based awards. Treasury stock also includes the reacquisition of shares associated with the vesting of employee share-based awards to satisfy tax withholding requirements. For additional information regarding share-based compensation, see
|
|
(c)
|
In November 2014, the company's Board of Directors authorized a
$500 million
share repurchase program. This program was substantially completed as of December 31, 2016. There were
no
share repurchases under this program during the year ended December 31, 2014.
|
|
Currency Translation
|
|
Accumulated
|
|
|||||||||||||
|
Derivatives
|
|
Adjustment
|
|
Benefit Plans
|
|
Other
|
|
|||||||||
|
Net Unrealized
|
|
Net Unrealized
|
|
Actuarial
|
|
Comprehensive
|
|
|||||||||
|
(MILLIONS OF DOLLARS)
|
(Losses)/Gains
|
|
(Losses)/Gains
|
|
Gains/(Losses)
|
|
(Loss)/Income
|
|
||||||||
|
Balance, December 31, 2013
|
$
|
—
|
|
$
|
(212
|
)
|
$
|
(7
|
)
|
$
|
(219
|
)
|
||||
|
Other comprehensive loss, net of tax
|
—
|
|
(124
|
)
|
(7
|
)
|
(a)
|
(131
|
)
|
|||||||
|
Pension plan transfers from Pfizer Inc.
(b)
|
—
|
|
—
|
|
(11
|
)
|
(11
|
)
|
||||||||
|
Balance, December 31, 2014
|
—
|
|
(336
|
)
|
(25
|
)
|
(361
|
)
|
||||||||
|
Other comprehensive (loss)/gain, net of tax
|
(2
|
)
|
(268
|
)
|
9
|
|
(261
|
)
|
||||||||
|
Balance, December 31, 2015
|
(2
|
)
|
(604
|
)
|
(16
|
)
|
(622
|
)
|
||||||||
|
Other comprehensive gain/(loss), net of tax
|
10
|
|
19
|
|
(7
|
)
|
|
22
|
|
|||||||
|
Divestiture of noncontrolling interest
(c)
|
$
|
—
|
|
$
|
2
|
|
$
|
—
|
|
$
|
2
|
|
||||
|
Balance, December 31, 2016
|
$
|
8
|
|
$
|
(583
|
)
|
$
|
(23
|
)
|
$
|
(598
|
)
|
||||
|
(a)
|
Includes the 2014 settlement charge associated with the 2012 sale of our Netherlands manufacturing facility. See
Note 13. Benefit Plans.
|
|
(b)
|
Relates to transfers of defined benefit pension plans from Pfizer Inc. and the reclassification from
Additional Paid in Capital
to
Accumulated Other Comprehensive Loss
. See
|
|
(c)
|
Reflects the divestiture of our share of our Taiwan joint venture. See
|
|
16.
|
Earnings per Share
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS AND SHARES, EXCEPT PER SHARE DATA)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Numerator
|
||||||||||||
|
Net income before allocation to noncontrolling interests
|
$
|
819
|
|
$
|
339
|
|
$
|
587
|
|
|||
|
Less: net (loss)/income attributable to noncontrolling interests
|
(2
|
)
|
—
|
|
4
|
|
||||||
|
Net income attributable to Zoetis Inc.
|
$
|
821
|
|
$
|
339
|
|
$
|
583
|
|
|||
|
Denominator
|
||||||||||||
|
Weighted-average common shares outstanding
|
495.715
|
|
499.707
|
|
501.055
|
|
||||||
|
Common stock equivalents: stock options, RSUs, DSUs and PSUs
|
2.510
|
|
2.312
|
|
0.970
|
|
||||||
|
Weighted-average common and potential dilutive shares outstanding
|
498.225
|
|
502.019
|
|
502.025
|
|
||||||
|
Earnings per share attributable to Zoetis Inc. stockholders—basic
|
$
|
1.66
|
|
$
|
0.68
|
|
$
|
1.16
|
|
|||
|
Earnings per share attributable to Zoetis Inc. stockholders—diluted
|
$
|
1.65
|
|
$
|
0.68
|
|
$
|
1.16
|
|
|||
|
17.
|
Commitments and Contingencies
|
|
A.
|
Legal Proceedings
|
|
•
|
Product liability and other product-related litigation, which can include injury, consumer, off-label promotion, antitrust and breach of contract claims.
|
|
•
|
Commercial and other matters, which can include product-pricing claims and environmental claims and proceedings.
|
|
•
|
Patent litigation, which typically involves challenges to the coverage and/or validity of our patents or those of third parties on various products or processes.
|
|
•
|
Government investigations, which can involve regulation by national, state and local government agencies in the United States and in other countries.
|
|
B.
|
Guarantees and Indemnifications
|
|
After
|
|
|||||||||||||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2017
|
|
2018
|
|
2019
|
|
2020
|
|
2021
|
|
2021
|
|
Total
|
|
||||||||||||||
|
Maturities
|
$
|
29
|
|
$
|
25
|
|
$
|
20
|
|
$
|
14
|
|
$
|
12
|
|
$
|
54
|
|
$
|
154
|
|
|||||||
|
18.
|
Segment, Geographic and Other Revenue Information
|
|
A.
|
Segment Information
|
|
•
|
Other business activities
, includes our CSS contract manufacturing results, as well as expenses associated with our dedicated veterinary medicine research and development organization, research alliances, U.S. regulatory affairs and other operations focused on the development of our products. Other R&D-related costs associated with non-U.S. market and regulatory activities are generally included in the international commercial segment.
|
|
•
|
Corporate
, which is responsible for platform functions such as business technology, facilities, legal, finance, human resources, business development, and communications, among others. These costs also include compensation costs and other miscellaneous operating expenses not charged to our operating segments, as well as interest income and expense.
|
|
•
|
Certain transactions and events such as (i)
Purchase accounting adjustments
, where we incur expenses associated with the amortization of fair value adjustments to inventory, intangible assets and property, plant and equipment; (ii)
Acquisition-related activities
, where we incur costs associated with acquiring and integrating newly acquired businesses, such as transaction costs and integration costs ; and (iii)
Certain significant items
, which comprise substantive, unusual items that, either as a result of their nature or size, would not be expected to occur as part of our normal business on a regular basis, such as certain costs related to becoming an independent public company, restructuring charges and implementation costs associated with our cost-reduction/productivity initiatives that are not associated with an acquisition, certain asset impairment charges, certain legal and commercial settlements and the impact of divestiture-related gains and losses.
|
|
•
|
Other unallocated
includes (i) certain overhead expenses associated with our global manufacturing operations not charged to our operating segments; (ii) certain costs associated with business technology and finance that specifically support our global manufacturing operations; (iii) certain supply chain and global logistics costs; and (iv) procurement costs.
|
|
Earnings
|
Depreciation and Amortization
(a)
|
|||||||||||||||||||||||
|
Year Ended December 31,
|
Year Ended December 31,
|
|||||||||||||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
2016
|
|
2015
|
|
2014
|
|
||||||||||||
|
U.S.
|
||||||||||||||||||||||||
|
Revenue
|
$
|
2,447
|
|
$
|
2,328
|
|
$
|
2,059
|
|
|||||||||||||||
|
Cost of Sales
|
551
|
|
551
|
|
482
|
|
||||||||||||||||||
|
Gross Profit
|
1,896
|
|
1,777
|
|
1,577
|
|
||||||||||||||||||
|
Gross Margin
|
77.5
|
%
|
76.3
|
%
|
76.6
|
%
|
||||||||||||||||||
|
Operating Expenses
|
388
|
|
389
|
|
401
|
|
||||||||||||||||||
|
Other (income)/deductions
|
—
|
|
(2
|
)
|
—
|
|
||||||||||||||||||
|
U.S. Earnings
|
1,508
|
|
1,390
|
|
1,176
|
|
$
|
27
|
|
$
|
24
|
|
$
|
33
|
|
|||||||||
|
International
|
||||||||||||||||||||||||
|
Revenue
(b)
|
2,390
|
|
2,386
|
|
2,676
|
|
||||||||||||||||||
|
Cost of Sales
|
833
|
|
873
|
|
964
|
|
||||||||||||||||||
|
Gross Profit
|
1,557
|
|
1,513
|
|
1,712
|
|
||||||||||||||||||
|
Gross Margin
|
65.1
|
%
|
63.4
|
%
|
64.0
|
%
|
||||||||||||||||||
|
Operating Expenses
|
501
|
|
570
|
|
685
|
|
||||||||||||||||||
|
Other (income)/deductions
|
2
|
|
2
|
|
2
|
|
||||||||||||||||||
|
International Earnings
|
1,054
|
|
941
|
|
1,025
|
|
44
|
|
46
|
|
50
|
|
||||||||||||
|
Total operating segments
|
2,562
|
|
2,331
|
|
2,201
|
|
71
|
|
70
|
|
83
|
|
||||||||||||
|
Other business activities
|
(309
|
)
|
(293
|
)
|
(318
|
)
|
25
|
|
26
|
|
28
|
|
||||||||||||
|
Reconciling Items:
|
||||||||||||||||||||||||
|
Corporate
|
(684
|
)
|
(606
|
)
|
(559
|
)
|
45
|
|
40
|
|
31
|
|
||||||||||||
|
Purchase accounting adjustments
|
(99
|
)
|
(57
|
)
|
(51
|
)
|
84
|
|
53
|
|
51
|
|
||||||||||||
|
Acquisition-related costs
|
(4
|
)
|
(21
|
)
|
(8
|
)
|
—
|
|
—
|
|
—
|
|
||||||||||||
|
Certain significant items
(c)
|
(57
|
)
|
(592
|
)
|
(205
|
)
|
7
|
|
6
|
|
5
|
|
||||||||||||
|
Other unallocated
|
(181
|
)
|
(217
|
)
|
(240
|
)
|
8
|
|
4
|
|
6
|
|
||||||||||||
|
Total Earnings
(d)
|
$
|
1,228
|
|
$
|
545
|
|
$
|
820
|
|
$
|
240
|
|
$
|
199
|
|
$
|
204
|
|
||||||
|
(a)
|
Certain production facilities are shared. Depreciation and amortization is allocated to the reportable operating segments based on estimates of where the benefits of the related assets are realized.
|
|
(b)
|
Revenue denominated in euros was
$632 million
in
2016
,
$593 million
in
2015
, and
$710 million
in
2014
.
|
|
(c)
|
For 2016, certain significant items primarily includes: (i) Zoetis stand-up costs of $
23 million
; (ii) charges related to our operational efficiency initiative and supply network strategy of
$10 million
; (iii) charges related to a commercial settlement in Mexico of
$14 million
; (iv) charges of
$10 million
associated with changes to our operating model; (v) a benefit of
$1 million
related to other restructuring charges/(benefits) and cost-reduction/productivity initiatives; and (vi) an impairment of finite-lived trademarks of
$1 million
related to a canine pain management product. Stand-up costs include certain nonrecurring costs related to becoming an independent public company, such as new branding (including changes to the manufacturing process for required new packaging), the creation of standalone systems and infrastructure, site separation, and certain legal registration and patent assignment costs.
|
|
(d)
|
Defined as income before provision for taxes on income.
|
|
B.
|
Geographic Information
|
|
As of December 31,
|
||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
||||
|
U.S.
|
$
|
985
|
|
$
|
916
|
|
||
|
International
|
396
|
|
391
|
|
||||
|
Property, plant and equipment, less accumulated depreciation
|
$
|
1,381
|
|
$
|
1,307
|
|
||
|
C.
|
Other Revenue Information
|
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Livestock:
|
||||||||||||
|
Cattle
|
$
|
1,653
|
|
$
|
1,680
|
|
$
|
1,747
|
|
|||
|
Swine
|
602
|
|
668
|
|
695
|
|
||||||
|
Poultry
|
457
|
|
525
|
|
568
|
|
||||||
|
Fish
|
90
|
|
5
|
|
—
|
|
||||||
|
Other
|
79
|
|
80
|
|
93
|
|
||||||
|
2,881
|
|
2,958
|
|
3,103
|
|
|||||||
|
Companion Animal:
|
||||||||||||
|
Horses
|
150
|
|
162
|
|
182
|
|
||||||
|
Dogs and Cats
|
1,806
|
|
1,594
|
|
1,450
|
|
||||||
|
1,956
|
|
1,756
|
|
1,632
|
|
|||||||
|
Contract Manufacturing
|
51
|
|
51
|
|
50
|
|
||||||
|
Total revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
(MILLIONS OF DOLLARS)
|
2016
|
|
2015
|
|
2014
|
|
||||||
|
Anti-infectives
|
$
|
1,255
|
|
$
|
1,305
|
|
$
|
1,398
|
|
|||
|
Vaccines
|
1,245
|
|
1,149
|
|
1,212
|
|
||||||
|
Parasiticides
|
659
|
|
651
|
|
708
|
|
||||||
|
Medicated feed additives
|
500
|
|
505
|
|
479
|
|
||||||
|
Other pharmaceuticals
|
988
|
|
910
|
|
783
|
|
||||||
|
Other non-pharmaceuticals
|
190
|
|
194
|
|
155
|
|
||||||
|
Contract manufacturing
|
51
|
|
51
|
|
50
|
|
||||||
|
Total revenue
|
$
|
4,888
|
|
$
|
4,765
|
|
$
|
4,785
|
|
|||
|
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA)
|
FIRST
|
|
SECOND
|
|
THIRD
|
|
FOURTH
|
|
||||||||
|
2016:
|
||||||||||||||||
|
Revenue
|
$
|
1,162
|
|
$
|
1,208
|
|
$
|
1,241
|
|
$
|
1,277
|
|
||||
|
Costs and expenses
(a)
|
828
|
|
897
|
|
904
|
|
1,026
|
|
||||||||
|
Restructuring charges and certain acquisition-related costs
|
2
|
|
(21
|
)
|
4
|
|
20
|
|
||||||||
|
Income before provision for taxes on income
|
332
|
|
332
|
|
333
|
|
231
|
|
||||||||
|
Provision for taxes on income
|
128
|
|
108
|
|
96
|
|
77
|
|
||||||||
|
Net income before allocation to noncontrolling interests
|
204
|
|
224
|
|
237
|
|
154
|
|
||||||||
|
Net loss attributable to noncontrolling interests
|
—
|
|
—
|
|
(2
|
)
|
—
|
|
||||||||
|
Net income attributable to Zoetis
|
$
|
204
|
|
$
|
224
|
|
$
|
239
|
|
$
|
154
|
|
||||
|
Earnings per common share--basic
|
$
|
0.41
|
|
$
|
0.45
|
|
$
|
0.48
|
|
$
|
0.31
|
|
||||
|
Earnings per common share--diluted
|
$
|
0.41
|
|
$
|
0.45
|
|
$
|
0.48
|
|
$
|
0.31
|
|
||||
|
2015:
|
||||||||||||||||
|
Revenue
|
$
|
1,102
|
|
$
|
1,175
|
|
$
|
1,214
|
|
$
|
1,274
|
|
||||
|
Costs and expenses
(a)
|
871
|
|
936
|
|
928
|
|
1,165
|
|
||||||||
|
Restructuring charges and certain acquisition-related costs
(b)
|
1
|
|
266
|
|
13
|
|
40
|
|
||||||||
|
Income before provision for taxes on income
|
230
|
|
(27
|
)
|
273
|
|
69
|
|
||||||||
|
Provision for taxes on income
|
65
|
|
9
|
|
83
|
|
49
|
|
||||||||
|
Net income/(loss) before allocation to noncontrolling interests
|
165
|
|
(36
|
)
|
190
|
|
20
|
|
||||||||
|
Net income/(loss) attributable to noncontrolling interests
|
—
|
|
1
|
|
1
|
|
(2
|
)
|
||||||||
|
Net income/(loss) attributable to Zoetis
|
$
|
165
|
|
$
|
(37
|
)
|
$
|
189
|
|
$
|
22
|
|
||||
|
Earnings per common share--basic
|
$
|
0.33
|
|
$
|
(0.07
|
)
|
$
|
0.38
|
|
$
|
0.04
|
|
||||
|
Earnings per common share--diluted
|
$
|
0.33
|
|
$
|
(0.07
|
)
|
$
|
0.38
|
|
$
|
0.04
|
|
||||
|
(a)
|
Costs and expenses in the fourth quarter reflect seasonal trends. For 2015, also reflects the Venezuela revaluation. For additional information, see
|
|
(b)
|
The second quarter of 2015 includes a charge for employee termination benefits associated with our operational efficiency initiative and supply network strategy. For additional information, see
|
|
Balance,
|
|
Balance,
|
|
|||||||||||||
|
Beginning of
|
|
End of
|
|
|||||||||||||
|
(MILLIONS OF DOLLARS)
|
Period
|
|
Additions
|
|
Deductions
|
|
Period
|
|
||||||||
|
Year Ended December 31, 2016
|
||||||||||||||||
|
Allowance for doubtful accounts
|
$
|
34
|
|
$
|
7
|
|
$
|
(11
|
)
|
$
|
30
|
|
||||
|
Year Ended December 31, 2015
|
||||||||||||||||
|
Allowance for doubtful accounts
|
32
|
|
9
|
|
(7
|
)
|
34
|
|
||||||||
|
Year Ended December 31, 2014
|
||||||||||||||||
|
Allowance for doubtful accounts
|
31
|
|
5
|
|
(4
|
)
|
32
|
|
||||||||
|
Item 9A.
|
Controls and Procedures
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
|
A.
|
(1) The financial statements and notes to financial statements are filed as part of this report in Item 8. Financial Statements and Supplementary Data.
|
|
Zoetis Inc.
|
|
|
By:
|
/S/ JUAN RAMÓN ALAIX
|
|
Juan Ramón Alaix
|
|
|
Chief Executive Officer and Director
|
|
|
Name
|
Title
|
Date
|
||
|
/S/ JUAN RAMÓN ALAIX
|
Chief Executive Officer and Director
|
February 16, 2017
|
||
|
Juan Ramón Alaix
|
(Principal Executive Officer)
|
|||
|
/S/ GLENN DAVID
|
Executive Vice President and
|
February 16, 2017
|
||
|
GLENN DAVID
|
Chief Financial Officer
(Principal Financial Officer and
Principal Accounting Officer)
|
|||
|
/S/ MICHAEL B. MCCALLISTER
|
Chairman and Director
|
February 16, 2017
|
||
|
Michael B. McCallister
|
||||
|
/S/ PAUL M. BISARO
|
Director
|
February 16, 2017
|
||
|
Paul M. Bisaro
|
||||
|
/S/ FRANK A. D'AMELIO
|
Director
|
February 16, 2017
|
||
|
Frank A. D’Amelio
|
||||
|
/S/ SANJAY KHOSLA
|
Director
|
February 16, 2017
|
||
|
Sanjay Khosla
|
||||
|
/s/ GREGORY NORDEN
|
Director
|
February 16, 2017
|
||
|
Gregory Norden
|
||||
|
/S/ LOUISE M. PARENT
|
Director
|
February 16, 2017
|
||
|
Louise M. Parent
|
||||
|
/S/ WILLIE M. REED
|
Director
|
February 16, 2017
|
||
|
Willie M. Reed
|
||||
|
/s/ ROBERT W. SCULLY
|
Director
|
February 16, 2017
|
||
|
Robert W. Scully
|
||||
|
/S/ WILLIAM C. STEERE, JR.
|
Director
|
February 16, 2017
|
||
|
William C. Steere, Jr.
|
||||
|
Exhibit 2.1
|
Share Purchase Agreement, dated as of November 2, 2015, by and among SalarLux Parent S.à.r.l., Salar Invest AS
|
|
|
and Zoetis Inc. (incorporated by reference to Exhibit 2.1 to Zoetis Inc.'s Current Report on Form 8-K filed on
|
||
|
November 2, 2015 (File No. 001-35797))
|
||
|
Exhibit 3.1
|
Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to Zoetis Inc.'s Quarterly
|
|
|
Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))
|
||
|
Exhibit 3.2
|
By-laws of the Registrant, amended and restated as of February 19, 2016 (incorporated by reference to Exhibit 3.2 to Zoetis
|
|
|
Inc.’s 2015 Annual Report on Form 10-K filed on February 24, 2016 (File No. 001-35797))
|
||
|
Exhibit 4.1
|
Specimen Class A Common Stock Certificate (incorporated by reference to Exhibit 4.1 of Zoetis Inc.’s registration
|
|
|
statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.2
|
Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company Americas, as trustee
|
|
|
(incorporated by reference to Zoetis Inc.'s registration statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.3
|
First Supplemental Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company
|
|
|
Americas, as trustee (incorporated by reference to Exhibit 4.3 of Zoetis Inc.'s registration statement on Form S-1
|
||
|
(File No. 333-183254))
|
||
|
Exhibit 4.4
|
Second Supplemental Indenture, dated November 13, 2015, between Zoetis Inc. and Deutsche Bank Trust Company
|
|
|
Americas, as trustee (incorporated by reference to Exhibit 4.1 to Zoetis Inc.’s Current Report on Form 8-K filed on
|
||
|
November 13, 2015 (File No. 001-35797))
|
||
|
Exhibit 4.5
|
Form of 1.875% Senior Notes due 2018 (incorporated by reference to Exhibit 4.5 of Zoetis Inc.'s registration statement on
|
|
|
Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.6
|
Form of 3.450% Senior Notes due 2020 (incorporated by reference to Exhibit 4.3 to Zoetis Inc.’s Current Report on
|
|
|
Form 8-K filed on November 13, 2015 (File No. 001-35797))
|
||
|
Exhibit 4.7
|
Form of 3.250% Senior Notes due 2023 (incorporated by reference to Exhibit 4.6 of Zoetis Inc.'s registration statement on
|
|
|
Form S-1 (File No. 333-183254))
|
||
|
Exhibit 4.8
|
Form of 4.500% Senior Notes due 2025 (incorporated by reference to Exhibit 4.4 to Zoetis Inc.’s Current Report on
|
|
|
Form 8-K filed on November 13, 2015 (File No. 001-35797))
|
||
|
Exhibit 4.9
|
Form of 4.700% Senior Notes due 2043 (incorporated by reference to Exhibit 4.7 of Zoetis Inc.'s registration statement on
|
|
|
Form S-1 (File No. 333-183254))
|
||
|
Exhibit 10.1
|
Global Separation Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to
|
|
|
Exhibit 10.1 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797))
|
||
|
Exhibit 10.2
|
Transitional Services Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to
|
|
|
Exhibit 10.2 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797))
|
||
|
Exhibit 10.3
|
Tax Matters Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to
|
|
|
Exhibit 10.3 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797))
|
||
|
Exhibit 10.4
|
Research and Development Collaboration and License Agreement, dated February 6, 2013, by and between Zoetis Inc.
|
|
|
and Pfizer Inc. (incorporated by reference to Exhibit 10.4 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on
|
||
|
March 28, 2013 (File No. 001-35797))
|
||
|
Exhibit 10.5
|
Employee Matters Agreement (incorporated by reference to Exhibit 10.5 of Zoetis Inc.'s registration statement on Form S-1
|
|
|
(File No. 333-183254))
|
||
|
Exhibit 10.6
|
Pfizer Inc. 2004 Stock Plan, as Amended and Restated (incorporated by reference to Exhibit 10.6 of Zoetis Inc.'s registration
|
|
|
statement on Form S-1 (File No. 333-183254))*
|
||
|
Exhibit 10.7
|
Pfizer Inc. Amended and Restated Nonfunded Supplemental Retirement Plan, together with all material Amendments
|
|
|
(incorporated by reference to Exhibit 10.7 of Zoetis Inc.'s registration statement on Form S-1 (File No. 333-183254))*
|
||
|
Exhibit 10.8
|
Patent and Know-How License Agreement (Zoetis as licensor), dated February 6, 2013, by and between Zoetis Inc. and
|
|
|
Pfizer Inc. (incorporated by reference to Exhibit 10.8 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on
|
||
|
March 28, 2013 (File No. 001-35797))
|
||
|
Exhibit 10.9
|
Patent and Know-How License Agreement (Pfizer as licensor), dated February 6, 2013, by and between Zoetis Inc. and
|
|
|
Pfizer Inc. (incorporated by reference to Exhibit 10.9 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on
|
||
|
March 28, 2013 (File No. 001-35797))
|
||
|
Exhibit 10.10
|
Trademark and Copyright License Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.10 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013
|
||
|
(File No. 001-35797))
|
||
|
Exhibit 10.11
|
Environmental Matters Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc.
|
|
|
(incorporated by reference to Exhibit 10.13 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013)
|
||
|
(File No. 001-35797))
|
||
|
Exhibit 10.12
|
Master Manufacturing and Supply Agreement, dated October 1, 2012, by and between Pfizer Inc. and Zoetis Inc. (Pfizer as
|
|
|
manufacturer) (incorporated by reference to Exhibit 10.14 of Zoetis Inc.'s registration statement on Form S-1
|
||
|
(File No. 333-183254))
|
||
|
Exhibit 10.13
|
Registration Rights Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to
|
|
|
Exhibit 10.15 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797))
|
||
|
Exhibit 10.14
|
Zoetis Inc. 2013 Equity and Incentive Plan (incorporated by reference to Exhibit 10.16 to Zoetis Inc.’s 2012 Annual Report
|
|
|
on Form 10-K filed on March 28, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.15
|
Sale of Business Severance Plan (incorporated by reference to Exhibit 10.17 to Zoetis Inc.’s 2012 Annual Report on
|
|
|
Form 10-K filed on March 28, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.16
|
Revolving Credit Agreement, dated as of December 21, 2016, among Zoetis Inc., the lenders party thereto and JPMorgan
|
|
|
Chase Bank, N.A., as administrative agent (incorporated by reference to Exhibit 10.1 of Zoetis Inc.'s Current Report
|
||
|
on Form 8-K filed on December 21, 2016 (File No. 001-35797))
|
||
|
Exhibit 10.17
|
Form of Indemnification Agreement for directors and officers (incorporated by reference to Exhibit 10.19 of Zoetis Inc.'s
|
|
|
registration statement on Form S-1 (File No. 333-183254))
|
||
|
Exhibit 10.18
|
Registration Rights Agreement, dated as of January 28, 2013, by and among Zoetis Inc. and Merrill Lynch, Pierce, Fenner &
|
|
|
Smith Incorporated, Barclays Capital Inc., J.P. Morgan Securities LLC and Deutsche Bank Securities Inc., as representatives
|
||
|
of the several initial purchasers (incorporated by reference to Exhibit 10.20 of Zoetis Inc.'s registration statement on Form
|
||
|
S-1 (File No. 333-183254))
|
||
|
Exhibit 10.19
|
Form of Restricted Stock Unit Award agreement (incorporated by reference to Exhibit 10.21 to Zoetis Inc.’s 2012 Annual
|
|
|
Report on Form 10-K filed on March 28, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.20
|
Form of Stock Option Award agreement (incorporated by reference to Exhibit 10.22 to Zoetis Inc.’s 2012 Annual Report
|
|
|
on Form 10-K filed on March 28, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.21
|
Form of Non-Employee Director Deferred Stock Unit Award agreement (incorporated by reference to Exhibit 10.22
|
|
|
on Form 10-K filed on March 28, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.22
|
Form of Cash Award agreement (incorporated by reference to Exhibit 10.24 to Zoetis Inc.’s 2012 Annual Report on
|
|
|
Form 10-K filed on March 28, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.23
|
Form of Performance Restricted Stock Unit Award Agreement, effective as of February 27, 2015 (incorporated by
|
|
|
reference to Exhibit 99.1 to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))*
|
||
|
Exhibit 10.24
|
Form of Restricted Stock Unit Award Agreement, effective as of February 27, 2015 (incorporated by reference to
|
|
|
Exhibit 99.2 to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))*
|
||
|
Exhibit 10.25
|
Form of Stock Option Award Agreement, effective as of February 27, 2015 (incorporated by reference to Exhibit 99.3
|
|
|
to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))*
|
||
|
Exhibit 10.26
|
Form of Cash Award Agreement, effective as of February 27, 2015 (incorporated by reference to Exhibit 99.4 to
|
|
|
Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))*
|
||
|
Exhibit 10.27
|
Non-Employee Director Deferred Compensation Plan (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s
|
|
|
Current Report on Form 8-K filed on May 7, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.28
|
Zoetis Executive Severance Plan (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s Quarterly Report on Form 10-Q
|
|
|
filed on August 14, 2013 (File No. 001-35797))*
|
||
|
Exhibit 10.29
|
Zoetis Supplemental Savings Plan, as amended and restated, effective September 15, 2014 (incorporated by reference to
|
|
|
Exhibit 10.4 to Zoetis Inc.'s Quarterly Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))*
|
||
|
Exhibit 10.30
|
Zoetis Equity Deferral Plan, effective November 1, 2014 (incorporated by reference to Exhibit 10.5 to Zoetis Inc.’s
|
|
|
Quarterly Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))*
|
||
|
Exhibit 10.31
|
Offer Letter between Zoetis Inc. and Paul Herendeen, dated July 31, 2014 (incorporated by reference to Exhibit 10.3
|
|
|
to Zoetis Inc.’s Quarterly Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))*
|
||
|
Exhibit 10.32
|
Letter Agreement, dated as of February 3, 2015, by and among Zoetis and Pershing Square Capital Management, L.P.
|
|
|
and certain affiliates thereof and Sachem Head Capital Management LP and certain affiliates thereof (incorporated by
|
||
|
reference to Exhibit 99.1 to Zoetis Inc.’s Current Report on Form 8-K filed on February 4, 2015 (File No. 001-35797))
|
||
|
Exhibit 12
|
Computation of Ratio of Earnings to Fixed Charges †
|
|
|
Exhibit 21.1
|
Subsidiaries of the Registrant †
|
|
|
Exhibit 23.1
|
Consent of KPMG LLP †
|
|
|
Exhibit 24.1
|
Power of Attorney (included as part of signature page) †
|
|
|
Exhibit 31.1
|
Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 †
|
|
|
Exhibit 31.2
|
Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 †
|
|
|
Exhibit 32.1
|
Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the
|
|
|
Sarbanes-Oxley Act of 2002 †
|
||
|
Exhibit 32.2
|
Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the
|
|
|
Sarbanes-Oxley Act of 2002 †
|
||
|
EX-101.INS
|
INSTANCE DOCUMENT
|
|
|
EX-101.SCH
|
SCHEMA DOCUMENT
|
|
|
EX-101.CAL
|
CALCULATION LINKBASE DOCUMENT
|
|
|
EX-101.LAB
|
LABELS LINKBASE DOCUMENT
|
|
|
EX-101.PRE
|
PRESENTATION LINKBASE DOCUMENT
|
|
|
EX-101.DEF
|
DEFINITION LINKBASE DOCUMENT
|
|
|
†
|
Filed herewith
|
|
*
|
Management contracts or compensatory plans or arrangements
|